Abstract
The pathophysiology of the tics that define Gilles de la Tourette syndrome (TS) is not well understood. Local disinhibition within the striatum has been hypothesized to play a pathogenic role. In support of this, experimental disinhibition by local antagonism of GABA-A receptors within the striatum produces tic-like phenomenology in monkey and rat. We replicated this effect in mice via local picrotoxin infusion into the dorsal striatum. Infusion of picrotoxin into sensorimotor cortex produced similar movements, accompanied by signs of behavioral activation; higher-dose picrotoxin in the cortex produced seizures. Striatal inhibition with local muscimol completely abolished tic-like movements after either striatal or cortical picrotoxin, confirming their dependence on the striatal circuitry; in contrast, cortical muscimol attenuated but did not abolish movements produced by striatal picrotoxin. Striatal glutamate blockade eliminated tic-like movements after striatal picrotoxin, indicating that glutamatergic afferents are critical for their generation. These studies replicate and extend previous work in monkey and rat, providing additional validation for the local disinhibition model of tic generation. Our results reveal a key role for corticostriatal glutamatergic afferents in the generation of tic-like movements in this model.
Introduction
Tics are involuntary stereotyped motor and vocal behaviors often associated with subjective premonitory urges; they are a defining symptom of Gilles de la Tourette syndrome (TS) and are frequently seen in other neuropsychiatric conditions. TS has childhood onset, with a prevalence among school aged children of 0.3–0.8%, and is diagnosed worldwide (Knight et al., 2012; Robertson and Stern, 1997; Scharf et al., 2012). Tics are often chronic, disruptive, and stigmatizing, producing substantial morbidity (Leckman, 2002). The pathophysiology of tic disorders is not well understood, and causative genes have proven elusive (State, 2011; Williams et al., 2013). The most effective pharmacological management of tics consists of antagonists of the dopamine (DA) D2 receptor, such as haloperidol. However, the substantial side effects of these agents limit their use, especially in children, and treatment drop-outs are common (Bloch, 2008).
Dysfunction of the cortico-basal ganglia circuitry is thought to be central to the pathophysiology of tic disorders (Leckman et al., 2010; Williams et al., 2013). Structural imaging has revealed a reduction in the size of caudate and putamen (Peterson et al., 2003) that correlates with disease persistence (Bloch et al., 2005). Functional imaging studies have similarly implicated this circuitry (Rickards, 2009). The sensorimotor cortex is thinned in children with TS, with cortical thickness negatively correlated with the severity of tic symptoms (Sowell et al., 2008). In contrast, regional volumes of dorsal prefrontal and parietal cortex are significantly increased in children with TS (Peterson et al., 2001); this may relate to compensatory responses or volitional tic suppression (Peterson et al., 1998). Structural abnormalities have also been described in the thalamus (Miller et al., 2010), cerebellum (Tobe et al., 2010), and elsewhere in the brain. Recently, post-mortem investigations have shown alterations in striatal microcircuitry in severe, refractory TS, with a reduction in the density of several populations of striatal interneuron (Kalanithi et al., 2005; Kataoka et al., 2010; Lennington et al., 2014).
It has been proposed that tics arise from foci of pathological disinhibition within the striatum (caudate-putamen) (Mink, 2001, 2003). In support of such a model, small, discrete strokes of the caudate and putamen have been observed to produce both motor and phonic tics (Kwak and Jankovic, 2002). Direct injections of GABA-A receptor antagonists into the monkey or rat striatum produce contralateral tic-like movements of limbs and face (Bronfeld et al., 2011; Bronfeld et al., 2013; Marsden et al., 1975; McCairn et al., 2009; McCairn et al., 2013; Tarsy et al., 1978; Worbe et al., 2013), providing experimental support for this concept as well as an animal model in which the consequences of local striatal inhibition can be examined (Pittenger, 2014).
Non-human primates, in which most work on local striatal disinhibiton has been performed, are well suited for electrophysiological studies, but less so for pharmacological or genetic investigations. Here we replicate the local striatal disinhibition model in mice using local striatal infusion of picrotoxin, producing phenomenology similar to that reported in non-human primates and in rats (Bronfeld et al., 2011; Bronfeld et al., 2013; McCairn et al., 2009; McCairn et al., 2013; Tarsy et al., 1978; Worbe et al., 2013). This permits pharmacological investigations of the model. Specifically, we investigated the ability of local modulation of glutamate and GABA within the corticostriatal circuitry to modulate tics produced by local striatal inhibition, to increase construct validity of the model and to probe the underlying neuronal mechanisms. Past studies of local disinhibition in non-human primates and rats have used the GABA-A antagonist bicuculine (Bronfeld et al., 2011; Bronfeld et al., 2013; McCairn et al., 2009; McCairn et al., 2013; Worbe et al., 2013). We used a more specific GABA-A receptor antagonist, picrotoxin, because bicuculine has been reported to also block calcium-activated potassium SK channels and produce epileptiform oscillations in the thalamic network (Kleiman-Weiner et al., 2009).
Materials and Methods
Subjects
Adult male C57Bl/6 mice, aged 2.5–5 months, were purchased from Jackson Laboratories (www.jax.org) and used in all experiments. Mice were housed under a 12/12 hr light/dark cycle under controlled temperature and humidity conditions. All procedures were performed in accordance with the NIH Guide for the Use of Experimental Animals and were approved and overseen by Yale University’s IACUC.
Drugs
Picrotoxin (PTX), muscimol (Musc), (RS)-4-(phosphonomethyl)-piperazine-2-carboxylic acid (PMPA), and D-amphetamine sulfate were all purchased from Sigma, St. Louis, MO. They were dissolved in sterile saline for intracranial or intraperitoneal administration.
Procedures
Stainless steel single- or double-injection guide cannulae (PlasticsOne, Roanoke, VA) were unilaterally implanted 1 week before experiments, using standard stereotaxic methods under ketamine/xylazine anesthesia. Initial mapping experiments (Figure 1A) used a range of targeting coordinates; subsequent experiments focused on three regions: AP+0.5, L −2.2, V −3.2 for dorsolateral striatum (DLS); AP+0.4, L −1.7, V −3.5 for central striatum (CES); or AP-0.1, L −2.0, V −1.5 for sensorimotor cortex (Cx) (all refer to the tip of injection cannula; see Figure 1A). For pharmacological and EEG experiments only DLS and Cx sites were used, as these were the sites where tics were most reliably elicited (see Figure 1). The total number of mice used for mapping and dose-response PTX experiments was 67. The number of animals for dose-response experiments in Cx, DLS, and CES is presented in Table 1.
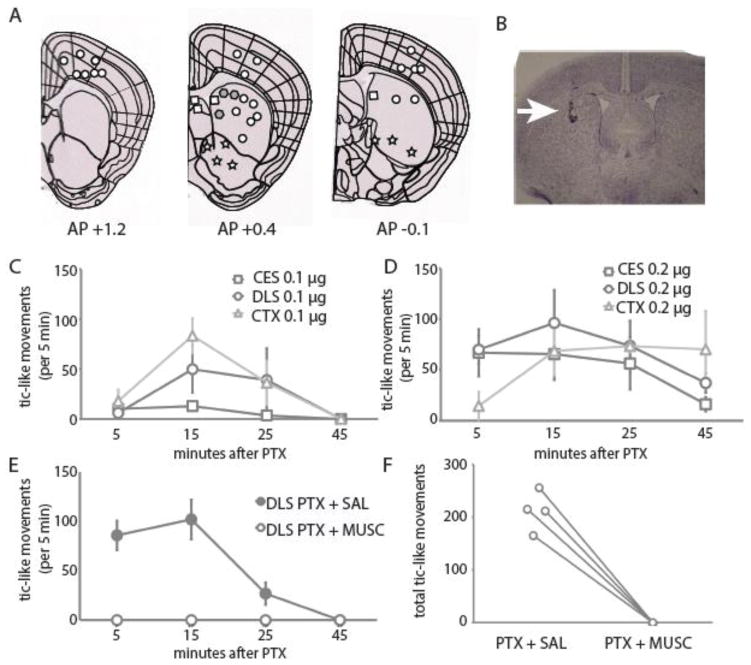
Figure 1.
A. Diagram of frontal sections (from www.brain-map.org) showing sites of injections and evoked effects in exploratory pilot experiments:
 tic-like movements;
tic-like movements;
 no apparent behavioral effect;
no apparent behavioral effect;
 locomotor activation, sniffing, hindpaw licking;
locomotor activation, sniffing, hindpaw licking;
 behavioral depression. B. Cresyl violet staining of a typical injection site in striatum after infusion of 0.3 uL toluidine blue 20 min before euthanasia. C. Time course of tics, quantified in 5 minute bins starting 5, 15, 25, and 45 min after 0.1 ug of PTX injected into CES, DLS, and Cx. D. Same for 0.2 ug of PTX. E. Pharmacological blockade of PTX-induced tic-like movements by muscimol (MUSC). Saline (SAL) or Musc 0.2 μg was injected 10 min before PTX using the same cannula, targeting the DLS (n = 4; all animals received both treatments, several days apart). F. All tic-like movements were eliminated by muscimol pretreatment.
behavioral depression. B. Cresyl violet staining of a typical injection site in striatum after infusion of 0.3 uL toluidine blue 20 min before euthanasia. C. Time course of tics, quantified in 5 minute bins starting 5, 15, 25, and 45 min after 0.1 ug of PTX injected into CES, DLS, and Cx. D. Same for 0.2 ug of PTX. E. Pharmacological blockade of PTX-induced tic-like movements by muscimol (MUSC). Saline (SAL) or Musc 0.2 μg was injected 10 min before PTX using the same cannula, targeting the DLS (n = 4; all animals received both treatments, several days apart). F. All tic-like movements were eliminated by muscimol pretreatment.
Table 1.
Latency to first tic and number of tics evoked by 0.1 and 0.2ug of PTX at three sites
| Latency (minutes; median and interquartile range) | 0.1ug | N = | 0.2ug | N = |
|---|---|---|---|---|
| Cx | 8.75 (6.4–12) | 11 | 9 (6.5–11.2) | 11 |
| DLS | 9 (6–11) | 7 | 6 (5–8) * | 9 |
| CES | 9.75 (6.5–13) | 4 | 6.25 (4.7–9) | 8 |
| Number of Tics (mean ± SEM)
| ||||
| Cx | 161.6 (34) | 11 | 226.6 (35) | 11 |
| DLS | 155.7 (46) | 7 | 248.4 (59) | 9 |
| CES | 13.3 (8.4) | 4 | 205.3 (68) | 8 |
Cx - Sensorimotor Cortex; DLS - Dorsolateral Striatum; CES - Central Striatum.
P<0.05 compared with Cx
Saline, picrotoxin (PTX), and other drugs were microinjected into the striatum or cortex in a fixed volume of 0.2 μL over 2 min using a microdrive and a 1.0 uL Hamilton syringe connected with a 30-cm Teflon tubing to the injection cannula (PlasticsOne, Roanoke, VA). During microinjection the animal was freely moving in the home cage. At the end of infusion the injector remained in place for an additional 1 min. Two Hamilton syringes were used for simultaneous double injections into striatum and cortex. For experiments requiring local pre-treatment with other pharmacological agents, the pre-treatment injection was performed 10 min before PTX injection into the same site, or a different site using double cannulae. A within-subjects design was used. The number of animals in each experiment is as follows: 4 in intra-DLS PTX/Musc, 7 in intra-DLS PTX/PMPA, 5 in intra-DLS PTX/intra-cortical Musc, 5 in intra-cortical PTX/intra-DLS Musc.
Tic-like movements of the head or limbs were counted over 5-min intervals at 5, 15, 25, and 45 min after PTX microinjection. Data are represented at each time point or as the total number of tics summed over all intervals.
Following all striatal targetings and most cortical targetings, 0.3 uL of toluidine blue was microinjected through the cannula 15 min prior to sacrifice and cannula placement was confirmed by visualization of the dye in brain slice.
EEG
EEG recordings were performed as previously described (Buchanan et al., 2014; Buchanan and Richerson, 2010). Briefly, mice were implanted with stainless steel guide cannulae (PlasticsOne, Roanoke, VA) into striatum as before, and an EEG head mount (PlasticsOne, Roanoke, VA) was secured to the skull with 4 epidural screws and dental cement. EEG was recorded from epidural screw electrodes over right frontal and parietal cortex with the aid of custom software written in Matlab. Two leads for EMG recording were implanted into the neck muscles, contralateral to the injection cannula. One week after surgery the animals were connected to the pre-amplifier (PlasticsOne, Roanoke, VA) and placed into a Plexiglass recording chamber. Baseline EEG was recorded for 30–40min followed by injection of PTX. Videos were recorded with two web cameras (Logitech Orbit AF) from the opposite sides, and video recording was temporally synchronized with EEG and EMG.
Four mice were tested with 2 PTX injections, spaced 1–2 weeks apart: low (0.1–0.2ug) and high (0.3–0.6ug) doses of PTX to compare tic- and seizure-associated EEG. In each experiment 10-s intervals of digitized data recording, corresponding to 5, 10, 20, and 40 min after PTX, were extracted in MatLab (Mathworks, Natick, MA). EEG was analyzed in epochs corresponding to tic-like motor events, identified by the corresponding EMG bursts. These events were frequently associated, especially at higher PTX doses, with complex discharges consistent with electrographic spikes lasting up to 70 msec, characterized by a brief positive sharp transient followed by a negative EEG deflection with an amplitude at least twice that of the baseline activity. Using these electrographic parameters, the EEG was re-evaluated for the presence of similar complex discharges in the absence of tic-like motor events.
Analysis
Results are presented as mean ± S.E.M. or medians and 25 to 75 percentile range, as appropriate. Between-subject designs were analyzed by one or two-way ANOVA followed by Tukey’s posthoc test. Latency data were analyzed by Kruskal-Wallis ANOVA followed by Dunn’s posthoc test. Within-subject designs, where same animals received repeated treatments, were analyzed by repeated measures (RM) ANOVA with Geisser-Greenhouse’s correction or paired t-test where appropriate. The level of significance was p < 0.05 for all analyses.
Results
Phenomenology
Local infusion of the GABA-A antagonist bicuculine into the dorsal striatum has previously been shown to produce tic-like phenomenology in monkey and rat (Bronfeld et al., 2011; Bronfeld et al., 2013; McCairn et al., 2009; McCairn et al., 2013; Tarsy et al., 1978). We sought to replicate this effect in mice, using the more specific GABA-A antagonist picrotoxin (PTX).
In exploratory experiments, we infused 0.1 or 0.2 μg PTX into targets throughout the corticostriatal circuitry and monitored the behavioral results (see Figure 1A). Infusion into the central and dorsolateral striatum produced intermittent, non-rhythmic, tic-like movements consisting of rapid stereotyped lifting of the contralateral forepaw or hindpaw or jerks of the head (see Supplementary Videos 1, 2). These are reminiscent of the tic-like phenomenology previously reported in non-human primate and in rat (Bronfeld et al., 2011; Bronfeld et al., 2013; McCairn et al., 2009; McCairn et al., 2013; Tarsy et al., 1978).
Infusion into the overlying sensorimotor cortex produced similar tic-like movements, accompanied by general behavioral activation, consisting of exploration, sniffing in the cage, and occasional licking of the hindpaws. PTX in the dorsomedial striatum, in contrast, produced no manifest behavioral effect or, in sites adjacent to the lateral ventricle (from which PTX might have spread broadly), to a general behavioral suppression. PTX in the ventral striatum produced locomotor activation accompanied by stereotypical sniffing and licking at the walls, without accompanying tic-like movements. Infusion of higher doses of PTX into the cortex (0.3–0.6 μg) frequently produced behaviorally evident seizures; seizures were also occasionally seen after cortical infusions at the 0.2 μg dose, or at striatal sites after higher doses (see below).
As the aim of this study is to characterize tic-like movements, for all subsequent experiments we performed PTX infusions only into the dorsal striatum and the sensorimotor cortex, where such movements were observed (see Supplementary Videos). An image of a typical injection site in striatum, marked by infusion of toluidine blue just before euthanasia, is shown in Fig. 1B.
To further characterize tic-like movements generated by local disinhibition in the central striatum (CES) and dorsolateral striatum (DLS) and the overlying sensorimotor cortex, we injected two doses of PTX into each site, 0.1 and 0.2 μg (always in 0.2 μl saline). Time courses of tic-like behaviors are shown in Figure 1C, D. At the striatal sites, the higher dose significantly reduced the latency to the first tic. Fewer tics were observed at the lower dose at the CES sites, relative to DLS and Cx, consistent with our qualitative observations in pilot experiments; while this comparison did not reach statistically significance (1-way ANOVA of tic number at 0.1 μg: p = 0.07), the observation led us to focus on DLS infusions for subsequent experiments. At the higher dose the number of tics was increased at all sites. These data are summarized in Table 1. Behaviorally evident seizures were occasionally seen after cortical injection, especially after the 0.2 μg dose; animals that developed seizures were excluded from analysis.
Injection of muscimol, a GABA-A receptor agonist (Johnston, 2014), into the DLS prior to PTX injection at the same site abrogated tic generation, confirming the dependence of the tics on GABAergic mechanisms (paired t-test: p = 0.0014.; Figure 1E, F).
Electroencephalographic correlates of tics
We examined the EEG correlates of tic-like movements after increasing intrastriatal doses of picrotoxin over a range of doses (0.1–0.6 μg; Figure 2). In 60% of observed 10-s intervals, tic-like movements were associated with electrographic complexes, which consisted of a brief positive transient followed by a negative deflection with an amplitude at least twice the baseline variability of the EEG trace. At the low PTX doses used in behavioral experiments (0.1–0.2 μg), many tic-like movements were not preceded by detectable spikes, especially early after PTX administration (Fig. 2A, D). At higher doses, spikes were clearer and preceded almost all tic-like movements (Fig. 2B). Tonic seizures emerged intermittently after injection of high doses of PTX (0.3–0.6 μg) into the striatum (note that these higher doses were not used in behavioral experiments). Behaviorally evident seizures were accompanied by characteristic electrographic activity (Fig. 2C); such seizure activity was not seen during tic-like movements, and tic-like movements were not discernible during seizures.
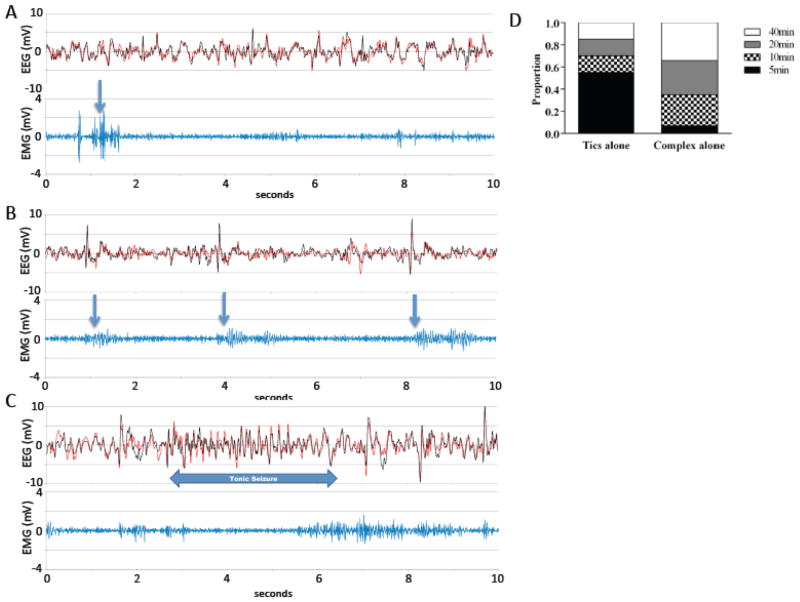
Figure 2.
EEG recordings from 2 electrodes (EEG1, 2), time-locked to EMG to identify tic-like movements (which were also identified on time-locked video), after intra-DLS PTX (n=4). A. Sample EEG and EMG 5 minutes after 0.2 ug of PTX; emission of tic-like movements, scored from EMG and video, is indicated by arrows. Tic-like movements frequent did not correlate with EEG complexes under these conditions (see 2D). B. Sample EEG from the same mouse 20 minutes after 0.4 ug PTX. Tics after this larger PTX dose were generally associated with pronounced electrographic complexes, as indicated. C. An episode of electrographic seizures 40 minutes after 0.4 ug intrastriatal PTX. D. Time distribution of tics not accompanied by EEG complexes and of the EEG complexes without tics (total number of 10s epochs = 48).
The relationship between phasic tic-like movements and electrographic complexes evolved during the hour after PTX injection. Tic-like movements not accompanied by detectable EEG complexes appeared mostly at 5 min after PTX, while the complexes without tic-like movements appeared later in the trial (Fig. 2D), especially after high doses of PTX.
Cortical contributions to tic-like movements after striatal PTX
The principal cells of the striatum, the medium spiny neurons, are GABAergic. If the production of tic-like movements after local PTX infused into the DLS results from the autonomous firing of these cells, it should be independent of afferent tone. On the other hand, if the tics result from a disinhibited response to afferent excitatory synaptic activity, they should be susceptible to local blockade of glutamate. To test this, we infused the NMDA receptor antagonist PMPA (0.2 μg in 0.2 μl) (Feng et al., 2005), or saline, into the DLS 10 minutes before PTX (0.2 μg in 0.2 μl). This dramatically attenuated tics (One-tailed paired t-test, t(6)=6.0, p < 0.0005; Figure 3A, B), confirming their dependence on afferent glutamatergic activity.
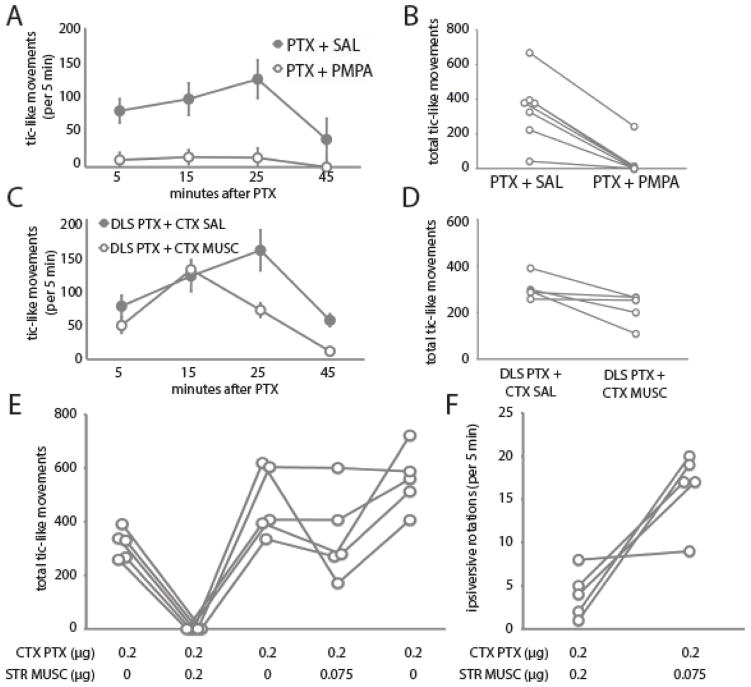
Figure 3.
Local pharmacological manipulation of PTX-induced tics. A, B. Saline or PMPA (0.2 μg in 0.2 μl) was injected 10 min before PTX into the same DLS site. All animals received both treatments, separated by several days; n = 7 animals. C, D. Antagonism by cortical MUSC of tics evoked by PTX from DLS. All animals received both treatments, separated by several days; n = 5 animals. E. Dose-dependent antagonism by striatal MUSC of tics evoked by PTX from Cx (n=5). Post-hoc comparisons by 1-tailed t-test (uncorrected): *** p ≤ 0.0001; ** p < 0.005; * p < 0.025; † p ≤ 0.1. F. Ipsiversive rotations were seen after DLS MUSC; they were increased in the fourth session, after the 0.075 μg dose. No ipsiversive rotations were seen in the other conditions.
We reasoned that a similar attenuation might be seen if cortical activity were pharmacologically inhibited. To test this, we infused the GABA-A agonist muscimol (MUSC; 0.2 μg in 0.2 μl) into the overlying sensorimotor cortex 10 minutes before infusing PTX (0.2 μg in 0.2 μl) into the DLS. As predicted, this attenuated the production of tic-like movements (One-tailed paired t-test, p < 0.03; Figure 3C, D). The suppression was less profound than after intrastriatal glutamate antagonism (compare to Figure 3A, B); this is most likely because not all sources of afferent excitatory tone to the PTX-infused zone of the striatum were inhibited by local cortical MUSC.
Striatal contributions to tic-like movements after cortical PTX
Cortical PTX produces tic-like movements, in addition to behavioral activation. To test whether these tic-like movements also depend on cortical projections to the striatum, we infused MUSC (or saline) into the DLS 10 minutes before infusing PTX into sensorimotor cortex (0.2 μg). The same cohort of 5 mice received PTX 5 times in this experiment, separated by 2–3 days, as shown in Figure 3E; PTX infusions #1, 3, and 5 were preceded by cortical saline, while #2 and #4 were preceeded by 0.2 μg (#2) or 0.075 μg (#4) of MUSC. Interestingly, the emission of tic-like movements increased across the three PTX-alone infusions, suggesting sensitization. 0.2 μg striatal MUSC completely abrogated tic-like movements (one-tailed paired t-test: p = 0.0001 vs. PTX session #1, p = 0.003 vs. PTX session #3). The effect of 0.075 μg MUSC was more subtle (p = 0.1 vs. PTX session #3, p = 0.024 vs. PTX session #5); there was a clear reduction in tic-like movements in three of the animals, but no alteration in the other two.
DLS MUSC, at both doses, produced ipsiversive rotations, as has been reported previously after striatal inactivation (Figure 3F). These were greater in session #4 (0.075 μg). Ipsiversive rotations were never seen with saline or PTX infusion into the DLS.
Discussion
Tics have been proposed to result from foci of pathological disinhibition within the striatum (Mink, 2001). This may relate to a deficit in local inhibitory tone, as suggested by a documented reduction in parvalbumin-expressing interneurons in post-mortem TS striatum (Kalanithi et al., 2005; Kataoka et al., 2010); these interneurons are a potent source of feed-forward inhibition in the striatum (Gittis et al., 2010). Additional support for this hypothesis derives from the recapitulation of tic-like phenomenology after local pharmacological disinhibition within the striatum (Bronfeld et al., 2013; Marsden et al., 1975; McCairn et al., 2009; McCairn et al., 2013; Tarsy et al., 1978; Worbe et al., 2013).
We have replicated this phenomenon in mouse, after microinfusion of low doses of picrotoxin into the dorsal striatum (Figure 1; Supplementary Video 1, 2). We observe intermittent rapid, stereotyped, repetitive, non-rhythmic movements of contralateral limbs or head that recapitulate key phenomenological characteristics of tics (Kurlan, 2010; Leckman, 2002). These movements seem to be always focal and not to interfere with ongoing motor behavior: mice could explore cage, eat pellets, or lie immobile while experiencing “tics”. Overall, this murine model presents good face validity and gives an advantage of easily quantifiable behavioral end-points that can be applied to studies with mutant or transgenic lines.
Tic-like movements were abolished by striatal infusion of the GABA-A agonist muscimol (Figure 1E, F), confirming the key role of striatal neuronal activity in the production of the phenomenon.
Similar tic-like movements can be elicited when PTX is infused into sensorimotor Cx overlying striatum (Figure 1). Deficient intracortical inhibition in Cx has been reported in TS patients (Ziemann et al., 1997), although the deficits in inhibitory tone are likely to be more subtle than those produced by PTX infusion. In contrast to DLS infusions, mice with Cx PTX infusions displayed behavioral activation, characterized by locomotion, licking hindpaws or food pellets, and sniffing, in addition to tics. This may reflect a spread of activity along the cortico-cortical connections into the prefrontal cortex, which projects to the ventral striatum and brainstem centers regulating locomotion and motivation (Swanson, 2000). Tic-like movements have been reported to be difficult to induce after cortical disinhibition in the rat (Tarsy et al., 1978) or to require larger doses of PTX than in striatum (Patel and Slater, 1987). However, we find Cx infusions in mice to produce tics similarly to DLS infusions (Figure 1C, D; Table 1). Unsurprisingly, cortical infusions were more likely to produce seizures, especially at higher doses of PTX.
Intra-striatal PTX infusion could also produce seizure at sufficiently high doses (see Figure 2C), possibly due to diffusion or reflux of infused PTX into the cortex. However, several observations suggest that the tic-like movements we observe at lower PTX doses are not due to cortical seizure activity. First, at the 0.1 and 0.2 μg doses, tic-like movements induced by intra-DLS PTX were often accompanied by no detectable EEG abnormalities (see Figure 2A). When an EEG correlate did emerge it consisted of a single spike, distinct from epileptiform discharges seen at higher doses of PTX (Figure 2B, C). These spikes dissociate from tic-like movements, especially during the first 5–10 min and at low PTX doses; this makes clear that they are not motion artifacts, and that they are not an essential correlate of tic production. Coordinated firing in the motor cortex has been observed after intra-striatal PTX in conjunction with tic-like movements in non-human primate (McCairn et al., 2009), albeit using a more sophisticated recording approach (cortical LFP using a high-impedance electrode). There are several reasons why we might see such discharges in this model (as in the non-human primate), whereas they have not been reported in TS patients. EEG recordings in the mouse are collected from electrodes affixed to the thin skull, in close proximity to the cortical surface, and therefore may be more sensitive to coherent cortical discharge than extracranial EEG recordings in patients. Coherent cortical discharge may also be greater in this model than in patients; PTX-induced disinhibition is likely to produce rather dramatic dysregulation within the basal ganglia (McCairn et al., 2009), whereas the lower-amplitude tics characteristic of most cases of TS may correspond to more focal effects that may be more difficult to detect in the EEG signal.
The convenience of the mouse model allowed us to test several hypotheses regarding the generation of these tic-like movements. First, we probed the interaction of cortical afferents with striatal activity in the generation of tics. The NMDA antagonist PMPA attenuated tics produced by striatal PTX, as did muscimol infusion into the overlying cortex (Figure 3A–D). These findings suggest that tic-like movements generated after striatal disinhibition do not result from spontaneous striatal activity but rather from enhanced responsivity to afferent glutamatergic synaptic input. These results are similar to those reported in a previous study, in which cooling or ablation of the cortex completely prevented striatal PTX-induced movements (Muramatsu et al., 1990), although the use of an anesthetized preparation in that study precludes direct comparison with our results. In aggregate, these results support a critical role for cortico-striatal interactions, as opposed to autonomous striatal activity, in the generation of tics in this model.
Any animal model of a human condition must contend with questions of face validity – whether the phenomena under investigation actually recapitulate the neurological symptoms they purport to (Macri et al., 2013). Previous studies using local injections of PTX or bicuculline have variably described consequent movements as dyskinesias (Muramatsu et al., 1990), myoclonus (Patel and Slater, 1987; Tarsy et al., 1978), or tics (Bronfeld et al., 2011; Bronfeld et al., 2013). Myoclonic jerks sometimes are difficult to differentiate from tics on clinical observation (Vercueil, 2006). Myoclonus is present in a variety of metabolic, toxic, and degenerative conditions and insults and can be associated with epilepsy (Borg, 2006). Certain features of the present model lead us to conclude that the tic-like movements we observe after striatal PTX recapitulate tics, rather than my myoclonus (see Supplementary Videos 1, 2). First, myoclonus has been associated with pathologies in non-striatal regions (Shibasaki and Hallett, 2005), while tics are more closely associated with pathologies of cortico-basal ganglia circuits (Albin and Mink, 2006; Leckman et al., 2010; Mink, 2001, 2003; Williams et al., 2013). We find that inhibition of striatum with muscimol can completely suppress tics evoked from either DLS or Cx (Figure 1E, F; Figure 3E), confirming that striatal activity is critical to the phenomenonology. Second, tics can be observed at rest, while myoclonus is more typically associated with movements or postural adjustments (unless it is related to epilepsy) or with sensory stimulation (Borg, 2006; Vercueil, 2006). We observe tic-like movements both at rest and during the execution of otherwise normal activities (see Supplementary Videos), providing further support for the interpretation that they recapitulate the mechanisms and phenomenology of tics.
Animal models are traditionally judged with respect to face validity (recapitulation of disease phenomenology), construct validity (recapitulation of putative causal mechanisms), and predictive validity (response to therapeutic interventions) (Pittenger, 2014; Willner, 1984). This PTX model satisfies the first two criteria: it produces phenomenology reminiscent of tics and recapitulates a hypothesized pathophysiological mechanism (Mink, 2001). Our mechanistic studies provide new evidence for the importance of gluatmatergic afferents in tic generation.
Supplementary Material
Highlights.
Disinhibition in the striatum produces contralateral tic-like movements in mice
Similar movements are produced by local disinhibition in sensorimotor cortex.
Cortico-striatal interactions are required for these tic-like movements
Acknowledgments
The authors gratefully acknowledge S. Wilber for assistance with mouse husbandry. These experiments were supported by the Alison Family Foundation (CP), R01MH091861 (CP), and the State of Connecticut through its support of the Ribicoff Research Facilities at the Connecticut Mental Health Center (CP). These experiments received no commercial support; the authors declare no commercial conflict of interest.
Footnotes
Author contributions
VP conducted all experiments, analyzed data, and wrote the manuscript. MX assisted with histological characterization of cannula placement and with data analysis. HRS assisted with EEG recordings. GFB supervised EEG recordings, analyzed data, and edited the manuscript. CP supervised all experiments and data analysis, analyzed and interpreted data, and wrote and edited the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albin RL, Mink JW. Recent advances in Tourette syndrome research. Trends in neurosciences. 2006;29:175–182. doi: 10.1016/j.tins.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Bloch MH. Emerging treatments for Tourette’s disorder. Curr Psychiatry Rep. 2008;10:323–330. doi: 10.1007/s11920-008-0052-z. [DOI] [PubMed] [Google Scholar]
- Bloch MH, Leckman JF, Zhu H, Peterson BS. Caudate volumes in childhood predict symptom severity in adults with Tourette syndrome. Neurology. 2005;65:1253–1258. doi: 10.1212/01.wnl.0000180957.98702.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg M. Symptomatic myoclonus. Neurophysiologie clinique = Clinical neurophysiology. 2006;36:309–318. doi: 10.1016/j.neucli.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Bronfeld M, Belelovsky K, Bar-Gad I. Spatial and temporal properties of tic-related neuronal activity in the cortico-basal ganglia loop. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:8713–8721. doi: 10.1523/JNEUROSCI.0195-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfeld M, Yael D, Belelovsky K, Bar-Gad I. Motor tics evoked by striatal disinhibition in the rat. Frontiers in systems neuroscience. 2013;7:50. doi: 10.3389/fnsys.2013.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan GF, Murray NM, Hajek MA, Richerson GB. Serotonin neurones have anticonvulsant effects and reduce seizure-induced mortality. The Journal of physiology. 2014;592:4395–4410. doi: 10.1113/jphysiol.2014.277574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan GF, Richerson GB. Central serotonin neurons are required for arousal to CO2. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16354–16359. doi: 10.1073/pnas.1004587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, Morley RM, Jane DE, Monaghan DT. The effect of competitive antagonist chain length on NMDA receptor subunit selectivity. Neuropharmacology. 2005;48:354–359. doi: 10.1016/j.neuropharm.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Gittis AH, Nelson AB, Thwin MT, Palop JJ, Kreitzer AC. Distinct roles of GABAergic interneurons in the regulation of striatal output pathways. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:2223–2234. doi: 10.1523/JNEUROSCI.4870-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston GA. Muscimol as an Ionotropic GABA Receptor Agonist. Neurochemical research. 2014 doi: 10.1007/s11064-014-1245-y. [DOI] [PubMed] [Google Scholar]
- Kalanithi PS, Zheng W, Kataoka Y, DiFiglia M, Grantz H, Saper CB, Schwartz ML, Leckman JF, Vaccarino FM. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:13307–13312. doi: 10.1073/pnas.0502624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka Y, Kalanithi PS, Grantz H, Schwartz ML, Saper C, Leckman JF, Vaccarino FM. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. The Journal of comparative neurology. 2010;518:277–291. doi: 10.1002/cne.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiman-Weiner M, Beenhakker MP, Segal WA, Huguenard JR. Synergistic roles of GABAA receptors and SK channels in regulating thalamocortical oscillations. Journal of neurophysiology. 2009;102:203–213. doi: 10.1152/jn.91158.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight T, Steeves T, Day L, Lowerison M, Jette N, Pringsheim T. Prevalence of tic disorders: a systematic review and meta-analysis. Pediatric neurology. 2012;47:77–90. doi: 10.1016/j.pediatrneurol.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Kurlan R. Clinical practice. Tourette’s Syndrome. N Engl J Med. 2010;363:2332–2338. doi: 10.1056/NEJMcp1007805. [DOI] [PubMed] [Google Scholar]
- Kwak CH, Jankovic J. Tourettism and dystonia after subcortical stroke. Movement disorders: official journal of the Movement Disorder Society. 2002;17:821–825. doi: 10.1002/mds.10207. [DOI] [PubMed] [Google Scholar]
- Leckman JF. Tourette’s syndrome. Lancet. 2002;360:1577–1586. doi: 10.1016/S0140-6736(02)11526-1. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Bloch MH, Smith ME, Larabi D, Hampson M. Neurobiological substrates of Tourette’s disorder. J Child Adolesc Psychopharmacol. 2010;20:237–247. doi: 10.1089/cap.2009.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennington JB, Coppola G, Kataoka-Sasaki Y, Fernandez TV, Palejev D, Li Y, Huttner A, Pletikos M, Sestan N, Leckman JF, et al. Transcriptome Analysis of the Human Striatum in Tourette Syndrome. Biological psychiatry. 2014 doi: 10.1016/j.biopsych.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macri S, Proietti Onori M, Laviola G. Theoretical and practical considerations behind the use of laboratory animals for the study of Tourette syndrome. Neuroscience and biobehavioral reviews. 2013;37:1085–1100. doi: 10.1016/j.neubiorev.2013.03.014. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Meldrum BS, Pycock C, Tarsy D. Focal myoclonus produced by injection of picrotoxin into the caudate nucleus of the rat. The Journal of physiology. 1975;246:96P. [PubMed] [Google Scholar]
- McCairn KW, Bronfeld M, Belelovsky K, Bar-Gad I. The neurophysiological correlates of motor tics following focal striatal disinhibition. Brain: a journal of neurology. 2009;132:2125–2138. doi: 10.1093/brain/awp142. [DOI] [PubMed] [Google Scholar]
- McCairn KW, Iriki A, Isoda M. Global dysrhythmia of cerebro-basal ganglia-cerebellar networks underlies motor tics following striatal disinhibition. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:697–708. doi: 10.1523/JNEUROSCI.4018-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AM, Bansal R, Hao X, Sanchez-Pena JP, Sobel LJ, Liu J, Xu D, Zhu H, Chakravarty MM, Durkin K, et al. Enlargement of thalamic nuclei in Tourette syndrome. Archives of general psychiatry. 2010;67:955–964. doi: 10.1001/archgenpsychiatry.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink JW. Basal ganglia dysfunction in Tourette’s syndrome: a new hypothesis. Pediatric neurology. 2001;25:190–198. doi: 10.1016/s0887-8994(01)00262-4. [DOI] [PubMed] [Google Scholar]
- Mink JW. The Basal Ganglia and involuntary movements: impaired inhibition of competing motor patterns. Archives of neurology. 2003;60:1365–1368. doi: 10.1001/archneur.60.10.1365. [DOI] [PubMed] [Google Scholar]
- Muramatsu S, Yoshida M, Nakamura S. Electrophysiological study of dyskinesia produced by microinjection of picrotoxin into the striatum of the rat. Neuroscience research. 1990;7:369–380. doi: 10.1016/0168-0102(90)90011-3. [DOI] [PubMed] [Google Scholar]
- Patel S, Slater P. Analysis of the brain regions involved in myoclonus produced by intracerebral picrotoxin. Neuroscience. 1987;20:687–693. doi: 10.1016/0306-4522(87)90119-9. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Anderson AW, Zhang H, Gatenby JC, Lacadie CM, Leckman JF, Gore JC. A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Archives of general psychiatry. 1998;55:326–333. doi: 10.1001/archpsyc.55.4.326. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Staib L, Scahill L, Zhang H, Anderson C, Leckman JF, Cohen DJ, Gore JC, Albert J, Webster R. Regional brain and ventricular volumes in Tourette syndrome. Archives of general psychiatry. 2001;58:427–440. doi: 10.1001/archpsyc.58.5.427. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Thomas P, Kane MJ, Scahill L, Zhang H, Bronen R, King RA, Leckman JF, Staib L. Basal Ganglia volumes in patients with Gilles de la Tourette syndrome. Arch Gen Psychiatry. 2003;60:415–424. doi: 10.1001/archpsyc.60.4.415. [DOI] [PubMed] [Google Scholar]
- Pittenger C. Animal models of Tourette syndrome and obsessive-compulsive disorder. In: LeDoux ME, editor. Animal Models of Movement Disorders. New York: Elsevier; 2014. [Google Scholar]
- Rickards H. Functional neuroimaging in Tourette syndrome. Journal of psychosomatic research. 2009;67:575–584. doi: 10.1016/j.jpsychores.2009.07.024. [DOI] [PubMed] [Google Scholar]
- Robertson MM, Stern JS. Gilles de la Tourette syndrome. British journal of hospital medicine. 1997;58:253–256. [PubMed] [Google Scholar]
- Scharf JM, Miller LL, Mathews CA, Ben-Shlomo Y. Prevalence of Tourette syndrome and chronic tics in the population-based Avon longitudinal study of parents and children cohort. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51:192–201. e195. doi: 10.1016/j.jaac.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki H, Hallett M. Electrophysiological studies of myoclonus. Muscle & nerve. 2005;31:157–174. doi: 10.1002/mus.20234. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Kan E, Yoshii J, Thompson PM, Bansal R, Xu D, Toga AW, Peterson BS. Thinning of sensorimotor cortices in children with Tourette syndrome. Nature neuroscience. 2008;11:637–639. doi: 10.1038/nn.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- State MW. The genetics of Tourette disorder. Current opinion in genetics & development. 2011;21:302–309. doi: 10.1016/j.gde.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain research. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- Tarsy D, Pycock CJ, Meldrum BS, Marsden CD. Focal contralateral myoclonus produced by inhibition of GABA action in the caudate nucleus of rats. Brain: a journal of neurology. 1978;101:143–162. doi: 10.1093/brain/101.1.143. [DOI] [PubMed] [Google Scholar]
- Tobe RH, Bansal R, Xu D, Hao X, Liu J, Sanchez J, Peterson BS. Cerebellar morphology in Tourette syndrome and obsessive-compulsive disorder. Annals of neurology. 2010;67:479–487. doi: 10.1002/ana.21918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercueil L. Myoclonus and movement disorders. Neurophysiologie clinique = Clinical neurophysiology. 2006;36:327–331. doi: 10.1016/j.neucli.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Williams K, Bloch MH, State MW, Pittenger C. Tourette syndrome and tic disorders. In: Charney DS, Buxbaum JD, Sklar P, Nestler EJ, editors. Neurobiology of Mental Illness. 4. New York: Oxford; 2013. [Google Scholar]
- Willner P. The validity of animal models of depression. Psychopharmacology. 1984;83:1–16. doi: 10.1007/BF00427414. [DOI] [PubMed] [Google Scholar]
- Worbe Y, Sgambato-Faure V, Epinat J, Chaigneau M, Tande D, Francois C, Feger J, Tremblay L. Towards a primate model of Gilles de la Tourette syndrome: anatomo-behavioural correlation of disorders induced by striatal dysfunction. Cortex; a journal devoted to the study of the nervous system and behavior. 2013;49:1126–1140. doi: 10.1016/j.cortex.2012.08.020. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Paulus W, Rothenberger A. Decreased motor inhibition in Tourette’s disorder: evidence from transcranial magnetic stimulation. The American journal of psychiatry. 1997;154:1277–1284. doi: 10.1176/ajp.154.9.1277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.