Abstract
Proteinuria is a hallmark of chronic kidney disease (CKD) and cardiovascular disease (CVD), and a good predictor of clinical outcome. Selective endothelin A (ETA) receptor antagonist used with renin-angiotensin system (RAS) inhibitors prevents development of proteinuria in CKD. However, whether the improvement in proteinuria would have beneficial effects on CVD, independent of RAS inhibition, is not well understood. In this study, we investigated whether atrasentan, an ETA receptor antagonist, has renal and cardiovascular effects independent of RAS inhibition. Male Dahl salt sensitive (DSS) rats, at six weeks of age, received water with or without different doses of atrasentan and/or enalapril under high salt (HS) diet or normal diet (ND) for 6 weeks. At the end of 12th week, atrasentan at a moderate dose significantly attenuated proteinuria and serum creatinine without reducing mean arterial pressure (MAP), thereby preventing cardiac hypertrophy and improving cardiac function. ACE inhibitor enalapril at a dose that did not significantly lowered BP, attenuated cardiac hypertrophy while moderately improving cardiac function without reducing proteinuria and serum creatinine level. Nonetheless, combined therapy of atrasentan and enalapril that does not altering BP exerted additional cardioprotective effect. Based on these findings, we conclude that BP independent monotherapy of ETA receptor antagonist attenuates the progression of CKD and significantly mitigates CVD independent of RAS inhibition.
Introduction
CKD has been recognized as a worldwide public health issue, affecting 6% to 11% of the population in the developed world [1,2]. It is an independent risk factor for CVD and is associated with increased morbidity and mortality [3,4]. Over 80% of CKD patients at the initiation of hemodialysis suffer from left ventricular hypertrophy (LVH), an abnormality strongly linked with increased mortality risk [5,6]. In similar context, the rate of cardiovascular-related mortality in CKD patients is 10–20 times higher than in the general population [7]. The increased risk of CVD in CKD patients are mainly associated with not only the high prevalence of traditional risk factors, such as hypertension and diabetes [1], but also with non-traditional risk factors, such as albuminuria, renal insufficiency, structural and functional abnormalities of the heart [1,8].
Proteinuria is a hallmark of CKD, and also a major factor for the progression to CKD [9]. Recent clinical studies have further revealed that proteinuria is an independent risk factor for cardiovascular events, and a predictor of mortality prognosis [3,9]. Importantly, reduction of proteinuria is associated with the improvements of cardiovascular outcome in those patients with and without CKD [10,11]. Current treatments for proteinuria focus on blood pressure (BP) reduction [12], ideally using ACEi and angiotensin receptor blockers, both of which are thought to decrease proteinuria to a greater extent than accounted for by BP lowering alone [11–13]. Nevertheless, many CKD patients have significant residual proteinuria, despite of an optimal treatment [14]. Thus, there is a great need for complementary treatments which can effectively augment the reduction of the progressive loss of kidney function and proteinuria [15–17].
Endothelins (ETs) are endothelial cell–derived vasoconstrictor and vasopressor [18,19], and mediate their biological activities through the endothelin receptors A (ETA) and B (ETB). The binding of ET to ETA receptor increases vasoconstriction and retention of sodium, subsequently leading to increased BP [20]. ETA receptor antagonists have been shown to be effective in abrogating proteinuria and kidney fibrosis in various models of rats with kidney damage [21]. A recent Phase 2 dose-ranging study showed that atrasentan, a highly selective ETA receptor antagonist, used in conjunction with RAS inhibitors, may reduce proteinuria and stall CKD progression in patients with diabetic nephropathy [22]. However, it has not been clearly identified whether improvement in proteinuria correlates with cardiovascular outcome, and whether the beneficial effects of atrasentan is independent to renin-angiotensin system (RAS) inhibition. In this study, with the Dahl salt sensitive (DSS) rat model, we examined the degree of proteinuria correlates with cardiovascular abnormalities, and tested our hypothesis that atrasentan, through inhibition of specific ETA receptor, has beneficial renal and cardiovascular effects that are independent of RAS inhibition.
Materials and Methods
Materials
Materials and chemicals were obtained from following sources: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma-Aldrich, USA), Dimethyl sulfoxide (Sigma-Aldrich, USA), Enalapril (Sigma-Aldrich, USA) and IDEXX Catalyst Test kit for creatinine (IDEXX, USA). Atrasentan was provided by Abbott Labs, Abbott Park, IL.
Animal model and experimental groups
Male DSS rats (Harlan Sprague–Dawley, Indianapolis, IN) were fed a normal diet until 6 weeks of age. To generate cardiac hypertrophy, animals were fed a high salt (HS) diet (6% NaCl) for the following 6 weeks as described previously [23,24]. To study the effects of atrasentan, rats fed HS diet were divided and treated for 6 weeks as follows: (i) HS + vehicle (V), (ii) HS + low dose atrasentan (2.5 mg/kg/day), (iii) HS + moderate dose atrasentan (5 mg/kg/day), (iv) HS + high dose atrasentan (10 mg/kg/day), (v) HS+ enalapril (10 mg/kg/day), (vi) HS + combined therapy of moderate dose atrasentan (5 mg/kg/day) and enalapril (10 mg/kg/day), in drinking water. Male and female have different baseline heart weights as well as different responses to the high salt diet. To avoid these gender-related variations, we used the same gender DSS rats for our study.
Atrasentan, selective ETA blocker (around 1000 times greater affinity for ETA vs ETB) provides maximum ETA blocked and selectivity at the dose 5 mg/kg/day in vivo [25] and significantly attenuated proteinuria streptozotocin-induced rat model of diabetes [26]. Based on the previous studies, we defined 2.5, 5.0 and 10.0 mg/kg/day as low, moderate and high doses for this study. In baseline atrasentan dose study, we found that low dose doesn’t have significant effect on renal and cardiac system, and high dose has a significant effect on BP. Moderate dose of atrasentan has no significant effect on BP but significantly attenuates reno-cardiac dysfunction. To meet the main objective of present study, whether reno-cardiac beneficial effect of BP independent dose of atrasentan is independent of RAS inhibition, moderate dose of atrasentan was used in combination with enalapril. Rats fed normal diet were divided and treated for 6 weeks as follows: (i) normal diet (ND) + V, and (ii) ND + high dose atrasentan (10 mg/kg/day) in drinking water. Data were obtained at the end of 12 weeks from HS+V and ND+V treated group, respectively. The dose of atrasentan and enalapril were adjusted weekly according to actual body weight. At the end of 12 weeks, we examined morphometric measures, cardiac function using noninvasive and invasive physiologic methods, histopathology, and biochemical/molecular changes related to cardiac stress. All procedures were submitted to, approved by and performed in accordance with the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee (IACUC) guidelines.
Blood pressure measurement and echocardiography
Direct BP was measured with a 1.4 F micro-tip pressure catheter (model SPR-671, Millar Instruments, Inc. Houston, TX) inserting into the carotid artery under isoflurane anesthesia. The data was recorded and analyzed with computer software (PowerLab, Chart 5, ADInstruments, CO). Echocardiography was performed under isoflurane anesthesia as described previously [23] at the end of study period on each group of animal.
Adult rat cardiomyocytes (ARCM) culture studies
ARCMs were isolated and cultured from 6-week-old female DSS rats by enzymatic dissociation using 0.3% collagenase, according to a previously published protocol [27,28]. To investigate the protective effects of atrasentan and enalapril against in vitro cardiac hypertrophy, ARCM cells were pre-treated with or without various concentrations of atrasentan and enalapril 30 minutes prior to the treatment with hypertrophic stimuli phenylephrine (100 μM). Cells were harvested after 48hrs of treatment to determine cardiomyocyte hypertrophy.
Biochemical analysis
Blood samples were collected at the end of study period from each group of animals. Then, serum samples were collected and serum creatinine levels were measured by IDEXX Catalyst Test kit for creatinine according to the manufacturer's instructions with Catalyst Dx Chemistry Analyzer (IDEXX, ME, USA). Urine samples were collected at the end of experiment and protein excretions were detected with Bradford protein assay.
Reverse transcription polymerase chain reaction (RT-PCR) for mRNA expression
Heart tissues were collected and processed for molecular analysis at the end of 12 weeks. Semi-quantitative RT-PCR was performed as described previously [24,29]. Ribosomal 18S acted as internal controls, and all RT-PCR signals were normalized to 18S expression.
Histological studies
Kidneys were fixed in 10% formalin and paraffin-embedded. Sections were stained with Masson-Trichrome (MT) at the Histology Core facility at Beth Israel Deaconess Medical Center. Tubulointerstitial injury was determined by evaluation of slides stained with MT to quantify the area of tissue fibrosis (blue) versus unstained region of image as described previously [24,29,30].
Statistical analysis
Data were expressed as means ± SEM. Comparisons between and within groups were conducted with unpaired Student’s t-tests and repeated-measures one-way ANOVA (followed by Bonferroni: compare all pairs of columns) using GraphPad Prism 5.0 (San Diego, CA), respectively.
Results
Atrasentan attenuate phenylephrine induced hypertrophy in ARCM
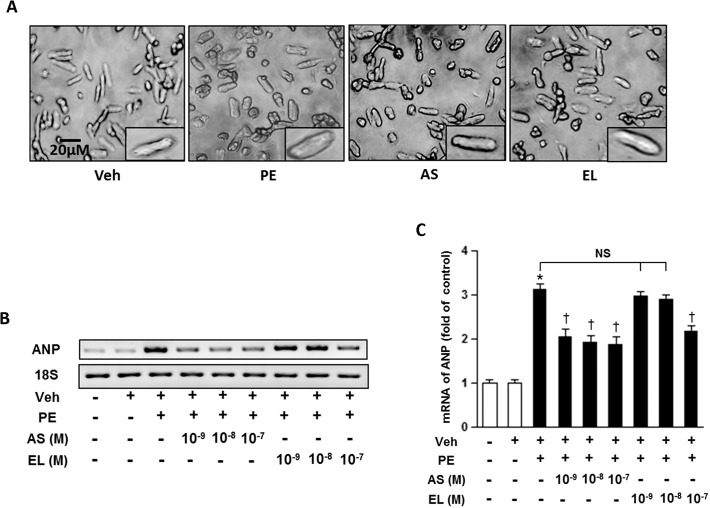
We examined the anti-hypertrophic effect of atrasentan and enalapril in ARCMs by pre-treating the cells with various concentrations of atrasentan and enalapril 30 minutes prior to treatment with phenylephrine (100 μM), a well-known hypertrophic stimulus. After exposing the cells to phenylephrine with various doses of atrasentan and enalapril for 48hrs, the cells were collected and analyzed for evidence of hypertrophy. There were significant increase in cell size (Fig. 1A) and atrial natriuretic peptide (ANP) mRNA expression in phenylephrine treated group (Fig. 1 B-C). Atrasentan significantly reduced the phenylephrine induced activation of ANP mRNA expression compare to the vehicle treatment even at a very low concentration of 10–9 M. Nonetheless, only high dose (10–7 M) of enalapril significantly attenuated phenylephrine induced hypertrophy.
Fig 1. Effect of atrasentan on cardiac hypertrophy in vitro.
A. Representative pictures of ARCM cultures. B. Representative mRNA expression of ANP. C. Quantitative analysis of mRNA expression of ANP. 18S expression is shown for internal loading control. The experiments performed in triplicate and results are expressed as means ± SEM. n = 3/group. *P < 0.05; *P < 0.05 vs. negative control group; † P < 0.05 vs. PE (100 nM) group. Veh, vehicle; PE, phenylephrine; AS, atrasentan; EL, enalapril.
Atrasentan effects on cardiac hypertrophy and dysfunction/heart failure
The DSS rat is a well-established animal model of high salt diet-induced cardiac hypertrophy and heart failure [23,27]. At 6 weeks of age, the male DSS rats received water with or without different dose of atrasentan and/or enalapril under HS diet or ND for 6 weeks. Organ weights were adjusted to the tibia length (TL) and body weight (BW). There were significant increases in heart weight (HW) as well as lung weight (LuW) in DSS rats receiving high salt diet with vehicle when compared with their normal diet littermates, which indicates that HS diet rats developed significant cardiac hypertrophy after 6 weeks of HS diet (Table 1).
Table 1. Morphometric analysis of DSS rats treated with atrasentan.
| Normal Diet | HS diet | |||||||
|---|---|---|---|---|---|---|---|---|
| Veh | AS 10 mg | Veh | AS 2.5 mg | AS 5 mg | AS 10 mg | EL 10mg | AS 5 mg + EL 10mg | |
| HW/BW, mg/g | 3.09±0.09 | 3.03±0.07 | 5.37±0.48* | 4.81±0.30 | 4.09±0.25 † | 3.70±0.20 † | 4.39±0.27 † | 3.84±0.17 † |
| LuW/BW, mg/g | 3.86±0.08 | 3.73±0.07 | 6.54±1.51* | 5.07±0.64 | 4.08±0.20 † | 3.88±0.10 † | 4.47±0.41 † | 3.93±0.11 † |
| HW/TL, mg/mm | 30.84±0.83 | 30.79±0.69 | 45.23±3.53* | 43.16±3.32 | 36.23±1.50 † | 35.8±1.58 † | 36.89±0.83 † | 34.16±0.95 † |
| LuW/TL, mg/mm | 37.41±1.10 | 35.10±1.60 | 48.25±6.47* | 43.32±5.49 | 37.48±1.29 † | 36.26±2.03 † | 37.98±2.24 † | 37.5±2.18 † |
The results are expressed as means ± SEM. n = 5–8 in each group.
*P < 0.05 vs. normal diet with vehicle treated group;
† P < 0.05 vs. high salt (6%) diet with vehicle treated group.
HS, high salt; Veh, vehicle; AS, atrasentan; EL, enalapril; HW, heart weight; BW, body weight; LuW, lung weight; TL, tibia length.
Among HS diet animals, all treated animal groups except for low dose atrasentan showed substantial attenuation of cardiac hypertrophy compared to the vehicle treated group (Table 1). The most benefit was seen in high dose atrasentan treated group. Enalapril treated group only showed a modest benefit. However, when combined with moderate dose of atrasentan, there was a significant and further decrease in cardiac hypertrophy and cardiac dysfunction suggesting an additive benefit of atrasentan in setting of RAS inhibition.
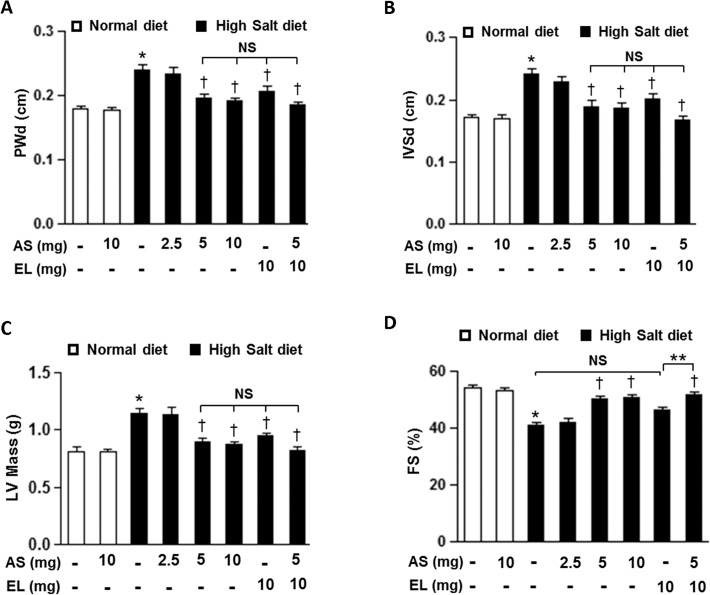
We further examined cardiac function with M-mode echocardiograms to evaluate the effectiveness of atrasentan in attenuation of cardiac hypertrophy and cardiac dysfunction. In HS+V rats, intraventricular septum thickness in diastole (IVSd), posterior wall thickness in diastole (PWd) of the left ventricle (LV) and LV mass were significantly increased (Fig. 2A-C) with significant decrease in fractional shortening (FS) as to compared to their ND littermates (Fig. 2D). Again, low dose atrasentan did not significantly affect the cardiac hypertrophy and cardiac dysfunction parameters in HS diet treated group. However, these cardiac parameters were all significantly improved in other treatment groups with the least benefit observed in enalapril only treated group. Of note, the group treated with combined therapy showed an additional beneficial effect and significantly increased these cardiac parameters over the enalapril treatment alone group.
Fig 2. Echocardiographic finding in the DSS rats.
A-D. Posterior wall thickness in diastole (PWd)(A), intraventricular septal wall thickness in diastole (IVSd)(B), left ventricular (LV) mass (C), and fractional shortening (FS)(D) of DSS rats from different groups. The results are expressed as means ± SEM. n = 5–8/group. *P < 0.05 vs. ND+V group; † P < 0.05 vs. HS+V group; **P < 0.05 vs. HS+EL group. NS, not significant; AS, atrasentan; EL, enalapril.
Atrasentan attenuates the fetal gene expression in the development of cardiac hypertrophy
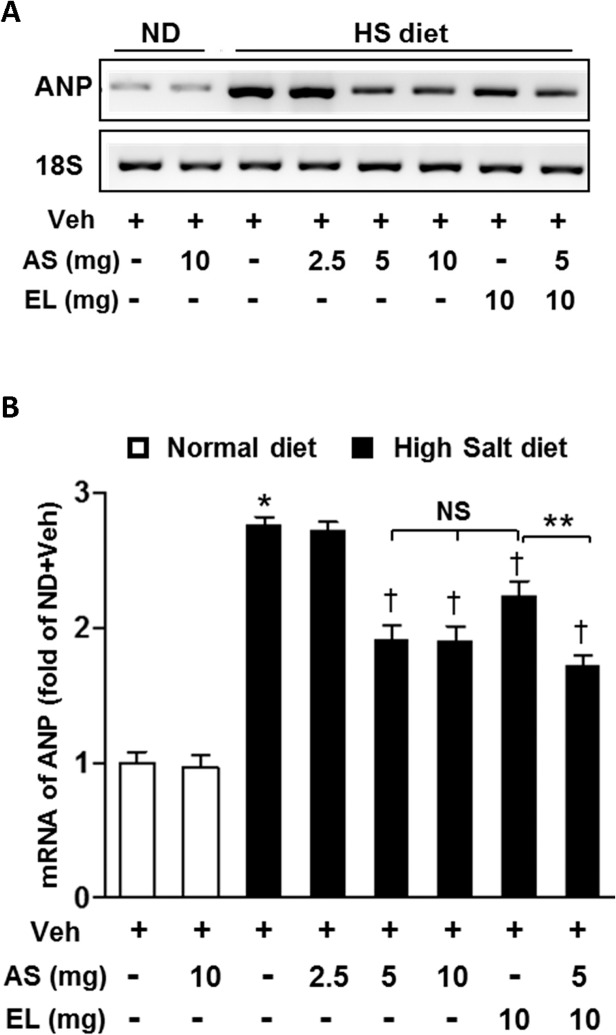
Atrial natriuretic peptide (ANP) is a well-established biochemical marker of cardiac hypertrophy and heart failure. For further confirmation, we examined the mRNA expression level of ANP in LV tissues. Ventricular ANP mRNA levels were significantly elevated in HS+V group than those in ND animals (Fig. 3A-B). These increases were significantly attenuated by all treatment groups, except for low dose atrasentan group. Enalapril treatment alone showed the minimal attenuation of HS-diet induced ANP activation. In groups treated with combination of a moderate dose atrasentan and low dose enalapril, there was an additive effect in further decreasing ANP compared with the group treated with enalapril alone.
Fig 3. Effect of atrasentan on ANP mRNA expression in vivo.
A. Representative mRNA expression of ANP in LV tissue. B. Quantitative analysis of mRNA expression of ANP. 18S expression is shown for internal loading control. The results are expressed as means ± SEM. n = 5–8 in each group. *P < 0.05 vs. ND+V group; † P < 0.05 vs. HS+V group; **P < 0.05 vs. HS+EL group. NS, not significant; Veh, vehicle; AS, atrasentan; EL, enalapril; HS, high salt; ND, normal diet.
Effects of atrasentan on renal impairment
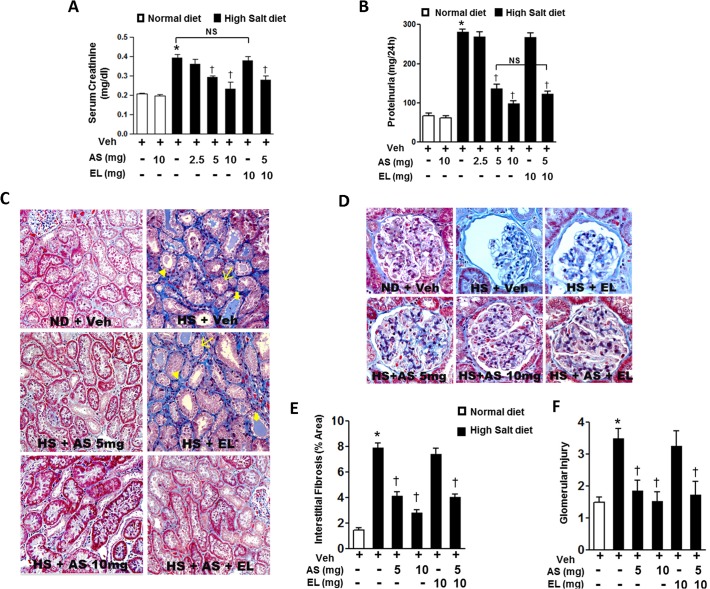
Salt-loaded DSS rats developed severe hypertension, proteinuria, glomerulosclerosis, and elevation of serum creatinine level in 6 weeks of HS diet [31]. In our study, there were significant differences in serum creatinine and proteinuria between ND and HS diet rats with vehicle (Fig. 4A-B), indicating marked hypertension associated with proteinuria and renal glomerular damage after 6 weeks of HS diet. The monotherapy with low dose atrasentan or enalapril did not significantly improve renal dysfunction and proteinuria in HS diet groups. However, moderate or high of atrasentan, and the combined therapy with atrasentan and enalapril significantly attenuated the elevated serum creatinine and proteinuria compared with the HS+V treated littermates.
Fig 4. Effect of atrasentan on kidney function in DSS rats.
A-B. Serum creatinine levels (A) and urinary protein levels (B) in DSS rats from different groups. The results are expressed as means ± SEM. n = 5–8/group. *P < 0.05 vs. ND+V group; † P < 0.05 vs. HS+V group. C-D. Representative photomicrographs of renal morphology (C) and glomerular injury (D) from: Dahl rats on normal diet with vehicle; high salt with vehicle, high salt diet with enalapril, high salt diet with moderate dose atrasentan, high salt diet with high dose atrasentan, and high salt diet with combined therapy (MT). n = 5–8 animals/group. E-F. Morphometric analysis of tubulointerstitial fibrosis (E) and glomerular injury (F). The results are expressed as means ± SEM. 20 random images per animal, n = 5–8 animals/group *P < 0.05 vs. ND+V group; † P < 0.05 vs. HS+V group. Veh, vehicle; EL, enalapril; ND, normal diet; HS, high salt; AS, atrasentan.
Morphological analysis of kidney sections of DSS rats fed ND demonstrated normal glomeruli and interstitium (Fig. 4C-D, top left panel). However, high salt diet induced interstitial fibrosis (arrowhead), dilatation of tubules with tubular atrophy (thin arrows) and presence of intratubular cast (thick arrows) (Fig. 4C, top right panel) and glomerular necrosis (Fig. 4D, top middle panel). Expansion of the interstitium, indicated by an increase in the distance between the tubules, was also evident. These findings suggest that the DSS rats on HS diet with vehicle developed global glomerular sclerosis, interstitial fibrosis, atrophy, and dilated tubules. In DSS rats fed HS diet and treated with moderate & high dose atrasentan as well as combined therapy, these pathological changes were attenuated with relatively normal appearing glomeruli and interstitium (Fig. 4C, middle left, lower left and right panel, and Fig. 4D, lower panel). No improvement of renal pathology was observed in monotherapy with enalapril compared with the HS+V treated littermates (Fig. 4C, middle right panel and Fig. 4D, top right panel). The extent of tubular injury and degree of interstitial fibrosis and glomerular injury were significantly reduced in the moderate and high dose atrasentan monotherapy groups and the combined therapy group, compared with the vehicle treated rats with HS diet (Fig. 4E-F).
Effects of atrasentan on hemodynamics
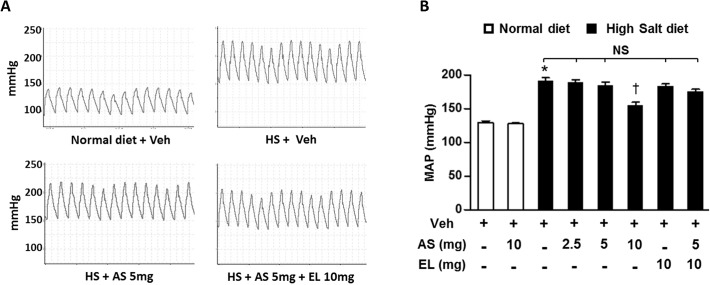
Excess salt intake is known to elevated mean arterial pressure (MAP) of DSS rats fed a HS diet compared to the rats fed a ND diet [23]. In our experiment, there was a 48% increase in MAP in HS+V group compared with ND diet group (Fig. 5A-B). In HS treatment groups, treatment with high dose atrasentan significantly decreased MAP when compared with their HS vehicle littermates. However, MAPs of other treatment groups were non-significant compared to the HS+V treated group. These data provide further evidence that high dose atrasentan significantly reduce BP, whereas moderate dose atrasentan or low dose enalapril alone or in combination do not significantly reduce BP. These findings suggest that the improvement in cardiac and renal dysfunctions by atrasentan—and to more modest extent in enalapril—is independent of lowering BP.
Fig 5. Hemodynamic finding in the DSS rats.
A. Representative blood pressure tracings of DSS rats from different groups, B. Quantitative analysis of mean arterial pressure (MAP) in DSS rats from different groups. The results are expressed as means ± SEM. n = 5–8/group. *P < 0.05 vs. ND+V group; † P < 0.05 vs. HS+V group. NS, not significant; Veh, vehicle; AS, atrasentan; EL, enalapril; HS, high salt.
Discussion
In this study with DSS rat model, we found that moderate and high dose of atrasentan had a substantial preventive effect on the progression of cardiac hypertrophy. Moreover, both atrasentan and enalapril, at a dose that does not alter BP, attenuated the clinical and biochemical evidence of heart failure. In fact, there was an additive anti-hypertrophic effect in combination therapy with atrasentan and enalapril suggesting a potential RAS-independent mechanism by atrasentan. Our study also revealed that moderate dose of atrasentan normalized serum creatinine level and restored normal renal architecture of glomeruli and interstitium without changing BP. However, enalapril, at a dose that does not alter BP, did not evoke any therapeutic effect on renal injury in hypertensive rats. The present study confirmed our hypothesis that in salt induced hypertension in DSS rats, the cardio-renal protective effect of atrasentan, an ETA receptor antagonist, is independent of BP and RAS.
Endothelin, an endothelial cell-derived potent vasoconstrictor, has 4 isoforms (ET-1 through 4)[19,32]. Among the 4 ETs, ET-1 plays a major role in the cardiovascular system [32,33]. It regulates vascular tone, exerts positive inotropic and chronotropic effects as well as causes cardiac hypertrophy [34,35] by binding to specific membrane receptors, ETA and ETB [36]. ET-1 expression level is elevated under certain pathological conditions such as heart failure, renal failure and salt sensitive hypertension [37–40]. ETA receptors are abundantly expressed in vascular smooth muscle cells and cardiomyocytes [41], and mediate the ET-1 concentration-dependent vasoconstriction and mitogenic actions of ET-1. However, ET activating ETB receptors located on the vascular endothelium causes vasodilation through the production of nitric oxide and prostacyclin [42,43]. Theoretically, selective ETA receptor antagonists should be more effective than nonselective ETA/ETB receptor antagonists, given the role played by ETB receptors in both vasodilation and ET-1 clearance [42].
Several studies also showed that ET-1 receptor antagonist prevented endothelial dysfunction, blocked renal damage, attenuated cardiovascular remodeling and heart failure, and improved survival rate [44,45]. In 2002, on the basis of convincing evidence of improved clinical status and survival in patients, US Food and Drug administration approved orally active ET receptor antagonist, bosentan (Tracleer), for the treatment of pulmonary arterial hypertension (PAH) [42]. Since then, several ET receptor antagonists have become available for the treatment of PAH/CKD including ambrisentan (Letairis, Volibris), sitaxsentan (Thelin), Avosentan, BQ123 and atrasentan [42,46]. Recently, Phase 3 clinical study called SONAR (Study Of Diabetic Nephropathy with Atrasentan) is ongoing to assess the effects of atrasentan—when added to standard of care—on progression of kidney disease in patients with stage 2 to 4 CKD and type 2 diabetes [47]. Atrasentan has been shown to be a potent and highly selective ETA receptor antagonist [25,48]. In this study, we demonstrated that atrasentan, a selective ETA receptor antagonist, alone has a significant anti-hypertrophic and cardioprotective effect even at a moderate dose without reducing BP. Interestingly, the high dose did not exert additional impact on cardiac hypertrophy and cardiac function compared with the moderate dose. It can be postulated that the higher dosages of the ETA receptor antagonists may have resulted in an additional blockade of the ETB receptors. Nonetheless, atrasentan has been shown to have 1000-fold greater affinity to human ETA receptors than ETB receptors [25].
RAS activation stimulates cardiomyocyte hypertrophy and fibroblast proliferation, thereby leading to left ventricular hypertrophy (LVH) and heart failure [49,50]. ACEi reduces the progression of RAS induced cardiac remodeling [51]. Enalapril maleate, one of the most commonly used ACEi [50], is non-hypertensive at a dose 10 mg/kg/day in spontaneously hypertensive rats (SHR)[52] and at least four times less potent in DSS rats than in spontaneous hypertensive rats (SHR) [53]. Our current study showed that enalapril dose that does not alter BP modestly limited LV remodeling without changing MAP. In comparison, the concomitant administration of atrasentan and enalapril, at a non-BP altering dose, considerably improved cardiac function and attenuated ANP expression level over enalapril monotherapy. Nonetheless, it did not illustrate any additional improvement of LV function and cardiac remodeling compared with atrasentan alone. In this context, the effect of ET blockade might be more important for cardiac and renal protection.
It is known that ET-1 induces cardiac hypertrophy through G-protein coupled receptors in cardiomyocytes and stimulates myocyte growth and myofibrillogenesis. ET-1 mainly uses the mitogenactivated protein kinase and phosphatidylinositol-3 kinase/AKT pathways to activate GSK3β, and the extracellular signal-regulated kinase 1/2 (ERK1/2) cascade [54,55]. In a healthy condition, ET-1 binding to ETA promotes vasoconstriction, cell proliferation, collagen synthesis, and matrix accumulation [46,56,57], whereas ETB activation pertains to vasodilation, anti-proliferation, and anti-fibrosis [46,58]. Selective ETA receptor blocker has been shown to decrease LV interstitial collagen deposition [58,59], fibrosis and matrix metalloproteinase activity [60,61] in hypertensive rats. ETB mediates vaso-relaxation by increasing the formation of prostacyclin and nitric oxide (NO) as well as the clearance of circulating ET-1[62]. In the case of highly selective ETA receptor antagonist, ETB remains active and therefore it can be regarded as “endogenous antagonistic receptors” which counteracts the effects of the ETA receptor [63]. Altogether, these may result in an increased cardiac output and may prevent deterioration of global LV function.
Growing evidence suggests that RAS and ET-1 [64,65] has an important role in the development and progression of CKD. In the past decade, several experimental and clinical trials have suggested that ET receptor antagonist alone and/or combination with a blocker of the RAS has unique nephroprotective effects with a reduction of up to 45% in albumin [22,46,66–68]. Proteinuria is a common feature of CKD [14] and has an important contribution to CVD risk in CKD [69]. ACEi and angiotensin receptor blockers are standard therapy to prevent CKD. Phase 2 clinical study indicates that low doses of atrasentan substantially reduce urinary albumin excretion in diabetic patients with minimal concern for fluid retention [22]. Though, fluid retention occurrence with atrasentan is dose dependent and associated with only the short-term administration, since no statistically significant edema was observed after a long-term administration [43,70]. However, whether improvement in proteinuria correlates with cardiovascular outcome, and whether the beneficial effects of atrasentan is independent of RAS inhibition is not yet known. In this study, after 6 weeks, DSS rat on high salt diet developed severe hypertension, proteinuria, and glomerulosclerosis [32,71–73]. Monotherapy of enalapril, at a dose that didn’t modify blood pressure, did not have a significant effect on salt-induced proteinuria and serum creatinine. This result concurs with the previous study which showed renoprotective effects of ACEi are BP dependent [74]. Conversely, atrasentan, even at a dose that didn’t alter BP, significantly decreased serum creatinine and proteinuria, and mitigated glomerular sclerosis, interstitial fibrosis, atrophy and dilatation of tubules, and tubular casts in salt-loaded DSS rats. As stated above, earlier studies suggest that chronic blockade of ETA reduces proteinuria and renal inflammation in various rat models without significant change in arterial pressure [15,64]. ETA antagonism induces regression of glomerulosclerosis and albuminuria independent of hypertension and CKD regression associated with the improvement of renal blood flow and cortical microvasculature structure in the stenotic kidney [46,75,76]. However, the combined therapy of non-antihypertensive dose of atrasentan and enalapril did exert additional effect over monotherapy of atrasentan.
Our present findings indicate that BP independent monotherapy of ETA receptor antagonist attenuated the progression of CKD and significantly mitigated CVD. This evidence proposes that proteinuria is strongly correlated with cardiovascular abnormalities in salt-loaded hypertensive rats, and that attenuation of proteinuria was indicative of reno- and cardioprotective effects. In BP independent combined therapy, main therapeutic index was exerted by ETA receptor antagonist and was independent of RAS inhibition. All these findings suggest that, in patient with CKD and CVD, BP independent monotherapy of ETA receptor antagonism may be considered a novel strategy.
Supporting Information
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported in part by research support from AbbVie (PMK) and Brain Korea 21 Plus program from Ministry of Education, Science and Technology, South Korea (MAS, PMK). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Chen SC, Su HM, Tsai YC, Huang JC, Chang JM, Hwang SJ, et al. (2013) Framingham risk score with cardiovascular events in chronic kidney disease. PLoS One 8: e60008 10.1371/journal.pone.0060008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Meguid El Nahas A, Bello AK (2005) Chronic kidney disease: the global challenge. Lancet 365: 331–340. [DOI] [PubMed] [Google Scholar]
- 3. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY (2004) Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305. [DOI] [PubMed] [Google Scholar]
- 4. Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, et al. (2004) Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol 15: 1307–1315. [DOI] [PubMed] [Google Scholar]
- 5. Parfrey PS, Foley RN, Harnett JD, Kent GM, Murray DC, Barre PE (1996) Outcome and risk factors for left ventricular disorders in chronic uraemia. Nephrol Dial Transplant 11: 1277–1285. [PubMed] [Google Scholar]
- 6. Silberberg JS, Barre PE, Prichard SS, Sniderman AD (1989) Impact of left ventricular hypertrophy on survival in end-stage renal disease. Kidney Int 36: 286–290. [DOI] [PubMed] [Google Scholar]
- 7. de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, et al. (2004) Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int 65: 2309–2320. [DOI] [PubMed] [Google Scholar]
- 8. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al. (2003) Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension 42: 1050–1065. [DOI] [PubMed] [Google Scholar]
- 9. Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, et al. (2001) Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 286: 421–426. [DOI] [PubMed] [Google Scholar]
- 10. de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, et al. (2004) Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation 110: 921–927. [DOI] [PubMed] [Google Scholar]
- 11. Ibsen H, Olsen MH, Wachtell K, Borch-Johnsen K, Lindholm LH, Mogensen CE, et al. (2005) Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: losartan intervention for endpoint reduction in hypertension study. Hypertension 45: 198–202. [DOI] [PubMed] [Google Scholar]
- 12. Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, de Jong PE, et al. (2003) Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med 139: 244–252. [DOI] [PubMed] [Google Scholar]
- 13. Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G (2000) Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 342: 145–153. [DOI] [PubMed] [Google Scholar]
- 14. Ruggenenti P, Perticucci E, Cravedi P, Gambara V, Costantini M, Sharma SK, et al. (2008) Role of remission clinics in the longitudinal treatment of CKD. J Am Soc Nephrol 19: 1213–1224. 10.1681/ASN.2007090970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gagliardini E, Corna D, Zoja C, Sangalli F, Carrara F, Rossi M, et al. (2009) Unlike each drug alone, lisinopril if combined with avosentan promotes regression of renal lesions in experimental diabetes. Am J Physiol Renal Physiol 297: F1448–1456. 10.1152/ajprenal.00340.2009 [DOI] [PubMed] [Google Scholar]
- 16. Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK (2008) Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med 358: 2433–2446. 10.1056/NEJMoa0708379 [DOI] [PubMed] [Google Scholar]
- 17. Sato A, Hayashi K, Saruta T (2005) Antiproteinuric effects of mineralocorticoid receptor blockade in patients with chronic renal disease. Am J Hypertens 18: 44–49. [DOI] [PubMed] [Google Scholar]
- 18. Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, Goto K, et al. (1989) The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci U S A 86: 2863–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, et al. (1988) A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332: 411–415. [DOI] [PubMed] [Google Scholar]
- 20. Hynynen MM, Khalil RA (2006) The vascular endothelin system in hypertension—recent patents and discoveries. Recent Pat Cardiovasc Drug Discov 1: 95–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bruzzi I, Remuzzi G, Benigni A (1997) Endothelin: a mediator of renal disease progression. J Nephrol 10: 179–183. [PubMed] [Google Scholar]
- 22. Kohan DE, Pritchett Y, Molitch M, Wen S, Garimella T, Audhya P, et al. (2011) Addition of atrasentan to renin-angiotensin system blockade reduces albuminuria in diabetic nephropathy. J Am Soc Nephrol 22: 763–772. 10.1681/ASN.2010080869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bodyak N, Ayus JC, Achinger S, Shivalingappa V, Ke Q, Chen YS, et al. (2007) Activated vitamin D attenuates left ventricular abnormalities induced by dietary sodium in Dahl salt-sensitive animals. Proc Natl Acad Sci U S A 104: 16810–16815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bae S, Yalamarti B, Ke Q, Choudhury S, Yu H, Karumanchi SA, et al. (2011) Preventing progression of cardiac hypertrophy and development of heart failure by paricalcitol therapy in rats. Cardiovasc Res 91: 632–639. 10.1093/cvr/cvr133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Opgenorth TJ, Adler AL, Calzadilla SV, Chiou WJ, Dayton BD, Dixon DB, et al. (1996) Pharmacological characterization of A-127722: an orally active and highly potent ETA-selective receptor antagonist. J Pharmacol Exp Ther 276: 473–481. [PubMed] [Google Scholar]
- 26. Saleh MA, Boesen EI, Pollock JS, Savin VJ, Pollock DM (2011) Endothelin receptor A-specific stimulation of glomerular inflammation and injury in a streptozotocin-induced rat model of diabetes. Diabetologia 54: 979–988. 10.1007/s00125-010-2021-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kang PM, Yue P, Liu Z, Tarnavski O, Bodyak N, Izumo S (2004) Alterations in apoptosis regulatory factors during hypertrophy and heart failure. Am J Physiol Heart Circ Physiol 287: H72–80. [DOI] [PubMed] [Google Scholar]
- 28. Kang PM, Haunstetter A, Aoki H, Usheva A, Izumo S (2000) Morphological and molecular characterization of adult cardiomyocyte apoptosis during hypoxia and reoxygenation. Circ Res 87: 118–125. [DOI] [PubMed] [Google Scholar]
- 29. Bae S, Singh SS, Yu H, Lee JY, Cho BR, Kang PM (2013) Vitamin D signaling pathway plays an important role in the development of heart failure after myocardial infarction. J Appl Physiol (1985) 114: 979–987. 10.1152/japplphysiol.01506.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Williams JM, Johnson AC, Stelloh C, Dreisbach AW, Franceschini N, Regner KR, et al. (2012) Genetic variants in Arhgef11 are associated with kidney injury in the Dahl salt-sensitive rat. Hypertension 60: 1157–1168. 10.1161/HYPERTENSIONAHA.112.199240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nagase M, Shibata S, Yoshida S, Nagase T, Gotoda T, Fujita T (2006) Podocyte injury underlies the glomerulopathy of Dahl salt-hypertensive rats and is reversed by aldosterone blocker. Hypertension 47: 1084–1093. [DOI] [PubMed] [Google Scholar]
- 32. Rich S, McLaughlin VV (2003) Endothelin receptor blockers in cardiovascular disease. Circulation 108: 2184–2190. [DOI] [PubMed] [Google Scholar]
- 33. Channick RN, Sitbon O, Barst RJ, Manes A, Rubin LJ (2004) Endothelin receptor antagonists in pulmonary arterial hypertension. J Am Coll Cardiol 43: 62S–67S. [DOI] [PubMed] [Google Scholar]
- 34. Pieske B, Beyermann B, Breu V, Loffler BM, Schlotthauer K, Maier LS, et al. (1999) Functional effects of endothelin and regulation of endothelin receptors in isolated human nonfailing and failing myocardium. Circulation 99: 1802–1809. [DOI] [PubMed] [Google Scholar]
- 35. Kakarla SK, Fannin JC, Keshavarzian S, Katta A, Paturi S, Nalabotu SK, et al. (2010) Chronic acetaminophen attenuates age-associated increases in cardiac ROS and apoptosis in the Fischer Brown Norway rat. Basic Res Cardiol 105: 535–544. 10.1007/s00395-010-0094-3 [DOI] [PubMed] [Google Scholar]
- 36. Yamamoto S, Matsumoto N, Kanazawa M, Fujita M, Takaoka M, Gariepy CE, et al. (2005) Different contributions of endothelin-A and endothelin-B receptors in postischemic cardiac dysfunction and norepinephrine overflow in rat hearts. Circulation 111: 302–309. [DOI] [PubMed] [Google Scholar]
- 37. Cody RJ, Haas GJ, Binkley PF, Capers Q, Kelley R (1992) Plasma endothelin correlates with the extent of pulmonary hypertension in patients with chronic congestive heart failure. Circulation 85: 504–509. [DOI] [PubMed] [Google Scholar]
- 38. Demuth K, Blacher J, Guerin AP, Benoit MO, Moatti N, Safar ME, et al. (1998) Endothelin and cardiovascular remodelling in end-stage renal disease. Nephrol Dial Transplant 13: 375–383. [DOI] [PubMed] [Google Scholar]
- 39. Ergul A (2000) Hypertension in black patients: an emerging role of the endothelin system in salt-sensitive hypertension. Hypertension 36: 62–67. [DOI] [PubMed] [Google Scholar]
- 40. Vuurmans TJ, Boer P, Koomans HA (2003) Effects of endothelin-1 and endothelin-1 receptor blockade on cardiac output, aortic pressure, and pulse wave velocity in humans. Hypertension 41: 1253–1258. [DOI] [PubMed] [Google Scholar]
- 41. Hosoda K, Nakao K, Hiroshi A, Suga S, Ogawa Y, Mukoyama M, et al. (1991) Cloning and expression of human endothelin-1 receptor cDNA. FEBS Lett 287: 23–26. [DOI] [PubMed] [Google Scholar]
- 42. Yan LD, Kong LL, Yong Z, Dong HJ, Chi MG, Pan XF, et al. (2010) Inhibition of endothelin-1 and hypoxia-induced pulmonary pressor responses in the rat by a novel selective endothelin-A receptor antagonist, di-n-butylaminocarbamyl-L-leucyl-D-tryptophanyl-D-4-chloro-Phe. J Cardiovasc Pharmacol 56: 246–254. 10.1097/FJC.0b013e3181e89f36 [DOI] [PubMed] [Google Scholar]
- 43. Raichlin E, Prasad A, Mathew V, Kent B, Holmes DR Jr., Pumper GM, et al. (2008) Efficacy and safety of atrasentan in patients with cardiovascular risk and early atherosclerosis. Hypertension 52: 522–528. 10.1161/HYPERTENSIONAHA.108.113068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chade AR, Krier JD, Textor SC, Lerman A, Lerman LO (2006) Endothelin-a receptor blockade improves renal microvascular architecture and function in experimental hypercholesterolemia. J Am Soc Nephrol 17: 3394–3403. [DOI] [PubMed] [Google Scholar]
- 45. Zhang Y, Li L, Hua Y, Nunn JM, Dong F, Yanagisawa M, et al. (2012) Cardiac-specific knockout of ET(A) receptor mitigates low ambient temperature-induced cardiac hypertrophy and contractile dysfunction. J Mol Cell Biol 4: 97–107. 10.1093/jmcb/mjs002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kohan DE, Barton M (2014) Endothelin and endothelin antagonists in chronic kidney disease. Kidney Int 86: 896–904. 10.1038/ki.2014.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chandrashekar K, Juncos LA (2014) Endothelin antagonists in diabetic nephropathy: back to basics. J Am Soc Nephrol 25: 869–871. 10.1681/ASN.2014020174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wessale JL, Adler AL, Novosad EI, Calzadilla SV, Dayton BD, Marsh KC, et al. (2002) Pharmacology of endothelin receptor antagonists ABT-627, ABT-546, A-182086 and A-192621: ex vivo and in vivo studies. Clin Sci (Lond) 103 Suppl 48: 112S–117S. [DOI] [PubMed] [Google Scholar]
- 49. Varagic J, Frohlich ED (2002) Local cardiac renin-angiotensin system: hypertension and cardiac failure. J Mol Cell Cardiol 34: 1435–1442. [DOI] [PubMed] [Google Scholar]
- 50. Tanaka R, Shimizu M (2012) The Relationship between Reactive Oxygen Species and Cardiac Fibrosis in the Dahl Salt-Sensitive Rat under ACEI Administration. Vet Med Int 2012: 105316 10.1155/2012/105316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Plante E, Gaudreau M, Lachance D, Drolet MC, Roussel E, Gauthier C, et al. (2004) Angiotensin-converting enzyme inhibitor captopril prevents volume overload cardiomyopathy in experimental chronic aortic valve regurgitation. Can J Physiol Pharmacol 82: 191–199. [DOI] [PubMed] [Google Scholar]
- 52. Piotrkowski B, Koch OR, De Cavanagh EM, Fraga CG (2009) Cardiac mitochondrial function and tissue remodelling are improved by a non-antihypertensive dose of enalapril in spontaneously hypertensive rats. Free Radic Res 43: 390–399. 10.1080/10715760902801517 [DOI] [PubMed] [Google Scholar]
- 53. Sharma JN, Fernandez PG, Kim BK, Idikio H, Triggle CR (1983) Cardiac regression and blood pressure control in the Dahl rat treated with either enalapril maleate (MK 421, an angiotensin converting enzyme inhibitor) or hydrochlorothiazide. J Hypertens 1: 251–256. [DOI] [PubMed] [Google Scholar]
- 54. Hong HM, Song EJ, Oh E, Kabir MH, Lee C, Yoo YS (2011) Endothelin-1- and isoproterenol-induced differential protein expression and signaling pathway in HL-1 cardiomyocytes. Proteomics 11: 283–297. 10.1002/pmic.201000018 [DOI] [PubMed] [Google Scholar]
- 55. Sugden PH (2003) An overview of endothelin signaling in the cardiac myocyte. J Mol Cell Cardiol 35: 871–886. [DOI] [PubMed] [Google Scholar]
- 56. Barton M (2008) Reversal of proteinuric renal disease and the emerging role of endothelin. Nat Clin Pract Nephrol 4: 490–501. 10.1038/ncpneph0891 [DOI] [PubMed] [Google Scholar]
- 57. Turner JM, Bauer C, Abramowitz MK, Melamed ML, Hostetter TH (2012) Treatment of chronic kidney disease. Kidney Int 81: 351–362. 10.1038/ki.2011.380 [DOI] [PubMed] [Google Scholar]
- 58. Xia QG, Reinecke A, Dorenkamp M, Daemen MJ, Simon R, Unger T (2006) Effects of endothelin ETA receptor blocker LU 135252 on cardiac remodeling and survival in a hypertensive rat model of chronic heart failure. Acta Pharmacol Sin 27: 1417–1422. [DOI] [PubMed] [Google Scholar]
- 59. Allan A, Fenning A, Levick S, Hoey A, Brown L (2005) Reversal of cardiac dysfunction by selective ET-A receptor antagonism. Br J Pharmacol 146: 846–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ammarguellat FZ, Gannon PO, Amiri F, Schiffrin EL (2002) Fibrosis, matrix metalloproteinases, and inflammation in the heart of DOCA-salt hypertensive rats: role of ET(A) receptors. Hypertension 39: 679–684. [DOI] [PubMed] [Google Scholar]
- 61. Callera GE, Montezano AC, Touyz RM, Zorn TM, Carvalho MH, Fortes ZB, et al. (2004) ETA receptor mediates altered leukocyte-endothelial cell interaction and adhesion molecules expression in DOCA-salt rats. Hypertension 43: 872–879. [DOI] [PubMed] [Google Scholar]
- 62. Luscher TF, Barton M (2000) Endothelins and endothelin receptor antagonists: therapeutic considerations for a novel class of cardiovascular drugs. Circulation 102: 2434–2440. [DOI] [PubMed] [Google Scholar]
- 63. Barton M (2010) Therapeutic potential of endothelin receptor antagonists for chronic proteinuric renal disease in humans. Biochim Biophys Acta 1802: 1203–1213. 10.1016/j.bbadis.2010.03.012 [DOI] [PubMed] [Google Scholar]
- 64. Dhaun N, Macintyre IM, Melville V, Lilitkarntakul P, Johnston NR, Goddard J, et al. (2009) Blood pressure-independent reduction in proteinuria and arterial stiffness after acute endothelin-a receptor antagonism in chronic kidney disease. Hypertension 54: 113–119. 10.1161/HYPERTENSIONAHA.109.132670 [DOI] [PubMed] [Google Scholar]
- 65. Kedzierski RM, Yanagisawa M (2001) Endothelin system: the double-edged sword in health and disease. Annu Rev Pharmacol Toxicol 41: 851–876. [DOI] [PubMed] [Google Scholar]
- 66. Andress DL, Coll B, Pritchett Y, Brennan J, Molitch M, Kohan DE (2012) Clinical efficacy of the selective endothelin A receptor antagonist, atrasentan, in patients with diabetes and chronic kidney disease (CKD). Life Sci 91: 739–742. 10.1016/j.lfs.2012.01.011 [DOI] [PubMed] [Google Scholar]
- 67. Parvanova A, van der Meer IM, Iliev I, Perna A, Gaspari F, Trevisan R, et al. (2013) Effect on blood pressure of combined inhibition of endothelin-converting enzyme and neutral endopeptidase with daglutril in patients with type 2 diabetes who have albuminuria: a randomised, crossover, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 1: 19–27. 10.1016/S2213-8587(13)70029-9 [DOI] [PubMed] [Google Scholar]
- 68. Wenzel RR, Littke T, Kuranoff S, Jurgens C, Bruck H, Ritz E, et al. (2009) Avosentan reduces albumin excretion in diabetics with macroalbuminuria. J Am Soc Nephrol 20: 655–664. 10.1681/ASN.2008050482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hillege HL, Fidler V, Diercks GF, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, et al. (2002) Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 106: 1777–1782. [DOI] [PubMed] [Google Scholar]
- 70. Andress DL, Coll B, Pritchett Y, Brennan J, Molitch M, Kohan DE (2012) Clinical efficacy of the selective endothelin A receptor antagonist, atrasentan, in patients with diabetes and chronic kidney disease (CKD). Life Sci 91: 739–742. 10.1016/j.lfs.2012.01.011 [DOI] [PubMed] [Google Scholar]
- 71. Nishikimi T, Akimoto K, Wang X, Mori Y, Tadokoro K, Ishikawa Y, et al. (2004) Fasudil, a Rho-kinase inhibitor, attenuates glomerulosclerosis in Dahl salt-sensitive rats. J Hypertens 22: 1787–1796. [DOI] [PubMed] [Google Scholar]
- 72. Yu M, Moreno C, Hoagland KM, Dahly A, Ditter K, Mistry M, et al. (2003) Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J Hypertens 21: 1125–1135. [DOI] [PubMed] [Google Scholar]
- 73. Ideishi M, Miura S, Sakai T, Maeda H, Kinoshita A, Sasaguri M, et al. (1994) Comparative effects of an angiotensin-converting enzyme inhibitor and an angiotensin II antagonist in Dahl rats. Blood Press Suppl 5: 99–104. [PubMed] [Google Scholar]
- 74. Griffin KA, Abu-Amarah I, Picken M, Bidani AK (2003) Renoprotection by ACE inhibition or aldosterone blockade is blood pressure-dependent. Hypertension 41: 201–206. [DOI] [PubMed] [Google Scholar]
- 75. Chade AR, Stewart NJ, Peavy PR (2014) Disparate effects of single endothelin-A and-B receptor blocker therapy on the progression of renal injury in advanced renovascular disease. Kidney Int 85: 833–844. 10.1038/ki.2013.477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kelsen S, Hall JE, Chade AR (2011) Endothelin-A receptor blockade slows the progression of renal injury in experimental renovascular disease. Am J Physiol Renal Physiol 301: F218–225. 10.1152/ajprenal.00089.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.