Abstract
Objectives
We have analyzed the parameters (bacterial translocation, immune activation and regulation, presence of HCV coinfection) which could be implicated in an inappropriate immune response from individuals with chronic HIV infection. The influence of them on the evolution of CD4+ T cell count has been investigated.
Patients and methods
Seventy HIV-infected patients [monoinfected by HIV (n = 20), HCV-coinfected (with (n = 25) and without (n = 25) liver cirrhosis)] and 25 healthy controls were included. Median duration of HIV infection was 20 years. HIV- and HCV-related parameters, as well as markers relative to bacterial translocation, monocyte and lymphocyte activation and regulation were considered as independent variables. Dependent variables were the increase of CD4+ T cell count during the follow-up (12 months).
Results
Increased values of bacterial translocation, measured by lipopolysaccharide-binding protein, monocyte and lymphocyte activation markers and T regulatory lymphocytes were detected in HIV-monoinfected and HIV/HCV coinfected patients. Serum sCD14 and IL-6 were increased in HIV/HCV-coinfected patients with liver cirrhosis in comparison with those with chronic hepatitis or HIV-monoinfected individuals. Time with undetectable HIV load was not related with these parameters. The presence of cirrhosis was negatively associated with a CD4+ T cell count increase.
Conclusion
In patients with a chronic HIV infection, a persistent increase of lipopolysaccharide-binding protein and monocyte and lymphocyte modifications are present. HCV-related cirrhosis is associated with more elevated serum concentrations of monocyte-derived markers. Cirrhosis influences the continued immune reconstitution of these patients.
Introduction
Antiretroviral treatment (ART) has been implicated in the transformation of human immunodeficiency virus (HIV) infection in a chronic entity. The undetectability of plasma HIV load in HIV-infected patients on ART is followed-up by immunological reconstitution, typically measured by circulating CD4+ cell counts [1,2]. However, a percentage of these patients, oscillating between 7% and 47%, shows an insufficient increase of CD4+ T cells even if plasma HIV load remains undetectable during prolonged periods [3,4]. Causes of discordant immune response are multiple. Among them it has been considered: 1) The persistence of immune activation [5,6]. Immune activation has been related with the following features: a) persistence of HIV replication in lymphatic nodes, although peripheral blood HIV replication was controlled [7]; b) bacterial translocation, related with the damage of intestinal barrier by the HIV [7], and c) existence of other coinfections, mainly cytomegalovirus infection [8]. 2) Immune activation induces an increase of regulatory T lymphocytes (Treg) (CD4+CD25highCD127lowFoxP3+) [9]. Cell contact and Treg-dependent secretion of transforming growth factor beta 1 (TGF-β1) modulate the lymphocyte proliferation, and it might contribute to the immune discordant response [10]. 3) Fibrosis of lymphatic nodes, related, among others, with the secretion of TGF-β1, interferes with the lymphocyte proliferation in these organs [11]. 4) Absence of an adequate tymopoiesis caused by either alteration of lymphocyte precursors or aging [12].
Hepatitis C virus infection is frequently detected in HIV-infected patients when the transmission risk is related with the parenteral use of drugs or hemoderivatives administration. HCV coinfection has been scarcely studied as a pathogenic factor of the immune discordant response [13,14], even though HCV might be implicated in it because of its effect on immune activation [15], the increase of Treg-dependent TGF-β1 secretion [16], or because of the bacterial translocation associated with portal hypertension, a situation typical of advanced phases of hepatopathy [17].
The absence of an adequate immune response is more complex in patients after several years of ART. Among individuals with virological suppression, CD4+ cell counts continue to increase throughout 5 years, although the rate of CD4 count increase diminishes with the prolongation of time since starting ART [18]. Cohort studies have observed that CD4 cell counts may reach a plateau after the first years of ART and that patients who started ART with CD4+ cell counts < 350 cells/mm3 were less likely to achieve normal levels [19,20,21]. Possible causes of this altered immune reconstitution are not clarified.
In this work, bacterial translocation and monocyte and lymphocyte activation and regulatory markers were analysed in a series of HIV-infected patients with a prolonged period of infection. The influence on these parameters of the time with ART-induced undetectable HIV load and the presence of HCV coinfection and its consequences was studied. In a different analysis, the influence of bacterial translocation and immune markers on the evolution of CD4+ T cell count after a 12-month period has also been prospectively investigated.
Patients and Methods
Patients and controls
Seventy HIV-infected patients were prospectively included. All subjects were consecutively recruited from a prospectively collected cohort of HIV-infected patients treated at the HIV outpatients’ clinics of a university hospital. Patients were distributed in two groups: those monoinfected by HIV (n = 20) and those with HCV-coinfection, grouped in those with (n = 25) and those without (n = 25) liver cirrhosis.
Inclusion criteria were: 1) Infection by HIV. 2) To be on ART. 3) Achievement and persistence of undetectable HIV load during at least 12 months. Exclusion criteria were as follows: (1) Active opportunistic or concomitant infections. (2) Neoplasias, including liver cancer at inclusion. (3) Evidence of active infection by hepatitis B virus (HbsAg negative), alcoholic hepatitis, or metabolic or autoimmune liver disease. Significant alcohol ingestion (higher than 50 g/day during at least 5 years) was also a criterion of exclusion. (4) Decompensation of liver cirrhosis. (5) Treatments which could have modified the determination of cytokines (pentoxyfilline, steroidal, or nonsteroidal anti-inflammatory or immunosuppressive drugs). (6) Treatment against chronic HCV infection. (7) Red blood cell or plasma transfusion in the month before inclusion in the study.
As a control group, we studied a sample of healthy subjects (n = 25) recruited from voluntary hospital workers, whose age and gender were comparable with those of the patients.
Definitions
Patients were followed-up in our hospital with clinical and analytical revision every three months. Positive serum antibodies against HIV were required for the diagnosis of HIV infection. Patients were classified according the 1993 Centers for Disease Control and Prevention classification of HIV infection. Spanish Group for AIDS Study guidelines (www.gesida.es) was used to indicate the antiretroviral treatment (ART). Plasma HIV RNA load lower than 50 copies/ml was considered as undetectable HIV load.
Positive serum antibodies against HCV and persistent (more than 6 months) HCV RNA were required for the diagnosis of chronic HCV infection. Diagnosis of chronic hepatitis or cirrhosis was established according to histological criteria when liver biopsy was performed [22], or by transient elastography, performed according to a standardized technique by one trained operator (JAGG) (according to data validated in HIV-HCV coinfected patients using liver biopsy as reference, patients with a liver stiffness > 14,6 kPa were classified as individuals with cirrhosis) [23].
Duration of HIV infection was estimated using an interviewer-assisted questionnaire assessing risk factors for HIV infection. The earliest exposure was designated as the time of acquisition [24].
Phenotypic studies
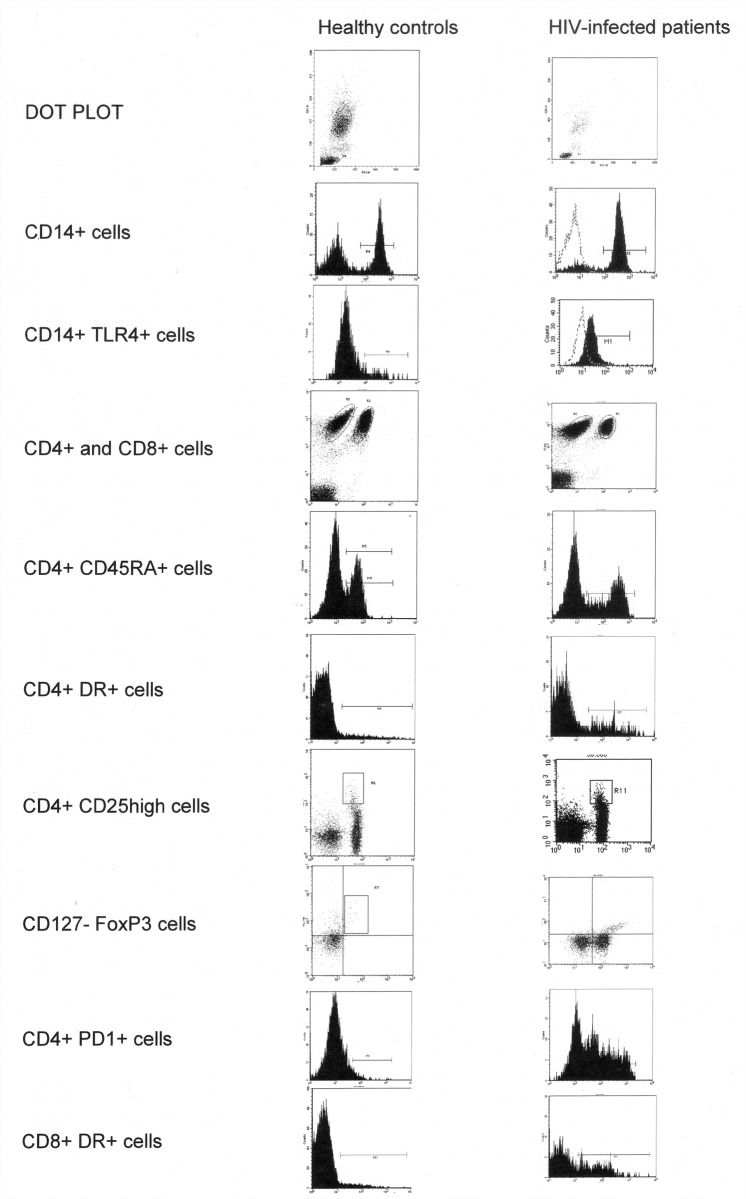
Blood samples, collected in pyrogen-free heparinized tubes (Biofreeze, Costar, EEUU), were taken at 8 am to minimize the influence of circadian rhythms. Peripheral blood mononuclear cells (PBMC) were obtained from heparinized venous blood by Ficoll-Hypaque (Lymphprep Nyegaard, Oslo, Norway) density gradient centrifugation. PBMC were incubated with combinations of fluorescein-CD127 (FITC), (Biolegend, San Diego CA, USA) phycoerythrin-CD25 (PE) (Becton-Dickinson, San Jose, CA, USA) and peridinin chlorophyll protein CD4 (PerCP) (Becton-Dickinson), fluorescein-CD45RA (FITC) (Becton-Dickinson), fluorescein-CD279 [–anti-PD1-] (FITC) (Becton-Dickinson), fluorescein-CD284 [–anti-TLR4-] (FITC) (Imgenex, San Diego CA, USA), and peridin chlorophyll protein CD14 (PerCP) (Becton-Dickinson) labelled monoclonal antibodies. The anti-human FOXP3 APC (eBioscience, San Diego CA, USA) was used for intracellular T cell immunophenotyping. Stained cells were washed, acquired, and analyzed by two-colour flow cytometry in a FACScalibur cytometer using Cell Quest and Paint-A-Gate software (Becton-Dickinson). FACS gating strategy and plots for all the cell subsets analysed are shown in Fig. 1.
Fig 1. FACS gating strategy and plots for all the cell subsets analysed in a representative healthy control and a HIV-infected patient.
Concentration of LBP and of pro- and anti-inflammatory molecules
Serum LBP was measured by immunometric sandwich assay (Immulite LBP; DPC, Los Angeles, CA). Serum concentrations of soluble CD14 (sCD14) and IL-6 were analysed using Milliplex MAP kit High Sensitivity Human Cytokine (Millipore, Billerica, MA, USA). Serum TGF-β1 levels were detected by Quantikine Human Immunoassay (R&D, Minneapolis, MN, USA).
Statistical analysis
Descriptive data were expressed as the median (25–75 interquartile range—IQR-) or as an absolute number (percentage). Qualitative variables were compared by the chi-square test or Fisher’s exact test when necessary. Quantitative variables were compared using the Mann-Whitney U test or ANOVA when necessary. The Spearman’s correlation test analysed the association between quantitative variables.
To analyse the influence of the time with plasma HIV undetectability on the bacterial translocation and immune response detected at the inclusion in the study, patients were grouped in function of the time (12–36, 36–60, > 60 months) with a continued plasma HIV load < 50 copies/ml. The influence on them of HCV coinfection and the presence or absence of liver cirrhosis was also investigated.
In a different analysis, the parameters influencing the increase of CD4+ T cells during a prospective 12-month period were considered. In function of the median of the increase of CD4+ T cells after 12 months of follow-up after inclusion, patients were classified in two groups: those with an adequate CD4+ T cell increase and those with an inadequate response. Demographic, HIV-related (CD4+ T cells at diagnosis and at inclusion in the study, protease inhibitor—based ART, previous time with undetectable HIV load) and HCV-related (percentage of patients with coinfection by HCV and of patients with liver cirrhosis) parameters, as well as parameters relative to bacterial translocation, monocyte and lymphocyte activation, and markers of Treg lymphocytes were considered as possible independent variables in this analysis. After a bivariant study, these variables with a significance lower than 0,1 were introduced in a linear regression study.
A p value <0.05 was considered significant. The statistical analysis was carried out using the SPSS 15.0 statistical software package (SPSS Inc., Chicago, IL, USA).
Ethical aspects
This study was performed according to the Helsinki Declaration. The project was approved by the University Hospital Puerta del Mar (Cádiz, Spain) ethical research committee. Written informed consent was obtained from each participant.
Results
Characteristics of the patients are shown in Table 1. Patients with chronic hepatitis secondary to HIV/HVC coinfection had higher CD4+ T cell count at inclusion than those with HIV monoinfection and those with liver cirrhosis. Due to these differences, data about lymphocyte subpopulations will be expressed as percentages.
Table 1. General characteristics of the HIV-infected patients (n = 70).
| Global (n = 70) | HIV-monoinfected patients (n = 20) | HIV/HVC coinfected patients | |||
|---|---|---|---|---|---|
| Global (n = 50) | Chronic hepatitis (n = 25) | Liver cirrhosis (n = 25) | |||
| General characteristics | |||||
| Age (years) | 47 (42–49) | 46 (39–49) | 47 (43–50) | 47 (43–51) | 47 (43–49) |
| Sex male (n, %) | 59 (84) | 16 (80) | 43 (86) | 20 (80) | 23 (92) |
| Previous parenteral drug use (n, %) | 44 (63) | 12 (60) | 32 (64) | 14 (56) | 18 (72) |
| Evolution of the infection (years) | 22 (19–26) | 22 (19–24) | 22 (20–27) | 22 (20–26) | 22 (19–27) |
| HIV-related characteristics | |||||
| CD4+ T cell/mm3 at diagnosis | 150 (87–267) | 182 (107–416) | 146 (65–253) | 160 (73–258) | 145 (64–234) |
| CD4+ T cell/mm3 at inclusion | 342 (252–535) | 305 (193–473) | 359 (277–540) | 519 (321–661) * | 339 (229–380) * |
| CD4+ T cell increase from diagnosis until inclusion | 181 (57–295) | 85 (-53; +183) | 202 (111–334) | 288 (177–399) | 172 (55–221) |
| CDC C stage (n, %) | 36 (51) | 12 (60) | 24 (48) | 14 (56) | 10 (40) |
| Months with undetectable HIV load | 42 (17–72) | 49 (16–102) | 39 (18–62) | 24 (16–60) | 48 (18–55) |
| Protease inhibitor—based HAART (n, %) | 38 (54) | 11 (55) | 27 (54) | 13 (52) | 14 (56) |
| HCV-related characteristics | |||||
| Liver stiffness (kPa) | 15 (10–27) | 10 (9–12) ** | 27 (17–33) ** | ||
| HCV genotype 1 or 4 (n, %) | 32 (64) | 18 (72) | 14 (56) | ||
| HCV viral load (x 1000) at inclusion | 2574 (401–4640) | 3130 (643–4404) | 2514 (572–4948) | ||
Quantitative variables are expressed as median (interquartile range).
* p = 0,003 HIV/HCV coinfected patients with cirrhosis vs HIV/HCV coinfected patients with chronic hepatitis.
** p < 0,001 HIV/HCV coinfected patients with cirrhosis vs HIV/HCV coinfected patients with chronic hepatitis.
Bacterial translocation and monocyte and lymphocyte parameters and relation with HCV coinfection
Serum levels of LBP were significantly elevated in HIV-infected patients with reference to healthy controls (p<0,001). Concentrations of LBP were similar in HIV-infected patients with or without HCV coinfection (with or without liver cirrhosis) (p>0,05) (Table 2).
Table 2. Bacterial translocation, monocyte and lymphocyte markers and Treg-derived parameters in healthy controls and HIV-infected patients with or without coinfection by HCV.
| Healthy controls (n = 25) | HIV- infected patients | |||||
|---|---|---|---|---|---|---|
| Global(n = 70) | HIV-mono-infected (n = 20) | HIV-HCV coinfected patients | ||||
| Global (n = 50) | Chronic hepatitis (n = 25) | Liver cirrhosis (n = 25) | ||||
| Bacterial translocation | ||||||
| LBP (ng/ml) | 4 (3–4) | 9 (6–14) * | 8 (5–14) | 10 (7–14) | 11 (8–12) | 9 (6–18) |
| Monocyte parameters | ||||||
| CD14+TLR4+ cells (% of total CD14+ cells) | 19 (11–28) | 83 (68–88) * | 83 (67–87) | 82 (68–88) | 84 (78–91) | 79 (59–85) |
| Serum soluble CD14 (x 100) (ng/ml) | 10 (5–12) | 19 (14–27) * | 15 (8–24) | 21 (16–28) | 19 (16–25) | 26 (16–30) ** |
| Serum IL-6 (pg/ml) | 2 (1–6) | 6 (2–15) | 6 (2–13) | 9 (2–15) | 6 (5–8) | 14 (8–18) ** |
| Lymphocyte parameters | ||||||
| CD4+ T cells (% of total T cells) | 58 (48–62) | 27 (19–34) * | 22 (19–36) | 29 (18–34) | 26 (24–33) | 29 (18–36) |
| CD4+DR+ cells (% of total CD4+T cells) | 12 (9–14) | 18 (14–25) * | 21 (16–66) | 17 (14–23) | 14 (12–21) | 18 (15–25) |
| CD4+CD45RA+ cells (% of total CD4+T cells) | 49 (36–62) | 37 (21–45) * | 31 (19–40) | 39 (22–45) | 42 (21–48) | 38 (22–45) |
| CD4+PD1+ cells (% of total CD4+T cells) | 5 (2–10) | 21 (8–55) * | 26 (12–81) | 17 (8–51) | 16 (5–70) | 17 (8–51) |
| CD8+ T cells (% of total T cells) | 24 (20–27) | 35 (30–45) * | 44 (19–57) | 36 (31–44) | 39 (31–45) | 35 (31–42) |
| CD8+DR+ cells (% of total CD8+T cells) | 22 (17–26) | 38 (39–44) * | 38 (35–81) | 39 (27–44) | 35 (30–43) | 42 (20–44) |
| CD4 / CD8 ratio | 1,7 (1,1–2,2) | 0,8 (0,5–1,0) * | 0,5 (0,4–2,1) | 0,9 (0,5–1,0) | 0,8 (0,5–1,0) | 0,8 (0,5–1,1) |
| T regulatory cells parameters | ||||||
| CD4+CD25highCD127lowFoxP3+ (% of total CD4+T cells) | 1 (0–2) | 7 (3–11) * | 8 (3–10) | 7 (3–12) | 5 (4–16) | 8 (3–14) |
| Serum TGF-β1 (pg/ml) | 38 (19–49) | 72 (52–97) * | 77 (58–121) | 72 (49–97) | 83 (58–115) | 70 (45–89) |
Variables are expressed as median (interquartile range).
* p < 0,05 comparing healthy controls with HIV-infected patients, global.
** p < 0,05 comparing patients with chronic hepatitis and cirrhosis.
Parameters indicative of monocyte activation (percentage of CD14+TLR4+ and serum sCD14 and IL-6 levels) were significantly increased in the overall group of HIV-infected patients (p<0,001) (Table 2). There was no significant difference in these parameters when patients with HIV monoinfection were compared with those with HIV/HCV coinfection with chronic hepatitis. Patients with cirrhosis showed significantly higher concentrations of serum sCD14 (p = 0,024) and IL-6 (p = 0,036) than those with chronic hepatitis.
The correlations between serum LBP levels and monocyte parameters (CD14+TLR4+ percentage and sCD14 and IL-6) were not significant (p>0,05 in each case).
At inclusion, HIV-infected patients showed a lower percentage of CD4+ T cells (p<0,001) and a higher percentage of CD8+ T cells (p = 0,005) than healthy controls, with a decreased CD4/CD8 ratio (p = 0,001). Percentages of activated CD4 (CD4+DR+) and CD8 (CD8+DR+) T lymphocytes were significantly higher in HIV-infected patients than in healthy controls (p = 0,041 and p = 0,048, respectively). HIV-infected patients showed lower percentages of naïve T CD4+ lymphocytes (CD4+CD45RA+) than healthy controls (p = 0,040). Finally, CD4+ T cells expressing the death receptor PD-1 were significantly increased in HIV-infected individuals with reference to healthy controls (p = 0,017). There was no significant difference in these parameters when patients with HIV monoinfection were compared with those with HIV/HCV coinfection. Likewise, there was no significant difference in these parameters when patients with chronic hepatitis were compared with those with liver cirrhosis (Table 2).
A significant correlation was established between serum LBP levels and the percentage of CD4+DR+ (r = 0,842, p = 0,004) and CD8+DR+ (r = 0,561, p = 0,016). A negative correlation was detected between percentages of activated CD4+ cells (CD4+DR+) and naïve CD4+ cells (CD4+CD45RA+) (r = -0,467, p = 0,025). No significant correlation was detected between CD4+DR+ percentage and monocyte (CD14+TLR4+, serum levels of sCD14 and IL-6) parameters (p>0,05 in each case).
The percentage of Treg lymphocytes and serum concentrations of TGF-β1 were significantly elevated in HIV-infected patients with reference to healthy controls (p<0,001 in each case). Treg percentage and serum TGF-β1 levels were similar in HIV/HCV coinfected patients (without differences between those with chronic hepatitis and those with liver cirrhosis) and HIV-monoinfected individuals (p>0,05, in each case). A significant correlation was demonstrated between the percentage of Treg lymphocytes and TGF-β1 (r = 0,921, p = 0,001) in these patients.
No significant correlation was established between CD4+DR+ and Treg percentages (p>0,05). Serum levels of TGF-β1 and IL-6 (r = -0,246, p = 0,043) were significantly correlated.
Relation between the time with undetectable HIV load and markers of bacterial translocation and lymphocyte or monocyte activation and regulation
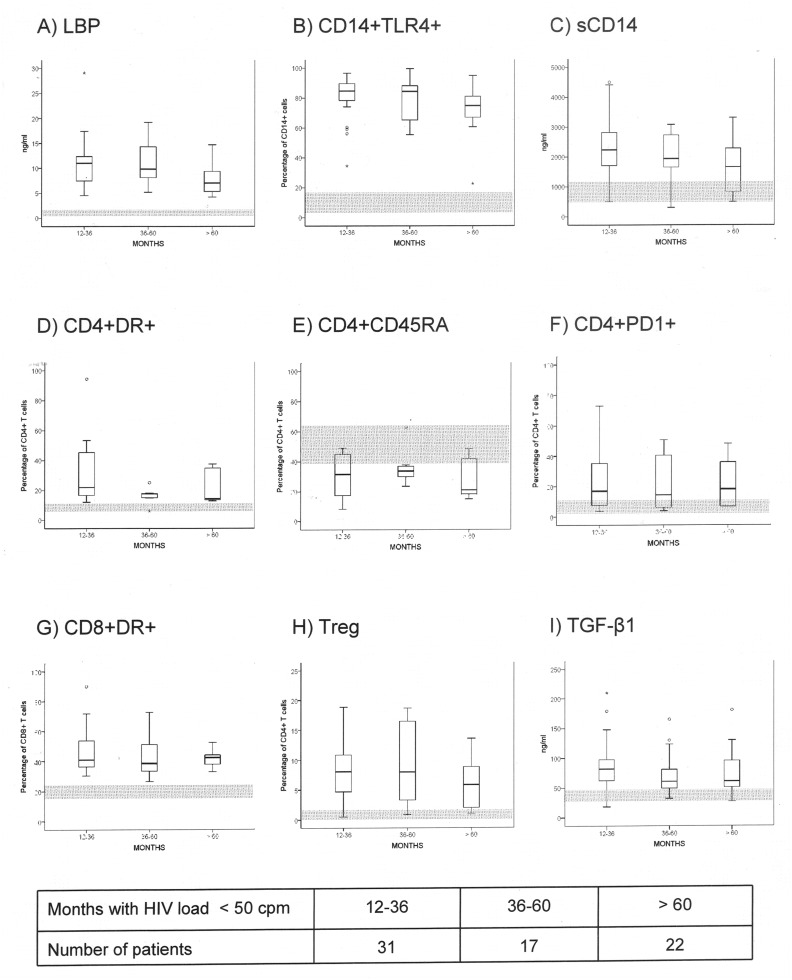
Patients included in the study had shown continuous undetectable HIV load during a median of 44 months (range: 13–132 months) and 21 patients (30%) of them showed a normal CD4+ T cell count (≤ 500/mm3). Even in patients with more than 60 months with undetectable HIV load, LBP, monocyte and lymphocyte activation markers, and Treg percentage and TGF-β1 persisted elevated in comparison with values of the healthy controls (Fig. 2).
Fig 2. Markers of bacterial traslocation (A) (serum levels of lipopolysaccharide binding protein—LBP-, ng/ml); monocyte derived parameters [toll-like receptor 4 expression on monocyte (B) (CD14+TLR4+, percentage of blood CD14+ cells) and soluble CD14 receptor (C) (sCD14, ng/ml)], T CD4+-related populations [activated CD4+ T cells (D) (CD4+DR+, percentage of blood T CD4+ cells), naïve T CD4+ cells (E) (CD4+CD45RA+, percentage of blood T CD4+ cells), CD4+ T cells expressing the dead receptor PD1 (F) (CD4+PD1+, percentage of blood T CD4+ cells)], activated T CD8+ cells (G) (CD8+DR+, percentage of blood T CD8+ cells), and regulatory T cells [Treg, CD4+CD25highCD127lowFoxP3+ T cells (H) (percentage of blood T CD4+ cells) and serum concentration of transforming growth factor beta 1 (I) (TGF-β1, ng/ml)] in patients with HIV load undetectable for 12–36, 36–60 or > 60 months.
Grey areas represent the normal values (range) of each parameter in healthy controls.
Implications of bacterial translocation and immune parameters on the magnitude of increase in CD4+ T cell count during the follow-up
Patients were followed-up during 12 months after the beginning of the study. The median increase of CD4+ T cell lymphocytes was 10 (IQR-74; +122)/mm3. Patients were distributed in two groups in function of that their increase of CD4+ T cells were higher or lower than 10 cells/mm3 (the value of the median). Patients with an increase of CD4+ T cells higher than the median showed a CD4+ T cell gain of 110/mm3 (IQR +40; +156), whereas those with an increase of CD4+ T cells lower than the median showed a CD4+ T cell decrease of-90/mm3 (IQR, -193; -31). The absolute CD4+ T cell count after 12 months of follow-up was 331 (IQR, 169; 469) cells/mm3 in patients with an increase lower than the median and 398 (IQR, 283; 671) cells/mm3 in those with an increase higher than the median (p<0,001).
Differential characteristics are shown in the Table 3. Only a higher CD4+ cell increase from HIV diagnosis until the beginning of the study and a higher CD4+ cell count at inclusion were significantly associated with a lower increase of CD4+ cells during the follow-up. The presence of cirrhosis approaches significance. Values of bacterial translocation and immune parameters were similar in patients with a higher or lower increase of CD4+ cells. When only HIV/HCV coinfected patients were considered, correlation among CD4+ cell count increase and liver stiffness was not significant (r = -0,175, p = 0,322). No significant correlation was established between time with undetectable HIV load and CD4+ cell increase (r = 0,113, p = 0,365).
Table 3. Lymphocyte and monocyte activation markers, T reg lymphocytes and bacterial translocation markers at inclusion in patients with an increase or decrease of CD4+ T cells/mm3 during the follow-up with reference to the median.
| Patients with an increase of CD4 T cells lower than 10 / mm3 (n = 37) | Patients with an increase of CD4 T cells higher than 10 / mm3 (n = 33) | P | |
|---|---|---|---|
| General characteristics | |||
| Age (years) | 47 (43–49) | 45 (42–51) | 0,533 |
| Sex male (n, %) | 32 (87) | 27 (82) | 0,745 |
| HIV-related characteristics | |||
| Time of evolution of HIV infection (years) | 22 (19–26) | 22 (19–27) | 0,892 |
| CD4+ T cell/mm3 count at diagnosis | 188 (89–310) | 146 (80–209) | 0,380 |
| CD4+ T cell/mm3 count at inclusion | 381 (315–558) | 300 (197–395) | 0,008 |
| Time with undetectable HIV load (months) | 39 (14–62) | 48 (14–72) | 0,504 |
| Protease inhibitor based HAART (n, %) | 19 (52) | 19 (58) | 0,420 |
| Increase of CD4+ T cell/mm3 count from nadir until inclusion | 221 (57–344) | 168 (50–283) | 0,047 |
| Increase of CD4+ T cell/mm3 count during follow-up | -90 (-193; -31) | 110 (+40; +156) | 0,001 |
| HCV-related parameters | |||
| Patients with HCV coinfection (% of total of patients) | 29 (78) | 21 (64) | 0,196 |
| Patients with liver cirrhosis (% of total of patients) | 17 (46) | 8 (24) | 0,081 |
| Bacterial translocation | |||
| LBP (ng/ml) | 8 (6–12) | 12 (7–14) | 0,760 |
| Monocyte and lymphocyte derived parameters | |||
| CD14+TLR4+ cells (% of total CD14+ cells) | 84 (75–90) | 78 (63–87) | 0,188 |
| Serum soluble CD14 (x 100) (ng/ml) | 21 (16–27) | 19 (9–26) | 0,318 |
| Serum IL-6 (pg/ml) | 9 (3–15) | 5 (1–13) | 0,270 |
| CD4+ T cells (% of total lymphocytes) | 30 (18–37) | 25 (20–30) | 0,286 |
| CD4+DR+ cells (% of total CD4+T cells) | 17 (14–23) | 20 (15–35) | 0,870 |
| CD4+CD45RA+ cells (% of total CD4+T cells) | 32 (25–47) | 28 (18–41) | 0,215 |
| CD4+PD1+ cells (% of total CD4+T cells) | 26 (7–42) | 34 (10–76) | 0,299 |
| CD8+ T cells (% of total T cells) | 34 (26–43) | 42 (33–52) | 0,094 |
| CD8+DR+ cells (% of total CD8+T cells) | 32 (19–43) | 43 (37–55) | 0,174 |
| CD4/CD8 ratio | 0,8 (0,5–1,4) | 0,6 (0,4–1,0) | 0,278 |
| T regulatory cells number and function | |||
| CD4+CD25highCD127lowFoxP3+ (% of total CD4+T cells) | 7 (4–14) | 7 (2–11) | 0,580 |
| Serum TGF- β1 (pg/ml) | 69 (49–96) | 75 (58–115) | 0,265 |
Quantitative variables are expressed as median (interquartile range).
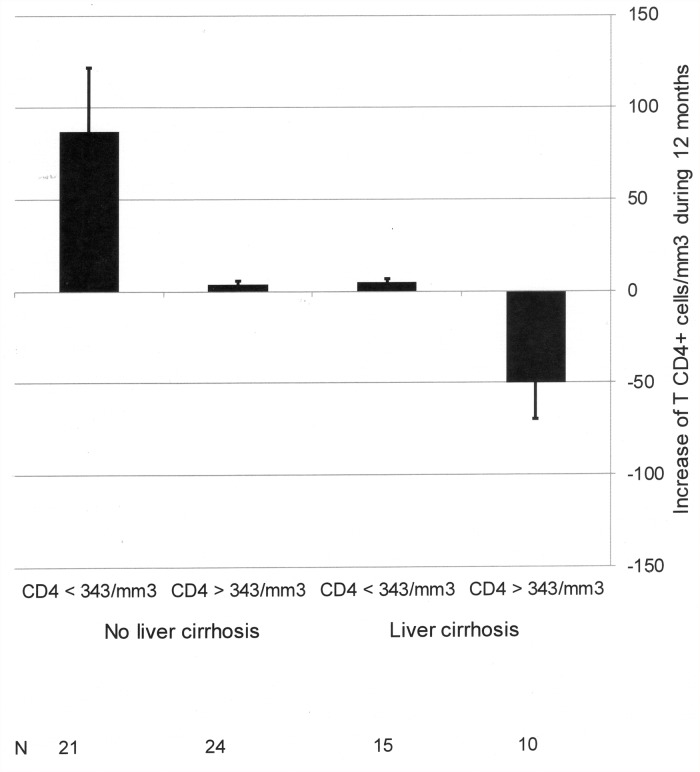
Logistic regression analysis of those factors associated with the increase of CD4+ cell count showed that a CD4+ cell count lower than the median (343/mm3) at the beginning of the study (Exp(B) 5,354, 95% confidence interval 1,681–17,052, p = 0,005) and the absence of cirrhosis (Exp(B) 4,648, 95% confidence interval 1,437–15,034, p = 0,010) were positively associated with the increase of CD4+ cell during the follow-up. The increase of CD4+ cell count in function of the presence or absence of these factors is shown in Fig. 3.
Fig 3. Increase in CD4+ T cell count during a follow-up of 12 months in HIV-infected patients with undetectable HIV viral load.
Patients have been classified in function of: 1) CD4+ T cell count at the beginning, and 2) presence or absence of liver cirrhosis.
Discussion
In a sample of chronically HIV-infected patients, we have studied their alterations in intestinal permeability, the monocyte and lymphocyte activation, and the regulatory T cells number.
Both HIV-monoinfected and HIV/HCV coinfected patients showed a state of bacterial translocation, as revealed by the increased LBP levels. LBP was selected as a measure of bacterial translocation because it is correlated with LPS concentration [25] but is more reliable than LPS due to its longer half-life [26]. Serum LBP levels in patients with or without HCV-coinfection were similar: it must be considered that decompensated cirrhosis, a situation in which bacterial translocation is characteristic [27], was an exclusion criterion.
Chronic monocyte and lymphocyte activation has been previously described in HIV-infected patients [28,29]. Markers of monocyte activation (sCD14 and IL-6) have been considered as powerful predictive parameters of evolution of HIV-infection, due to both AIDS- and non-AIDS related entities [30,31], and they have been proposed in risk stratification strategies [32].
As it must be expected in a situation of chronic antigenemia [33,34,35], an increase of regulatory T lymphocytes (Treg) and serum TGF-β1 concentration was observed. High frequencies of Tregs can down-regulate immune activation but also benefit HIV-specific responses, thereby favouring persistence of HIV infection [36,37]. Interestingly, our results demonstrate that serum concentration of TGF-β1, the main molecule secreted by Treg, significantly correlate with those of IL-6, suggesting that monocyte activation might be a powerful stimulating factor of secretion of TGF-β1.
The proportion of naïve T cells was decreased and the percentage of cells prone to apoptosis, as revealed by the expression of the programmed death receptor PD-1 on CD4+ cells, was increased in HIV-infected patients, with independence of the evolution of CD4+ cell number. Recent in vitro and in vivo studies, including those performed in HIV-infected individuals, have shown the importance of the PD-1 interaction with its ligand PD-L1 in the pathogenesis of decreased T cell proliferation and increased apoptosis [38].
Our work has analysed if these abnormalities in intestinal permeability and in monocyte and lymphocyte functions are related with the time of undetectable HIV load as well as with the presence of HCV coinfection. Antiretroviral treatment decreases but does not normalize bacterial translocation markers when it is initiated in patients with recent HIV infection [28]. Interestingly, in patients such as those in our series with a more chronic HIV infection, LBP values kept on having increased and similar to baseline levels even in patients with more than 5 years of continuous undetectable HIV load.
A rapid reduction in both activated CD4+ and CD8+ cell populations coincides with the rapid first phase of immune reconstitution and control of HIV viremia, supporting the hypothesis that ART reduces inflammation and subsequent redistribution of CD4+ and CD8+ cells [7,8,28]. However, in patients with chronic HIV infection with a time of infection of more than 20 years, such as those studied in the present work, the immune activation state is maintained.
The influence of HCV coinfection and liver cirrhosis was also considered. Data of our work have demonstrated that both sCD14 and IL-6 are even more increased in those HIV-infected patients with HCV-related cirrhosis. Previous results of our group had detected the elevation of these markers in patients with decompensated liver cirrhosis, having been attributed to the situation of bacterial translocation associated with the portal hypertension [17,34]. But in the present work, the elevation of sCD14 and IL-6, but not of CD14+TLR4+ percentage or lymphocyte activation, was also evident in patients with HCV-related compensated cirrhosis. There was no significant difference between patients with HIV monoinfection and those with chronic hepatitis. Moreover, HCV load was similar in HIV/HCV coinfected patients with chronic hepatitis and liver cirrhosis (data not shown), discarding the possibility of an effect directly attributed to the hepatitis virus on monocyte activation. A possible explanation for this finding has been provided by the experimental demonstration of a monocyte activation state directly associated with the liver cirrhosis: effectively, monocyte activation is present in hepatic lymph nodes, and serum IL-6 is increased in cirrhotic rats previously to the development of portal hypertension [39]. Thus, both HCV-related liver cirrhosis and HIV-induced intestinal barrier lesion could be operative and justify the increased values of sCD14 and IL-6 in HIV/HCV cirrhotic patients. This double influence on monocyte activation could explain the absence of significant correlation among sCD14 or IL-6 and LBP, observed in this and in a previous study [40]. Taking into account the predictive value of sCD14 and IL-6, this can also explain the increased mortality of HIV/HCV coinfected patients detected by some authors [41,42] and the beneficial impact of HCV eradication in cirrhotic patients on survival [43].
Finally, the influence of the several HIV- and HCV-related parameters, as well as the alterations in immune responses, on the evolution of CD4+ cell count has been studied. Among individuals with virological suppression, CD4+ cell counts continue to increase throughout 5 years [18], although patients who started ART with CD4+ cell counts < 350 cells/mm3 were less likely to achieve normal levels [19,20,21,44]. Effectively, a notable percentage (40%) of the patients did not increase the CD4+ cell count after 5 years of undetectable HIV load [45]. These findings led to the conclusion that not all patients might eventually respond to ART by achieving a CD4 count in the normal range. As it has been discussed, patients in our series showed several characteristics previously associated with a poor immune reconstitution: immune activation, increased expression of PD-1, decreased proportion of naïve CD4+ T cells, and elevated percentages of Treg [5,10,11]. These parameters were similar in groups in which occurred or not occurred the increase of CD4+ T cell counts, implicating that this alteration of the innate and adaptive immune systems is not a primary causative factor of the absence of CD4+ cell increase in this population with a prolonged period of infection.
Furthermore, HCV infection was considered as a factor implicated in the presence or absence of increase of CD4+ cell count. HCV coinfection has been previously analysed in the pathogenesis of discordant immune response, discarding its independent contribution to it [6,18]. However, the importance of the cirrhosis, and not of every histological type of HCV-related liver disease, has not been previously studied. The presence of cirrhosis was negatively associated with an increase of CD4+ cell count. This is the first time in which the influence of cirrhosis has been considered a parameter implicated in the altered immune recuperation of HIV-infected individuals. It is possible that the increase of serum levels of sCD14 and IL-6, particularly elevated in cirrhotic patients, compromises the immune reconstitution, although they were not independent factors associated with it.
In conclusion, this work has demonstrated that in patients with a chronic HIV infection, a persistent increase in intestinal permeability and immune modifications are present. HCV-related cirrhosis is associated with more elevated serum concentrations of monocyte-derived markers (sCD14 and IL-6). Furthermore, cirrhosis influences the continued immune reconstitution of these patients.
Data Availability
All relevant data are within the paper.
Funding Statement
This work has been performed with grants from the Instituto de Salud Carlos III (PI08/0869 and PI11/00605). José A. Girón-González has a grant for “Intensificación de Actividad Investigadora” 2013, Servicio Andaluz de Salud, Spain. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. DeHovitz JA, Kovacs A, Feldman JG, Anastos K, Young M, Cohen M, et al. The relationship between virus load response to highly active antiretroviral therapy and change in CD4 cell counts: A report from the Women’s Interagency HIV Study. J Infect Dis. 2000;182: 1527–1530. [DOI] [PubMed] [Google Scholar]
- 2. Lange CG, Lederman MM. Immune reconstitution with antiretroviral therapies in chronic HIV-1 infection. J Antimicrob Chemother. 2003;51: 1–4. [DOI] [PubMed] [Google Scholar]
- 3. Florence E, Lundgren J, Dreezen C, Fisher M, Kirk O, Blaxhult A, et al. Factors associated with a reduced CD4 lymphocyte count response to HAART despite full viral suppression in the EuroSIDA Study. HIV Med. 2003;4: 255–262. [DOI] [PubMed] [Google Scholar]
- 4. Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: causes and consequences. J Pathol. 2008;214: 231–241. [DOI] [PubMed] [Google Scholar]
- 5. Pitrak DL, Bolanos J, Hershow R, Novak RM. Discordant CD4 T lymphocyte responses to antiretroviral therapy for HIV infection are associated with ex-vivo rates of apoptosis. AIDS. 2001;15: 1317–1319. [DOI] [PubMed] [Google Scholar]
- 6. Negredo E, Massanella M, Puig J, Perez-Alvarez N, Gallego-Escuredo JM, Villaroya J, et al. Nadir CD4 T cell count as predictor and high CD4 T cell intrinsic apoptosis as final mechanism of poor CD4 T cell recovery in virologically suppressed HIV-infected patients: Clinical implications. Clin Infect Dis. 2010;50: 1300–1308 10.1086/651689 [DOI] [PubMed] [Google Scholar]
- 7. Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200: 749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hsu DC, Kerr SJ, Iampornsin T, Pett SL, Avihingsanon A, Thongpaeng P, et al. Restoration of CMV-specific-CD4 T cells with ART occurs early and is greater in those with more advanced immunodeficiency. PLoS One. 2013;8: e77479 10.1371/journal.pone.0077479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aandahl EM, Michaelsson J, Moretto WJ, Hecht FM, Nixon DF. Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J Virol. 2004;78: 2454–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shevach EM. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity. 2006;25: 195–201 [DOI] [PubMed] [Google Scholar]
- 11. Schacker TW, Reilly C, Beilman GJ, Taylor J, Skarda D, Krason D, et al. Amount of lymphatic tissue fibrosis in HIV infection predicts magnitude of HAART-associated change in peripheral CD4 cell count. AIDS. 2005;19: 2169–2171. [DOI] [PubMed] [Google Scholar]
- 12. Ping Y, Kourtis AP, Kirschner DE. Reconstitution of thymic function in HIV-1 patients treated with highly active antiretroviral therapy. Clin Immunol. 2003;106: 95–105. [DOI] [PubMed] [Google Scholar]
- 13. Miller MF, Haley C, Koziel MJ, Rowley CF. Impact of hepatitis C virus on immune restoration in HIV-infected patients who start highly active antiretroviral therapy: a meta-analysis. Clin Infect Dis. 2005;41: 713–720. [DOI] [PubMed] [Google Scholar]
- 14. Antonucci G, Girardi E, Cozzi-Lepri A, Capobianchi MR, De Luca A, Puoti M, et al. Role of hepatitis C virus (HCV) viremia and HCV genotype in the Immune recovery from highly active antiretroviral therapy in a cohort of antiretroviral-naive HIV-infected individuals. Clin Infect Dis. 2005;40: e101–e109. [DOI] [PubMed] [Google Scholar]
- 15. Kovacs A, Al-Harthi L, Christensen S, Mack W, Cohen M, Landay A. CD8(+) T cell activation in women coinfected with human immunodeficiency virus type 1 and hepatitis C virus. J Infect Dis. 2008;197: 1402–1407. 10.1086/587696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boettler T, Spangenberg HC, Neumann-Haefelin C, Panther E, Urbani S, Ferrari C, et al. T cells with a CD4+ CD25+ regulatory phenotype suppress in vitro proliferation of virus-specific CD8+ T cells during chronic hepatitis C virus infection. J Virol. 2005;79: 7860–7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Oca Arjona MM, Marquez M, Soto MJ, Rodriguez-Ramos C, Terron A, Vergara A, et al. Bacterial translocation in HIV-infected patients with HCV cirrhosis: implication in hemodynamic alterations and mortality. J Acquir Immune Defic Syndr. 2011;56: 420–427. 10.1097/QAI.0b013e31820ef408 [DOI] [PubMed] [Google Scholar]
- 18. Mocroft A, Phillips AN, Gatell J, Ledergerber B, Fisher M, Clumeck N, et al. Normalisation of CD4 counts in patients with HIV-1 infection and maximum virological suppression who are taking combination antiretroviral therapy: an observational cohort study. Lancet. 2007;370: 407–413. [DOI] [PubMed] [Google Scholar]
- 19. Moore DM, Hogg RS, Yip B, Craib K, Wood E, Montaner JS. CD4 percentage is an independent predictor of survival in patients starting antiretroviral therapy with absolute CD4 cell counts between 200 and 350 cells/microL. HIV Med. 2006;7: 383–388. [DOI] [PubMed] [Google Scholar]
- 20. Gras L, Kesselring AM, Griffin JT, van Sighem AI, Fraser C, Ghani AC, et al. CD4 cell counts of 800 cells/mm3 or greater after 7 years of highly active antiretroviral therapy are feasible in most patients starting with 350 cells/mm3 or greater. J Acquir Immune Defic Syndr. 2007;45: 183–192. [DOI] [PubMed] [Google Scholar]
- 21. Robbins GK, Spritzler JG, Chan ES, Asmuth DM, Gandhi RT, Rodriguez BA, et al. Incomplete reconstitution of T cell subsets on combination antiretroviral therapy in the AIDS Clinical Trials Group protocol 384. Clin Infect Dis. 2009;48: 350–61. 10.1086/595888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19: 1513–1520 [PubMed] [Google Scholar]
- 23. Macías J, Girón-González JA, González-Serrano M, Merino D, Cano P, Mira JA, et al. Prediction of liver fibrosis in HIV/HCV-coinfected patients by simple noninvasive indexes. Gut. 2006;55: 409–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Graham CS, Baden LR, Yu E, Mrus JM, Carnie J, Heeren T, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: A meta-analysis. Clin Infect Dis. 2001;33: 562–569 [DOI] [PubMed] [Google Scholar]
- 25. Abad-Fernández M, Vallejo A, Hernández-Novoa B, Díaz L, Gutiérrez C, Madrid N, et al. Correlation between different methods to measure microbial translocation and its association with immune activation in long-term suppressed HIV-1-infected individuals. J Acquir Immune Defic Syndr. 2013;64: 149–53. 10.1097/QAI.0b013e31829a2f12 [DOI] [PubMed] [Google Scholar]
- 26. Schumann RR, Latz E. Lipopolysaccharide binding protein. Chem Immunol. 2000;74: 42–60. [DOI] [PubMed] [Google Scholar]
- 27. Albillos A, de la Hera A, Gonzalez M, Moya JL, Calleja JL, Monserrat J, et al. Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatology. 2003;37: 208–217. [DOI] [PubMed] [Google Scholar]
- 28. Jiang W, Lederman MM, Hunt P, Sieg SF, Haley K, Rodriguez B, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis. 2009;199: 1177–1185. 10.1086/597476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Paiardini M, Muller-Trutwin M. HIV-associated chronic immune activation. Immunol Rev. 2013;254: 78–101. 10.1111/imr.12079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5: e203 10.1371/journal.pmed.0050203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203: 780–90 10.1093/infdis/jiq118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boulware DR, Hullsiek KH, Puronen CE, Rupert A, Baker JV, French MA, et al. Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J Infect Dis. 2011;203: 1637–1646. 10.1093/infdis/jir134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Márquez M, Fernández-Gutiérrez C, Montes-de-Oca M, Blanco MJ, Brun F, Rodriguez-Ramos C, et al. Chronic antigenic stimuli as a possible explanation for the immunodepression caused by liver cirrhosis. Clin Exp Immunol. 2009; 158: 219–229. 10.1111/j.1365-2249.2009.04005.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hunt PW. HIV and inflammation: mechanisms and consequences. Curr HIV/AIDS Rep. 2012;9: 139–147 10.1007/s11904-012-0118-8 [DOI] [PubMed] [Google Scholar]
- 35. Hazenberg MD, Otto SA, van Benthem BH, Roos MT, Coutinho RA, Lange JM, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17: 1881–1888. [DOI] [PubMed] [Google Scholar]
- 36. Gaardbo JC, Nielsen SD, Vedel SJ, Ersbøll AK, Harritshøj L, Ryder LP, et al. Regulatory T cells in human immunodeficiency virus-infected patients are elevated and independent of immunological and virological status, as well as initiation of highly active anti-retroviral therapy. Clin Exp Immunol. 2008;154: 80–86. 10.1111/j.1365-2249.2008.03725.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rallón NI, López M, Soriano V, García-Samaniego J, Romero M, Labarga P, et al. Level, phenotype and activation status of CD4+FoxP3+ regulatory T cells in patients chronically infected with human immunodeficiency virus and/or hepatitis C virus. Clin Exp Immunol. 2009;155: 35–43. 10.1111/j.1365-2249.2008.03797.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rosignoli G, Lim CH, Bower M, Gotch F, Imami N. Programmed death (PD)-1 molecule and its ligand PD-L1 distribution among memory CD4 and CD8 T cell subsets in human immunodeficiency virus-1-infected individuals. Clin Exp Immunol. 2009;157: 90–97 10.1111/j.1365-2249.2009.03960.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Úbeda M, Muñoz L, Borrero MJ, Díaz D, Francés R, Monserrat J, et al. Critical role of the liver in the induction of systemic inflammation in rats with preascitic cirrhosis. Hepatology. 2010;52: 2086–2095. 10.1002/hep.23961 [DOI] [PubMed] [Google Scholar]
- 40. Romero-Sánchez M, González-Serna A, Pacheco YM, Ferrando-Martínez S, Machmach K, García-García M, et al. Different biological significance of sCD14 and LPS in HIV-infection: importance of the immunovirology stage and association with HIV-disease progression markers. J Infect. 2012;65: 431–438. 10.1016/j.jinf.2012.06.008 [DOI] [PubMed] [Google Scholar]
- 41. Greub G, Ledegerber B, Battegay M, Grob P, Perrin L, Furrer H, et al. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study. Lancet. 2000;356: 1800–1805. [DOI] [PubMed] [Google Scholar]
- 42. De Luca A, Bugarini R, Lepri AC, Puoti M, Girardi E, Antinori A, et al. Coinfection with hepatitis viruses and outcome of initial antiretroviral regimens in previously naive HIV-infected subjects. Arch Intern Med. 2002;162: 2125–2132. [DOI] [PubMed] [Google Scholar]
- 43. Mira JA, Rivero-Juárez A, López-Cortés LF, Girón-González JA, Téllez F, de los Santos-Gil I, et al. Benefits from sustained virologic response to pegylated interferon plus ribavirin in HIV/hepatitis C virus-coinfected patients with compensated cirrhosis. Clin Infect Dis. 2013;56: 1646–1653. 10.1093/cid/cit103 [DOI] [PubMed] [Google Scholar]
- 44. Kaufmann GR, Furrer H, Ledergerber B, Perrin L, Opravil M, Vernazza P, et al. Characteristics, determinants, and clinical relevance of CD4 T cell recovery to <500 cells/ul in HIV type-1 infected individuals receiving potent antiretroviral therapy. Clin Infect Dis. 2005;41: 361–372. [DOI] [PubMed] [Google Scholar]
- 45. Gras L, Kesselring AM, Griffin JT, van Sighem AI, Fraser C, Ghani AC, et al. CD4 cell counts of 800 cells/mm3 or greater after 7 years of highly active antiretroviral therapy are feasible in most patients starting with 350 cells/mm3 or greater. J Acquir Immune Defic Syndr. 2007;45: 183–192 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.