Abstract
The objective of the present study was to compare two components of executive functioning, response monitoring and inhibition in bipolar disorder (BP) and schizophrenia (SZ). The saccadic countermanding task is a translational paradigm optimized for detecting subtle abnormalities in response monitoring and response inhibition. We have previously reported countermanding performance abnormalities in SZ, but the degree to which these impairments are shared by other psychotic disorders is unknown. 18 BP, 17 SZ, and 16 demographically-matched healthy controls (HC) participated in a saccadic countermanding task. Performance on the countermanding task is approximated as a race between movement generation and inhibition processes; this model provides an estimate of the time needed to cancel a planned movement. Response monitoring was assessed by the reaction time (RT) adjustments based on trial history. Like SZ patients, BP patients needed more time to cancel a planned movement. The two patient groups had equivalent inhibition efficiency. On trial history-based RT adjustments, however, we found a trend towards exaggerated trial history-based slowing in SZ compared to BP. Findings have implications for understanding the neurobiology of cognitive control, for defining the etiological overlap between schizophrenia and bipolar disorder and for developing pharmacological treatments of cognitive impairments.
Keywords: saccades, response inhibition, response monitoring, cognitive control, stop signal, countermanding
1. Introduction
Executive functioning refers to cognitive abilities involved in the control of thought and action. Despite strong empirical support for executive functioning impairments in schizophrenia that predict functional outcome (Bilder et al., 2000; Hutton et al., 1998), evidence for stable impairments in executive functioning in bipolar disorder, as measured by standard neuropsychological tests, is equivocal. Although current diagnostic classification considers schizophrenia and bipolar disorder to be distinct disorders, there is ample evidence for neurobiological overlap (e.g. Lichtenstein et al., 2009; Maier et al., 2006; Moskvina et al., 2009). Mapping the overlapping and unique cognitive markers in these two clinical populations can contribute to our understanding of shared etiology and pathophysiology of bipolar disorder and schizophrenia.
Although a recent meta-analysis reported executive function impairments of medium to large effect sizes in euthymic bipolar patients, particularly response inhibition (Bora et al., 2009), a subsequent large-scale study found that impairments in response inhibition were largely symptom-dependent (Langenecker et al., 2010). Along with differences in clinical status of participant across studies, heterogeneity of tasks used to assess executive function likely also gives rise to discrepant findings across studies, as different tasks place different demands on various subdivisions of executive functioning. As an alternative to these standard neuropsychological tests, a translational approach that applies simple experimental tasks that have been performed by humans and non-human primates under similar conditions is valuable in outlining precise cognitive phenotypes and making specific hypotheses about the etiology of putative deficits.
One such translational paradigm that has been used to explore the cellular basis of executive functioning in non-human primate studies, is the saccadic countermanding task (Hanes and Schall, 1995). In this task, participants must make a speeded eye movement to a target unless a stop-signal is presented at some delay following the initial target. On these trials, participants must inhibit the prepared eye movement. The time needed to cancel a movement, the stop signal reaction time (SSRT), can be estimated from the distribution of RTs on no-stop signal trials and the probability of making a saccade given that a stop signal occurred, assuming a race between STOP and GO processes (Logan and Cowan, 1984). Along with response inhibition, trial-by-trial adjustments in response speed have been used to measure response monitoring (e.g. Emeric et al., 2007). Neural activity necessary to accomplish the preparation and inhibition of saccades has been identified in the frontal eye fields (FEF; Brown et al., 2008; Hanes et al., 1998) and the superior colliculus (SC; Paré and Hanes, 2003). In contrast, neurons in the medial frontal cortex display performance monitoring signals such as those associated with errors, reward and conflict (Ito et al., 2003; Stuphorn et al., 2000). These performance-monitoring signals may contribute to specific behavioral adjustments based on trial history.
In a previous study, we found that, compared with controls, patients with schizophrenia had longer SSRT, which was associated with occupational functioning (Thakkar et al., 2011). To our knowledge, performance on the saccadic countermanding task has not been investigated in individuals with bipolar disorder. Although there are data from the manual (keypress) version of this task, the results are mixed. Generally, impairments in adult bipolar patients on the manual countermanding task appear to be state-related. Strakowski, et al. (2009) reported that BP in a manic/mixed episode had longer SSRT; however, SSRT in these same patients had normalized after converting to depression or euthymia (Strakowski, et al., 2010).
Inhibition of eye movements has been measured in bipolar disorder using the antisaccade task. Similar to the saccadic countermanding task, participants are required to inhibit a saccade to a visual target; however, in the antisaccade task, participants are instructed to saccade to the mirror location in the opposite hemifield. There is robust evidence for higher antisaccade error rates and longer antisaccade latency in patients with schizophrenia (see Clementz, 1998; Gooding and Basso, 2008; Hutton and Ettinger, 2006 for reviews). Compared to controls, elevated antisaccade error rates have also been reported in bipolar disorder (Gooding and Tallent, 2001; Harris et al., 2009; Katsanis et al., 1997; Martin et al., 2007; McDowell and Clementz, 1997; Tien et al., 1996; but see Crawford et al., 1995) and mixed affective groups comprising mainly bipolar patients (Sereno and Holzman, 1995). With regards to diagnostic specificity of antisaccade performance, results are mixed. Some studies report greater anitisaccade errors in schizophrenia patients compared to bipolar patients (Crawford et al., 1995; Gooding and Tallent, 2001; McDowell and Clementz, 1997), and others report no difference between the two groups (Harris et al., 2009; Katsanis et al., 1997; Martin et al., 2007). Given evidence for temporal instability of antisaccade deficits in bipolar disorder (Gooding et al., 2004), differences across studies could be attributed to differences in clinical status of study samples. It is important to note, however, that the antisaccade and saccadic countermanding tasks provide different information about two dissociable aspects of response inhibition: proactive inhibition and reactive inhibition. Proactive inhibition, also referred to as action restraint, refers to the ability to prepare to inhibit based on advance information. Reactive inhibition, or action cancellation, refers to the ability to rapidly interrupt an ongoing action plan. Although the antisaccade and countermanding task tax both aspects of inhibition, the countermanding task provides much more information about reactive inhibition ability.
In a previous study, we also observed idiosyncratic response monitoring in SZ. Although SZ and controls both slowed down following both cancelled and non-cancelled trials, SZ slowed down nearly twice as much following correctly inhibited trials. That is, they were more influenced by the prior trial than controls. In contrast to response inhibition, response monitoring has not been investigated in bipolar disorder to our knowledge. Despite evidence for general executive dysfunction (Bora et al., 2009), there are no published studies that have examined history-based adjustments in response speed in bipolar disorder.
To summarize, we previously observed slower response inhibition and exaggerated trial history effects in schizophrenia during the saccadic countermanding task. The aim of the current study was to investigate the diagnostic specificity of these findings by investigating cognitive control of gaze during the same task in bipolar disorder.
2. Methods and Materials
2.1 Participants
Individuals who met the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for bipolar disorder or schizophrenia were recruited from outpatient psychiatric facilities in Nashville, TN. Diagnoses were confirmed using structured clinical interviews (SCID-IV; First et al., 1995). All but two BP were medicated with mood stabilizers, antidepressants, atypical antipsychotics, or a combination. All SZ were medicated with a combination of atypical antipsychotic medications, mood stabilizers, and antidepressants. Detailed information about medication is presented in Supplementary Data 1. Healthy, unmedicated control subjects (HC) without a personal and self-reported family history of DSM-IV Axis I disorders were recruited from the same community by advertisements. Personal history of Axis I disorders was also assessed using the SCID-IV in HC. The SZ and HC samples are identical to those published in Thakkar, et al. (2011).
Clinical symptoms were assessed with the Brief Psychiatric Rating Scale (BPRS; Overall and Gorham, 1962; BP and SZ), Hamilton Rating Scale for Depression (HRSD; Hamilton, 1980; BP only), Young Mania Rating Scale (YMRS; Young et al., 1978; BP only), the Scale for the Assessment of Positive Symptoms (SAPS; Andreasen, 1984; SZ only), and the Scale for the Assessment of Negative Symptoms (SANS; Andreasen, 1983; SZ only). Subscale scores of the BPRS were calculated based on Ventura, et al. (2000): Positive, Negative, Depression-Anxiety, and Manic-Excitement. Social and occupational functioning was assessed by the 79-item Social Functioning Scale (SFS; Birchwood et al., 1990), which assesses seven areas: social engagement, interpersonal communication, frequency of daily living activities, competence of daily living activities, recreational activities, social activities, and occupational activity. The North American Adult Reading Test, (NAART; Blair and Spreen, 1989) or Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999) were used to assess IQ.
All participants were screened to exclude self-reported substance use, neurological disorders, history of head injury, inability to fixate, and excessive sleepiness. All subjects had normal or corrected-to-normal vision. Two SZ were excluded based on countermanding task performance, as outlined in the Statistical Methods section. Analyses were conducted on the remaining 18 BP, 17 SZ, and 16 HC; demographic data are presented in Table 1. The three groups were matched for age, sex, and handedness. Years of education were significantly higher in HC than SZ and BP. Estimated IQ was higher in HC than SZ, but not BP. BP and SZ were matched on all demographic variables, including social and occupational functioning, and general psychiatric symptoms as indexed by BPRS score. However, SZ were taking a significantly higher antipsychotic dose and showed a non-significant trend towards longer length of illness. Ten out of the 18 bipolar patients had a lifetime history of psychosis. All participants gave written informed consent approved by the Vanderbilt Institutional Review Board, and the study was carried out in accordance with the provisions of the World Medical Association Declaration of Helsinki. Participants were compensated for their time.
Table 1.
| HC (n=16) |
BP (n=18) |
SZ (n=17) |
HC v SZ | HC v BP | SZ v BP | ||||
|---|---|---|---|---|---|---|---|---|---|
| t | p | t | p | t | p | ||||
| Age | 34.9 (7.9) | 32.0 (8.4) | 36.0 (7.7) | 0.4 | 0.7 | 1.0 | 0.3 | 1.5 | 0.15 |
| Sex | 7F / 9M | 10F / 8M | 6 F/11 M | ϕ=0.2 | 0.7 | ϕ=0.7 | 0.5 | ϕ=1.45 | 0.31 |
| IQ | 110.5 (4.6) | 105.8 (10.2) | 102.6 (10.8) | 2.7 | 0.01 | 1.7 | 0.1 | 0.90 | 0.37 |
| Years of Education | 16.2 (2.1) | 13.4 (2.3) | 13.4 (1.9) | 4.0 | <0.001 | 3.6 | 0.001 | 0.13 | 0.9 |
| Handedness | 59.7 (67.7) | 69.4 (31.3) | 51.5 (55.0) | 0.4 | 0.7 | 0.5 | 0.6 | 1.2 | 0.24 |
| SFS Total | 156.8 (14.6) | 132.3 (18.4) | 132.3 (17.9) | 3.4 | 0.002 | 4.3 | <0.001 | 0.007 | 0.99 |
| SFS Employment | 9.7 (0.7) | 6.6 (3.3) | 5.2 (3.8) | 4.6 | <0.001 | 3.6 | 0.001 | 1.16 | 0.26 |
| Years of Illness | n/a | 10.6 (7.8) | 15.7 (8.3) | 1.9 | 0.07 | ||||
| CPZ Equivalent | n/a | 160.7 (275.8) | 383.7 (354.3) | 2.02 | 0.05 | ||||
| BPRS | n/a | 12.8 (7.6) | 11.8 (7.1) | 0.4 | 0.7 | ||||
| YMARS | n/a | 8.5 (7.9) | n/a | ||||||
| HRSD | n/a | 10.1 (6.7) | n/a | ||||||
| SAPS | n/a | n/a | 13.8 (19.1) | ||||||
| SANS | n/a | n/a | 20.8 (16.7) | ||||||
2.2 Apparatus and Stimuli
Eye position was monitored using the EyeLink II eyetracker (SR Research, Canada) at a sampling rate of 250 Hz with average gaze position error <0.5°, noise limited to <0.01° RMS. Saccades were detected on-line using a velocity criterion (35°/sec). Subjects were seated 57cm from the computer monitor with their head in a chinrest. The fixation and targets subtended 1° and were light gray (34 cd/m2) on a darker gray (18 cd/m2) background.
2.3 Design and Procedure
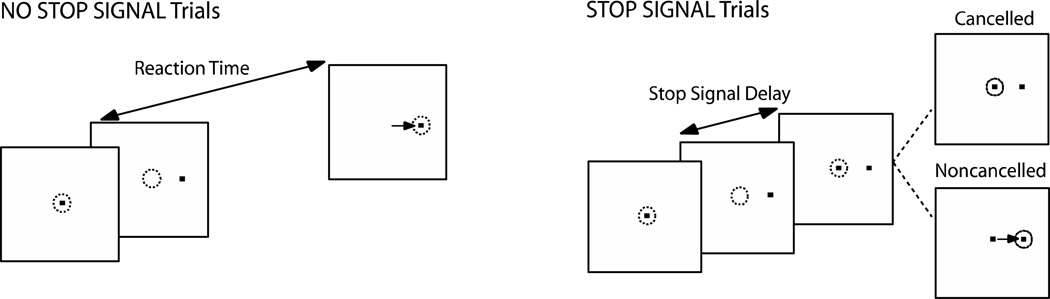
Participants performed a saccadic countermanding task (Figure 1). Seventy percent of the trials were no-stop-signal trials. These trials required subjects to fixate on the central fixation spot until it disappeared (after a random delay between 500–1000 ms) and a peripheral target appeared at one of two randomly selected locations (left or right) equidistant (8.5°) from the central fixation spot. Participants were instructed to look directly at the target as quickly as possible. The remaining 30% of trials were stop-signal trials. These trials were initially identical to the no-stop-signal trials, but the fixation spot was re-illuminated after a variable delay (stop signal delay; SSD) following target presentation, cuing subjects to inhibit a saccade to the target. Stop signal trials were labeled cancelled or non-cancelled based on whether subjects inhibited or failed to inhibit the saccade, respectively. Response inhibition becomes more difficult with increasing SSDs. SSDs were dynamically adjusted using a 1-up/1-down tracking procedure, thereby ensuring successful inhibition on 50% of the stop signal trials (Osman et al., 1986). The initial SSD was set at 225ms and increased or decreased by 47ms when the subject succeeded or failed to inhibit, respectively. The testing session consisted of a practice block of 60 trials, and 4 experimental blocks of 120 trials each.
Figure 1. Saccadic countermanding task.
Dotted circles indicate gaze position, and the arrow indicates the direction of the saccade. Trials begin with the presentation of a central fixation spot. After the fixation spot disappears, a target appears simultaneously at a non-central location. On stop signal trials, the fixation spot is re-illuminated at some delay, referred to as stop signal delay (SSD), following target onset. Fixation re-illumination is cue for the subject to withhold a saccade to the target. Trials in which the subject is successful in maintaining fixation are referred to as cancelled trials, and trials in which the subject makes a saccade to the target are referred to as non-cancelled trials. For the remaining majority of trials (no-stop signal trials), fixation is not re-illuminated, and the subject is instructed to make a saccade to the target.
Behavioral performance was evaluated through measurements of saccadic RT on no-stop-signal and non-cancelled trials, and mean SSD. At each SSD, we quantified the proportion of trials in which a participant successfully inhibited a saccade. The proportion of cancelled trials at each delay is referred to as the inhibition function.
Performance in the stop signal task can be accounted for by a mathematical model that assumes a race between independent processes that generate (GO process) and inhibit (STOP process) the movement (Logan and Cowan, 1984). The response is executed if the GO process finishes before the STOP process, and inhibited if the STOP process finishes first. The latency of the GO process can be measured directly from the observable RTs, but the latency of the STOP process is estimated. The independent race model provides an estimate of SSRT. According to the race model, on each trial, the RT of the STOP and GO process are random variables. If, on a particular stop signal trial, the GO RT is less than the sum of the SSRT and SSD, the GO process ‘wins’, and the response is executed. Likewise, if GO RT is greater than the sum of STOP RT and SSD, the STOP process ‘wins’, and the response is inhibited. The trials that escape inhibition are from the fastest portion of the no-stop signal RT distribution. Thus, the race model accounts for the finding that the proportion of non-cancelled trials increases with increasing SSD and that non-cancelled RTs are shorter than no-stop signal RTs.
There are several published methods of calculating SSRT (Band et al., 2003; Logan & Cowan, 1984; Logan et al., 2014; Matzke et al., 2013), the ‘mean method’ and ‘integration method’ being the most widely used. In studies where a tracking procedure is used to adjust the SSD, the mean method is most frequently applied. However, a recent study found that the mean method tended to overestimate SSRT as distribution of no-stop RTs became increasingly skewed (Verbruggen et al., 2013), potentially resulting in spurious group differences in SSRT. Thus, although our previously published study of countermanding performance in SZ patients used the mean method to calculate SSRT, in the current study we estimated SSRT using the integration method. In this method, the finishing time of the STOP process is estimated by integrating the GO RT distribution to obtain the RT value at which the area under the curve equals the probability of failing to inhibit at a particular delay between the GO signal and the signal to stop or change the response. SSRT is calculated by subtracting that delay from the finishing time of the STOP process. Since we used the dynamic tracking procedure, SSRT was calculated by sorting the no-stop RTs and finding the RT corresponding to the proportion of noncancelled trials. Then the mean SSD was subtracted from this RT.
The slope of the inhibition function is thought to reflect variability in the STOP and GO RT and the ability to trigger an inhibitory response. Since variability in GO RT does not reflect inhibition ability, the slope can be corrected for variability in GO RT by applying a Z-transformation to the SSDs (Logan et al., 1997). This transformation expresses the SSDs in terms of the latency relative to finishing times of GO and STOP processes standardized with respect to variability in GO RT using the equation:
To index response monitoring, RT was examined as a function of trial history. Mean RT was computed separately for no-stop-signal trials preceding and following no-stop-signal trials, correctly cancelled stop signal trials, and non-cancelled stop signal trials (i.e. stop-task errors; Nelson et al., 2010; Bissett and Logan, 2011). RTs on no-stop signal trials preceding and following two consecutive stop signal trials were included in this analysis only if the response on the two stop signal trials was the same (i.e. if both trials were cancelled or non-cancelled). Post-cancelled slowing was calculated as the difference between mean RT for no-stop signal trials preceding and following a cancelled trial. Likewise, post-error slowing was calculated as the difference between mean RT for no-stop signal trials preceding and following an erroneously noncancelled (error) trial.
2.4 Statistical Methods
Fisher’s exact tests, independent t-tests, and repeated measures ANOVAs were used where appropriate. All tests were two-tailed except otherwise specified. Subjects were excluded from analyses if the adaptive tracking procedure in the stop signal task was ineffective, defined by a proportion of successfully inhibited responses lying outside a 95% binomial confidence interval around p = 0.5.
3. Results
3.1 Probability of inhibition
The dynamic tracking procedure was successful, and the mean proportion of non-cancelled trials was 49%. The three groups did not differ in the proportion of non-cancelled trials (F(2,48)=2.16, p=0.13). For each subject, the estimated slope of the inhibition function plotted against ZRFT was calculated (Supplementary Data 2). There was no group difference in the slope of the Z-transformed inhibition function (F(2,48) = 1.2, p = 0.31). This provides evidence for equal variability in the inhibitory process across groups and suggests that both patient groups were sufficiently able to maintain the instruction to inhibit a response upon presentation of the stop-signal.
3.2 No-stop-signal and non-cancelled RT
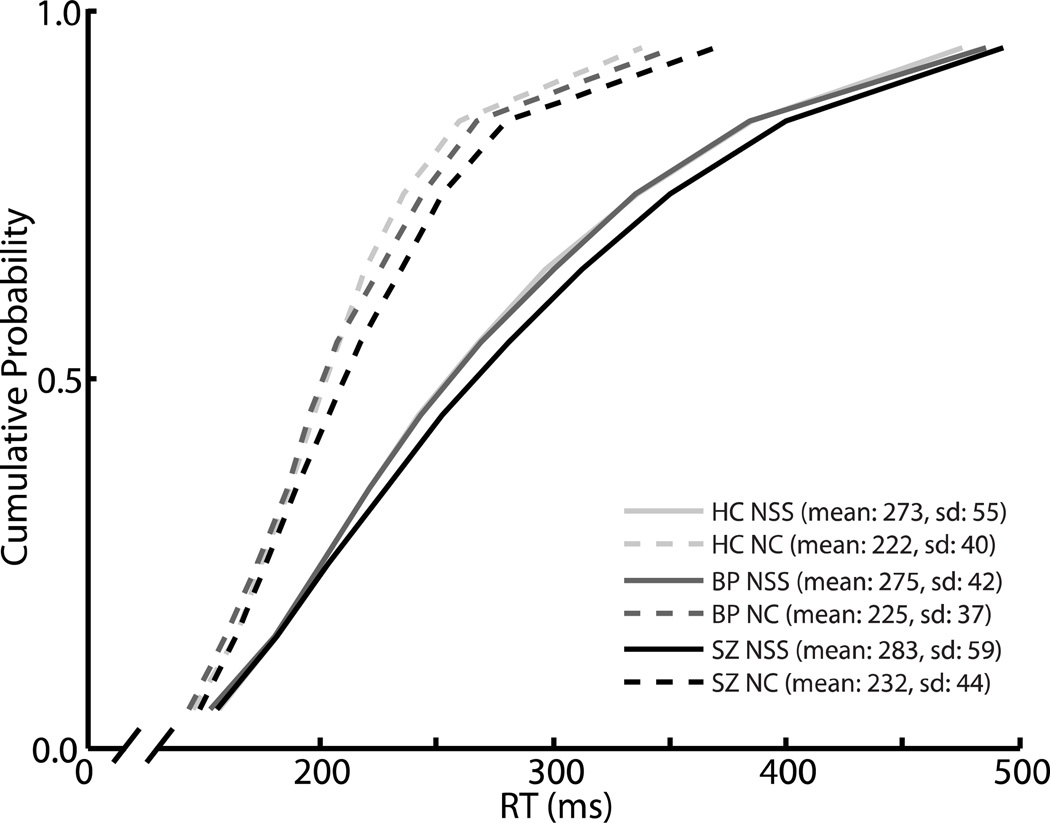
The effect of trial type (no-stop signal or non-cancelled) on RT was assessed with a mixed-model ANOVA with group as a between-subjects variable and trial type as a within-subjects variable. There was a significant effect of trial type (F(1,48) = 188.9, p < 0.0001), with no-stop-signal trials being slower than non-cancelled trials. This finding is consistent with race model logic and indicates that only the fastest GO processes were fast enough to escape inhibition. There was no main effect of group (F(2,48) = 0.24, p = 0.79) or group-by-trial type interaction effect (F(2,48) = 0.03, p = 0.97), indicating equal speed of response initiation across the three groups. Cumulative distributions of RTs are presented in Figure 2.
Figure 2. RT distributions.
Vincentized cumulative distributions of saccade latencies in no-stop signal (dotted lines) and non-cancelled (solid lines) trials for healthy controls (light gray), bipolar patients (dark gray), and schizophrenia patients (black). For each subject, mean RT for each decile was calculated. Then, for all three groups, the mean RT at each decile was averaged across participants.
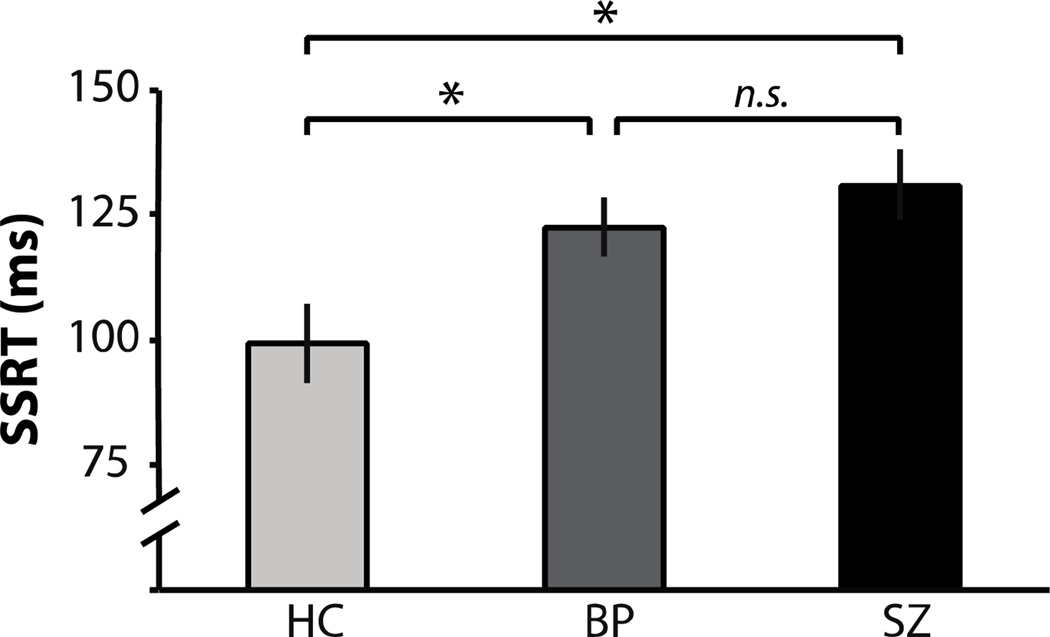
3.3 SSRT
SSRT results are presented in Figure 3. There was a significant effect of group on SSRT (F(2,48)=5.4, p=0.008). SSRT was significantly longer in BP than HC (t(32)=2.4, p=0.02, d=0.82). As previously reported (Thakkar et al., 2011) using a different estimation method, SSRT was significantly longer in SZ than HC (t(31)=3.0, p=0.005, d=1.05). Finally, SSRT did not differ significantly between the two patient groups (t(33)=0.9, p=0.36, d=0.3).
Figure 3. SSRT.
Mean SSRT (plus standard error) for healthy controls (light gray), bipolar patients (dark gray), and schizophrenia patients (black).
3.4 RT adjustments across three trials in sequence
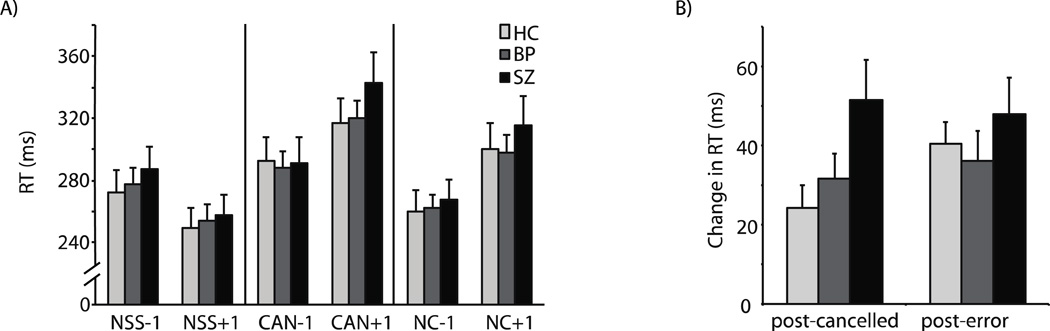
See Supplementary Data 3 for detailed analysis of trial history effects. Consistent with previous studies (e.g. Bissett and Logan, 2011; Nelson et al., 2010; Thakkar et al., 2011), we observed that relative to the n-1th trial, participants slow down on the no-stop trial immediately following both cancelled and noncancelled trials, and speed up following consecutive no-step trials (Figure 4). A one-way ANOVA was conducted to assess group differences in post-cancelled and post-error slowing and speeding following no-stop signal trials. There was a main effect of group on post-cancelled slowing (F(2,48)=3.35, p=0.04). As we reported in Thakkar, et al. (2011), SZ slowed down significantly more following cancelled trials than HC (HC: mean=24 ms, s.d.=22; SZ: mean=51, s.d.=42; t(31)=2.3, p=0.03, d=0.79). In addition, they tended to slow down more following cancelled trials than BP (BP: mean=32 ms, s.d.=26; t(33)=1.69, p=0.10, d=0.57).
Figure 4. Trial history effects.
Mean no-stop signal RT (plus standard error) as a function of trial history. A) Mean no-stop signal RT (with standard error) for trials following (+1) and preceding (−1) no-stop signal (NSS), cancelled (CAN) and non-cancelled (NC) trials for healthy controls (light gray), bipolar patients (dark gray), and bipolar patients (black). B) Mean post-cancelled and post-error slowing.
3.5 Symptoms, social functioning, and medication
We previously reported a correlation between longer SSRT and greater negative symptom severity in this schizophrenia sample (Thakkar et al., 2011). In this study, Spearman rank-correlation coefficients were used to evaluate the association between the severity of mania and depression using the YMRS and HRSD, respectively, and countermanding performance (SSRT, post-cancelled slowing, post-error slowing) in bipolar patients. In addition, correlations between BPRS total and subscale scores and performance were calculated for patient groups, combined and separately. No significant relationships were observed between BPRS scores and countermanding performance in either patient group; however, at a statistical trend level, longer SSRT was associated with greater Negative scores in bipolar patients (rs=0.43, p=0.08). and greater Total scores in schizophrenia patients (rs=0.44, p=0.08). Combined across patient groups, greater scores on the Negative subscale were associated with less postcancelled slowing (rs=−0.34, p=0.05).
In our previous study, we also performed a median split on SFS employment scores in schizophrenia patients and found that those with poorer occupational functioning had longer SSRT than patients with higher occupational functioning. Since SFS employment scores were also bimodally distributed in bipolar patients, a median split was performed on the scores, and independent t-tests were conducted to compare behavioral measures in those scoring high and low on occupational functioning. There was a trend for greater post-cancelled slowing in the low compared to high employment group, (t(16) = 2.1, p = 0.056, d=0.94). That is, higher occupational functioning was associated with less slowing following correctly inhibited saccades. There was no significant difference in any other behavioral measure between employment groups, and no significant relationship between SFS total score and countermanding task performance was observed.
Finally, we investigated potential relationships between countermanding performance and medication. First, we correlated standardized antipsychotic medication dosages (CPZ equivalents) with countermanding performance (no-stop trial RTs, SSRT, post-cancelled slowing, post-error slowing). Due to non-normal distributions of CPZ equivalent dosages, spearman rank-correlation coefficients were used. No significant correlations were observed in BP (all rs’s<0.31, p’s>0.22), SZ (all rs’s<0.12, p’s>0.64), or combined across groups (all rs’s<0.26, p’s>0.14). To further explore the role of medication, we divided patients into those who were and were not taking antipsychotics, mood stabilizers, and antidepressants and examined differences in countermanding performance between medication groups, for both patient groups separately and collapsed across patient groups. These results are presented in full in Supplementary Data 4. Of particular interest, SSRT did not differ significantly between antipsychotic, antidepressant, or mood stabilizer medication groups, within the BP sample, and effect sizes for medication group differences were small. The only significant difference between medication groups was in post-cancelled slowing. We found reduced post-cancelled slowing in patients who were not taking mood stabilizers compared to those that were, both in the BP sample (t(16)=2.4, p=0.03) and collapsed across patient groups (t(33)=2.3, p=0.03).
4. Discussion
In a previously published manuscript using this schizophrenia sample (Thakkar et al., 2011), we reported longer SSRT in SZ compared with HC. SSRT measures the time needed to cancel a movement and is derived from a race model of response inhibition; longer SSRT in patients indicates that they need more time to put the brakes on their planned actions, so to speak. Slower SSRT was associated with negative symptom severity and poorer occupational functioning, attesting to its clinical relevance. We also reported that SZ slow down nearly twice as much as HC following a trial in which they successfully inhibited. The current study sought to examine the diagnostic specificity of these findings by investigating countermanding performance in individuals with bipolar disorder.
First, we found evidence for poorer inhibition efficiency in BP, as indexed by longer SSRT relative to HC. These findings are consistent with reports of impaired response inhibition in BP (see Introduction), and particularly with more recent findings of impaired countermanding of manual movements in adults with bipolar disorder (Ethridge et al., 2014; Strakowski et al., 2009; Strakowski et al., 2010). In line with these previous countermanding sstudies, we also did not observe any relationship between inhibition speed (SSRT) and clinical symptomatology in bipolar patients, suggesting that longer SSRT represents a trait-like impairment. We did not, however, observe evidence for diagnostic specificity of longer SSRT, as this measure did not differ between schizophrenia and bipolar patients. Although it is possible that we were underpowered to detect subtle differences in SSRT, we do not think this is the case as this finding is consistent with a study of over 500 patients with either schizophrenia spectrum or bipolar disorder using the manual countermanding task (Ethridge et al., 2014). Rather, this study provides evidence that longer SSRT, alone, cannot distinguish bipolar and schizophrenia patients. This somewhat in contrast to findings that general neurocognitive functioning of BP patients is impaired relative to healthy controls but is better than in SZ (Hill et al., 2013). Despite group differences in the speed of stopping, there was no overall group effect on speed of responding, as measured by RTs on no-stop and incorrectly non-compensated trials.
Second, we observed group differences in response monitoring. We found in a previous study that SZ had greater post-cancelled slowing than HC, indicating a bigger effect of inhibition on the subsequent response speed. There was no difference in post-cancelled slowing between HC and BP, and the effect size was small. However, there was a trend for greater post-cancelled slowing in SZ compared to BP; although it did not reach significance, the effect size was large. Interestingly, although there was no relationship between post-cancelled slowing and clinical symptoms or social functioning in SZ, occupational functioning was associated with post-cancelled slowing in BP. Bipolar patients with low occupational functioning showed greater post-cancelled slowing. That is, those BP whose trial history effects more closely resembled those of the SZ had poorer work outcomes. Trend level findings of greater post-cancelled slowing in SZ versus BP is suggestive of diagnostic specificity, and it is possible that greater influence of the prior trial on current behavior is specific to schizophrenic pathology. Importantly, both patient groups were matched on clinical symptom severity and social functioning, indexed by BPRS and SFS scores, which bolsters the argument that these trend-level idiosyncratic trial history effects specifically vary as a function of diagnosis, rather than general psychiatric symptoms and functional status.
A major advantage of the saccadic countermanding task has over standard neuropsychological measures to study executive functioning in psychiatric populations and also over the manual version of this task is the large body of primate neurophysiology work that has described how single neurons can implement executive control of gaze. This body of work indicates that neurons in FEF and SC must modulate in order for a saccade to be inhibited (Hanes et al., 1998; Paré and Hanes, 2003). Activity in basal ganglia pathways can directly inhibit movement-related activity in SC and can inhibit FEF indirectly via the thalamus (see Hikosaka et al., 2000 for review). Direct stimulation of neurons in the striatum, the input node of the basal ganglia, can suppress contralateral saccades (Watanabe and Munoz, 2010), rodent neurophysiology work has shown that activity in various nodes of the basal ganglia determines whether response can be inhibited during stop-signal task performance (Schmidt et al., 2013), and a recent fMRI study found that striatal activation was associated with faster SSRT in a modified oculomotor countermanding paradigm (Thakkar et al., 2014). Thus, slowed SSRT in both patient groups might have its basis in abnormalities in a circuit involving SC, FEF, and basal ganglia. Results from the current study provide a basis for investigating the neural underpinnings of the observed impairments in the speed of inhibition in bipolar disorder and provide strong motivation for examining specific frontal and subcortical oculomotor networks in future studies.
Given the apparent similarities between the saccadic countermanding task and the antisaccade task, which has already been used extensively in schizophrenia and bipolar disorder, one might question the added clinical utility of the saccadic countermanding task. It is important to note, however, that these differ in important ways. In the antisaccade task, subjects know before each trial whether they will be required to engage inhibitory processes and make an antisaccade. In the countermanding or stop-signal task, the subject is given no advance information instructing them whether a stop signal will be presented. Although proactive and reactive inhibition are certainly involved in both tasks (see Verbruggen and Logan, 2009), SSRT primarily reflects reactive inhibition and gives an estimate of the time required to enact response inhibition, which antisaccade error rate, the main task outcome measure, does not. Thus, we argue that SSRT can provide more specific information about putative impairments in the reactive inhibition of actions. Although seemingly a subtle distinction, proactive and reactive inhibitory processes are dissociable. Proactive inhibition places a larger demand on working memory; subjects must maintain a representation over time of the cue that indicates the imminent need for response control in order to prepare the appropriate action. Indeed, fMRI studies suggest that brain activation related to proactive and reactive inhibition are at least partly separable (Zandbelt et al., 2013), although there is also evidence for significant overlap between these two networks (Aron, 2011; Chikazoe et al., 2009; Jahfari et al., 2010). Further, pharmacological manipulations in both humans and rodents have been found to have differing effects on proactive and reactive inhibition. For example, serotonergic manipulations affect proactive, but not reactive, inhibition (see Eagle et al., 2008 for review). On the other hand, modafinil, an atypical stimulant, has been found to affect reactive, but not proactive, inhibition (Eagle et al., 2007; Turner et al., 2004). Thus, describing the specific aspects of response inhibition that are spared and impaired in schizophrenia and bipolar disorder have important implications for pharmacological treatment of these cognitive deficits.
This study should be considered in light of several limitations. First, our sample size was relatively small, and the study is underpowered to detect potentially subtle relationships between clinical status and cognitive control abilities. Additionally, bipolar patients with a history of psychosis have been found to fare worse, cognitively, than those without a history of psychosis (Martinez-Aran et al., 2008), and response inhibition has been found to vary as a function of clinical status in bipolar disorder (Gooding et al., 2004; Langenecker et al., 2010). Because of our modest sample size, further studies are needed to examine stop-signal task performance as a function of symptom severity and psychosis history in bipolar disorder.
A second limitation of the present study is the unclear role of psychotropic medications in the inhibition and monitoring of saccades. All but one participant in the schizophrenia sample and approximately half of the bipolar sample were using antipsychotic medication, which are dopamine antagonists. Additionally, approximately half of the bipolar group and a quarter of schizophrenia patients were taking mood stabilizers. Although in bipolar patients there was no significant difference in SSRT or post-cancelled slowing between those patients who were and were not taking antipsychotics, we know from the rodent literature that D1 and D2 antagonists injected into the striatum affects SSRT. D1 antagonists reduce SSRT, and D2 antagonists prolong SSRT (Eagle et al., 2011). Although the therapeutic effect of antipsychotic medications is attributed to D2 receptor blockade, commonly prescribed antipsychotic medications also have, to varying degrees, affinity for D1 receptors (see Miller, 2009 for review) and the ratio of D1:D2 occupancy varies widely across different atypical antipsychotic drugs (Tauscher et al, 2004). Given the opposite effects of D1 and D2 receptor antagonists on response inhibition speed, it is difficult to formulate concrete hypotheses about the effect of antipsychotics on SSRT. Arguing against the possibility that antipsychotic medications are giving rise to altered SSRT in patient groups is the absence of a significant relationship between standardized antipsychotic dose and countermanding performance measures and absence of a difference in countermanding performance measures between bipolar patients that were and were not receiving antipsychotic treatment. Additionally, atypical antipsychotic medication has been found to improve inhibitory performance, as measured with the antisaccade task (Harris et al., 2006). Further, based on studies in which healthy subjects are administered antipsychotic medication, it is unlikely that medication effects are giving rise to longer post-cancelled slowing in patients with SZ relative to HC and BP. Various antipsychotic medications administered in single doses either show no effect on trial history-based slowing or lead to a reduction in slowing (de Bruijn et al., 2006; Zirnheld et al., 2004). With regard to mood stabilizers, we also did not observe any differences between patients with bipolar disorder that were and were not administered mood stabilizers. Lithium, but not other mood stabilizers, has been obseved to negatively affect SSRT (Strakowski et al., 2009) and psychotmotor speed in bipolar patients (Wingo et al., 2009); however only one schizophrenia patient and one bipolar patient in the current sample were taking lithium.
Generally, in post-hoc analyses of antipsychotic and mood stabilizing drug effects in affective disorder patients, niether visually guided saccade production nor cognitive control of saccades are adversely affected in a consistent manner across studies (Katsanis et al., 1997; Reilly et al., 2008). Given these findings, combined with the absence of signifcant drug effects on SSRT in our own sample, we would argue against a major confounding effect of medication. However, such cross-sectional studies are not suited for rigorously examining medication effects on cognitive performance, as relevant clinical factors and medication status are likely not orthogonal, and future studies are needed.
To conclude, patients with both bipolar disorder and schizophrenia patients receiving standard treatment show similar impairments in the reactive control of action. On the other hand, exaggerated behavioral adjustments as a function of the prior trial tend to be specific to schizophrenia patients. Given the rich neurophysiology data from non-human primates performing this exact task under similar experiment settings, these findings have implications for understanding the neurobiology of cognitive control in schizophrenia and bipolar, for defining the etiological overlap between schizophrenia and bipolar disorder, and for developing pharmacological treatments of cognitive impairments in major mental illnesses.
Supplementary Material
Highlights.
Controls and patients with bipolar disorder or schizophrenia performed the stop-signal task
Group differences in inhibition speed and dynamic adjustments in performance were explored
Performance of bipolar patients fell midway between controls and schizophrenia patients
Results suggest that cognitive control abilities map onto a spectrum of psychosis-proneness
Acknowledgements
The authors would like to thank Dr. Stefan Heckers and his research group for their help with recruitment and Dr. Natasha Matthews and Amanda Cumming for their assistance with clinical interviews and subject recruitment. This work was supported in part by MH073028 (SP), the Gertrude Conaway Vanderbilt Chair (SP), F31-MH085405-01 (KNT), Netherlands Organization for Scientific Research Rubicon grant (KT), P30-EY08126 (JDS, GDL), MH055806 (JDS), the E. Bronson Ingram Chair in Neuroscience (JDS), MH073878 (GDL), and NICHD Grant P30 HD15052 to the Vanderbilt Kennedy Center for Research on Human Development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
All authors developed the study concept and contributed to the study design. Data collection and analyses were performed by K.N. Thakkar under the supervision of S. Park, G.D. Logan, and J.D. Schall. K.N. Thakkar drafted the paper and S. Park, J.D. Schall, and G.D. Logan provided critical revisions. All authors contributed to and approved the final version of the paper for submission.
Conflicts of interest
The authors declare no conflicts of interest.
References
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) Iowa City, IA: The University of Iowa; 1983. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) Iowa City, IA: The University of Iowa; 1984. [Google Scholar]
- Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biological Psychiatry. 2011;69:e55–e68. doi: 10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band GP, van der Molen MW, Logan GD. Horse-race model simulations of the stop-signal procedure. Acta Psychologica. 2003;112:105–142. doi: 10.1016/s0001-6918(02)00079-3. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Goldman RS, Robinson D, Reiter G, Bell L, Bates JA, Pappadopulos E, Willson DF, Alvir JM, Woerner MG, Geisler S, Kane JM, Lieberman JA. Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. American Journal of Psychiatry. 2000;157:549–559. doi: 10.1176/appi.ajp.157.4.549. [DOI] [PubMed] [Google Scholar]
- Birchwood M, Smith J, Cochrane R, Wetton S, Copestake S. The Social Functioning Scale. The development and validation of a new scale of social adjustment for use in family intervention programmes with schizophrenic patients. British Journal of Psychiatry. 1990;157:853–859. doi: 10.1192/bjp.157.6.853. [DOI] [PubMed] [Google Scholar]
- Bissett PG, Logan GD. Balancing cognitive demands: Control adjustments in the stop-signal paradigm. Journal of Experimental Psychology: Learning, Memory and Cognition. 2011;37:392–404. doi: 10.1037/a0021800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair JR, Spreen O. Predicting Premorbid IQ: A revision of the National Adult Reading Test. The Clinical Neuropsychologist. 1989;3:129–136. [Google Scholar]
- Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. Journal of Affective Disorders. 2009;113:1–20. doi: 10.1016/j.jad.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Brown JW, Hanes DP, Schall JD, Stuphorn V. Relation of frontal eye field activity to saccade initiation during a countermanding task. Experimental Brain Research. 2008;190:135–151. doi: 10.1007/s00221-008-1455-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikazoe J, Jimura K, Hirose S, Yamashita K, Miyashita Y, Konishi S. Preparation to inhibit a response complements response inhibition during performance of a stop-signal task. Journal of Neuroscience. 2009;29:15870–15877. doi: 10.1523/JNEUROSCI.3645-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz BA. Psychophysiological measures of (dis)inhibition as liability indicators for schizophrenia. Psychophysiology. 1998;35:648–668. [PubMed] [Google Scholar]
- Crawford TJ, Haeger B, Kennard C, Reveley MA, Henderson L. Saccadic abnormalities in psychotic patients. I. Neuroleptic-free psychotic patients. Psychological Medicine. 1995;25:461–471. doi: 10.1017/s0033291700033389. [DOI] [PubMed] [Google Scholar]
- Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. American Journal of Psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- de Bruijn ER, Sabbe BG, Hulstijn W, Ruigt GS, Verkes RJ. Effects of antipsychotic and antidepressant drugs on action monitoring in healthy volunteers. Brain Research. 2006;1105:122–129. doi: 10.1016/j.brainres.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Bari A, Robbins TW. The neuropsychopharmacology of action inhibition: cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacology. 2008;199:439–456. doi: 10.1007/s00213-008-1127-6. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Tufft MR, Goodchild HL, Robbins TW. Differential effects of modafinil and methylphenidate on stop-signal reaction time task performance in the rat, and interactions with the dopamine receptor antagonist cis-flupenthixol. Psychopharmacology. 2007;192:193–206. doi: 10.1007/s00213-007-0701-7. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Wong JC, Allan ME, Mar AC, Theobald DE, Robbins TW. Contrasting roles for dopamine D1 and D2 receptor subtypes in the dorsomedial striatum but not the nucleus accumbens core during behavioral inhibition in the stop-signal task in rats. Journal of Neuroscience. 2011;31:7349–7356. doi: 10.1523/JNEUROSCI.6182-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeric EE, Brown JW, Boucher L, Carpenter RH, Hanes DP, Harris R, Logan GD, Mashru RN, Pare M, Pouget P, Stuphorn V, Taylor TL, Schall JD. Influence of history on saccade countermanding performance in humans and macaque monkeys. Vision Research. 2007;47:35–49. doi: 10.1016/j.visres.2006.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethridge LE, Soilleux M, Nakonezny PA, Reilly JL, Hill SK, Keefe RSE, Gershon ES, Pearlson GD, Tamminga CA, Keshavan MS, Sweeney JA. Behavioral response inhibtion in psychotic disorders: diagnositc specificity, familiarity and relation to generalized cognitive deficit. Schizophrenia Research. doi: 10.1016/j.schres.2014.08.025. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I disorders. New York: Biometrics Research Department; 1995. [Google Scholar]
- Gooding DC, Basso MA. The tell-tale tasks: a review of saccadic research in psychiatric patient populations. Brain and Cognition. 2008;68:371–390. doi: 10.1016/j.bandc.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooding DC, Mohapatra L, Shea HB. Temporal stability of saccadic task performance in schizophrenia and bipolar patients. Psychological Medicine. 2004;34:921–932. doi: 10.1017/s003329170300165x. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Tallent KA. The association between antisaccade task and working memory task performance in schizophrenia and bipolar disorder. Journal of Nervous and Mental Disease. 2001;189:8–16. doi: 10.1097/00005053-200101000-00003. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Rating depressive patients. J. Clin. Psychiatry. 1980;41:21–24. [PubMed] [Google Scholar]
- Hanes DP, Patterson WF, 2nd, Schall JD. Role of frontal eye fields in countermanding saccades: visual, movement, and fixation activity. Journal of Neurophysiology. 1998;79:817–834. doi: 10.1152/jn.1998.79.2.817. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Schall JD. Countermanding saccades in macaque. Visual Neuroscience. 1995;12:929–937. doi: 10.1017/s0952523800009482. [DOI] [PubMed] [Google Scholar]
- Harris MS, Reilly JL, Keshavan MS, Sweeney JA. Longitudinal studies of antisaccades in antipsychotic-naïve first-episode schizophrenia. Psychological Medicine. 2006;36:485–494. doi: 10.1017/S0033291705006756. [DOI] [PubMed] [Google Scholar]
- Harris MS, Reilly JL, Thase ME, Keshavan MS, Sweeney JA. Response suppression deficits in treatment-naive first-episode patients with schizophrenia, psychotic bipolar disorder and psychotic major depression. Psychiatry Research. 2009;170:150–156. doi: 10.1016/j.psychres.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol. Rev. 2000;80:953–978. doi: 10.1152/physrev.2000.80.3.953. [DOI] [PubMed] [Google Scholar]
- Hill SK, Reilly JL, Keefe RSE, Gold JM, Bishop JR, Gershon ES, Tamminga CA, Pearlson GD, Keshavan MS, Sweeney JA. Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (BSNIP) study. American Journal of Psychaitry. 2013;170:1275–1284. doi: 10.1176/appi.ajp.2013.12101298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes O, Bose S, Turkheimer F, Valli I, Egerton A, Stahl D, Valmaggia L, Allen P, Murray R, McGuire P. Progressive increase in striatal dopamine synthesis capacity as patients develop psychosis: a PET study. Molecular Psychiatry. 2011;16:885–886. doi: 10.1038/mp.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton SB, Ettinger U. The antisaccade task as a research tool in psychopathology: A critical review. Psychophysiology. 2006;43:302–313. doi: 10.1111/j.1469-8986.2006.00403.x. [DOI] [PubMed] [Google Scholar]
- Hutton SB, Puri BK, Duncan LJ, Robbins TW, Barnes TRE, Joyce EM. Executive function in first-episode schizophrenia. Psychological Medicine. 1998;28:463–473. doi: 10.1017/s0033291797006041. [DOI] [PubMed] [Google Scholar]
- Ito S, Stuphorn V, Brown JW, Schall JD. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science. 2003;302:120–122. doi: 10.1126/science.1087847. [DOI] [PubMed] [Google Scholar]
- Jahfari S, Stinear CM, Claffey M, Verbruggen F, Aron AR. Responding with restraint: what are the neurocognitive mechanisms? Journal of Cognitive Neuroscience. 2010;22:1479–1492. doi: 10.1162/jocn.2009.21307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsanis J, Kortenkamp S, Iacono WG, Grove WM. Antisaccade performance in patients with schizophrenia and affective disorder. Journal of Abnormal Psychology. 1997;106:468–472. doi: 10.1037//0021-843x.106.3.468. [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Saunders EF, Kade AM, Ransom MT, McInnis MG. Intermediate: cognitive phenotypes in bipolar disorder. Journal of Affective Disorders. 2010;122:285–293. doi: 10.1016/j.jad.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, Hultman CM. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and action: A theory of an act of control. Psychological Review. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- Logan GD, Schachar R, Tannock R. Impulsivity and inhibitory control. Psycholoical Science. 1997;8:60–64. [Google Scholar]
- Logan GD, Van Zandt T, Verbruggen F, Wagenmakers EJ. On the ability to inhibit thought and action: General and special theories of an act of control. Psychological Review. 2014;121:66–95. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- Maier W, Zobel A, Wagner M. Schizophrenia and bipolar disorder: differences and overlaps. Current Opinions in Psychiatry. 2006;19:165–170. doi: 10.1097/01.yco.0000214342.52249.82. [DOI] [PubMed] [Google Scholar]
- Martin LF, Hall MH, Ross RG, Zerbe G, Freedman R, Olincy A. Physiology of schizophrenia, bipolar disorder, and schizoaffective disorder. American Journal of Psychiatry. 2007;164:1900–1906. doi: 10.1176/appi.ajp.2007.06010017. [DOI] [PubMed] [Google Scholar]
- Martinez-Aran A, Torrent C, Tabares-Seisdedos R, Salamero M, Daban C, Balanza-Martinez V, Sanchez-Moreno J, Manuel Goikolea J, Benabarre A, Colom F, Vieta E. Neurocognitive impairment in bipolar patients with and without history of psychosis. Journal of Clinical Psychiatry. 2008;69:233–239. doi: 10.4088/jcp.v69n0209. [DOI] [PubMed] [Google Scholar]
- Matzke D, Dolan CV, Logan GD, Brown SD, Wagenmakers E-J. Bayesian parametric estimation of stop-signal reaction time distributions. Journal of Experimental Psychology: General. 2013;142:1047–1073. doi: 10.1037/a0030543. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Clementz BA. The effect of fixation condition manipulations on antisaccade performance in schizophrenia: studies of diagnostic specificity. Experimental Brain Research. 1997;115:333–344. doi: 10.1007/pl00005702. [DOI] [PubMed] [Google Scholar]
- Miller R. Mechanisms of action of antipsychotic drugs of different classes, refractoriness to therapeutic effects of classical neuroleptics, and individual variation in sensitivity to their actions: Part I. Current Neuropharmacology. 2009;7:302–314. doi: 10.2174/157015909790031229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskvina V, Craddock N, Holmans P, Nikolov I, Pahwa JS, Green E, Owen MJ, O'Donovan MC. Gene-wide analyses of genome-wide association data sets: evidence for multiple common risk alleles for schizophrenia and bipolar disorder and for overlap in genetic risk. Molecular Psychiatry. 2009;14:252–260. doi: 10.1038/mp.2008.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MJ, Boucher L, Logan GD, Palmeri TJ, Schall JD. Nonindependent and nonstationary response times in stopping and stepping saccade tasks. Attention, Perception & Psychophysics. 2010;72:1913–1929. doi: 10.3758/APP.72.7.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman A, Kornblum S, Meyer D. The Point of No Return in Choice Reaction Time: Controlled and Ballistic Stages of Response Preparation. Journal of Experimental Psychology: Human Perception and Performance. 1986;12:243–258. doi: 10.1037//0096-1523.12.3.243. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Report. 1962;10:799–812. [Google Scholar]
- Paré M, Hanes DP. Controlled movement processing: superior colliculus activity associated with countermanded saccades. Journal of Neuroscience. 2003;23:6480–6489. doi: 10.1523/JNEUROSCI.23-16-06480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly JL, Lencer R, Bishop JR, Keedy S, Sweeney JA. Pharmacological treatment effects on eye movement control. Brain and Cognition. 2008;68:415–435. doi: 10.1016/j.bandc.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Leventhal DK, Mallet N, Chen F, Berke JD. Canceling actions involves a race between basal ganglia pathways. Nature Neuroscience. 2013;16:1118–1124. doi: 10.1038/nn.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno AB, Holzman PS. Antisaccades and smooth pursuit eye movements in schizophrenia. Biological Psychiatry. 1995;37:394–401. doi: 10.1016/0006-3223(94)00127-O. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Fleck DE, DelBello MP, Adler CM, Shear PK, McElroy SL, Keck PE, Moss Q, Cerullo MA, Kotwal R, Arndt S. Characterizing impulsivity in mania. Bipolar Disorders. 2010;11:41–51. doi: 10.1111/j.1399-5618.2008.00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski SM, Fleck DE, DelBello MP, Adler CM, Shear PK, Kotwal R, Arndt S. Impulsivity across the course of bipolar disorder. Bipolar Disorders. 2010;12:285–297. doi: 10.1111/j.1399-5618.2010.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuphorn V, Taylor TL, Schall JD. Performance monitoring by the supplementary eye field. Nature. 2000;408:857–860. doi: 10.1038/35048576. [DOI] [PubMed] [Google Scholar]
- Thakkar KN, Schall JD, Boucher L, Logan G, Park S. Response inhibition and response monitoring in a saccadic countermanding task in schizophrenia. Biological Psychiatry. 2011;69:55–62. doi: 10.1016/j.biopsych.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar KN, van den Heiligenberg FMZ, Kahn RS, Neggers SFW. Frontal-subcortical circuits involved in reactive control and monitoring of gaze. Journal of Neuroscience. 2014;34:8918–8929. doi: 10.1523/JNEUROSCI.0732-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauscher J, Hussain T, Agid O, Verhoeff NPL, Wilson AA, Houle S, Kapur S. Equivalent occupancy of dopamine D1 and D2 receptors with clozapine: differentiation from other atypical antipsychotics. American Journal of Psychiatry. 2004;161:1620–1625. doi: 10.1176/appi.ajp.161.9.1620. [DOI] [PubMed] [Google Scholar]
- Tien AY, Ross DE, Pearlson G, Strauss ME. Eye movements and psychopathology in schizophrenia and bipolar disorder. Journal of Nervous and Mental Disorders. 1996;184:331–338. doi: 10.1097/00005053-199606000-00001. [DOI] [PubMed] [Google Scholar]
- Turner DC, Clark L, Dowson J, Robbins TW, Sahakian BJ. Modafinil improves cognition and response inhibition in adult attention-deficit/hyperactivity disorder. Biological Psychiatry. 2004;55:1031–1040. doi: 10.1016/j.biopsych.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Ventura J, Nuechterlein KH, Subotnik KL, Gutkind D, Gilbert EA. Symptom dimensions in recent-onset schizophrenia and mania: a principal components analysis of the 24-item Brief Psychiatric Rating Scale. Psychiatry Research. 2000;97:129–135. doi: 10.1016/s0165-1781(00)00228-6. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Chambers CD, Logan GD. Fictitious inhibitory differences: how skewness and slowing distort the estimation of stopping latencies. Psychological Science. 2013;24:352–362. doi: 10.1177/0956797612457390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Proactive adjustments of response strategies in the stop-signal paradigm. Journal of Experimental Psychology: Human Perception and Performance. 2009;35:835–854. doi: 10.1037/a0012726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Munoz DP. Saccade suppression by electrical microstimulation in monkey caudate nucleus. Journal of Neuroscience. 2010;30:2700–2709. doi: 10.1523/JNEUROSCI.5011-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- Wingo AP, Wingo TS, Harvey PD, Baldessarini RJ. Effects of lithium on cognitive performance: a meta-analysis. Journal of Clinical Psychiatry. 2009;70:1588–1597. doi: 10.4088/JCP.08r04972. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Zandbelt BB, Bloemendaal M, Neggers SF, Kahn RS, Vink M. Expectations and violations: Delineating the neural network of proactive inhibitory control. Human Brain Mapping. 2013;34:2015–2024. doi: 10.1002/hbm.22047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirnheld PJ, Carroll CA, Kieffaber PD, O'Donnell BF, Shekhar A, Hetrick WP. Haloperidol impairs learning and error-related negativity in humans. Journal of Cognitive Neuroscience. 2004;16:1098–1112. doi: 10.1162/0898929041502779. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.