Abstract
Body composition refers to the amount of fat and lean tissues in our body; it is a science that looks beyond a unit of body weight, accounting for the proportion of different tissues and its relationship to health. Although body weight and body mass index are well-known indexes of health status, most researchers agree that they are rather inaccurate measures, especially for elderly individuals and those patients with specific clinical conditions. The emerging use of imaging techniques such as dual energy x-ray absorptiometry, computerized tomography, magnetic resonance imaging, and ultrasound imaging in the clinical setting have highlighted the importance of lean soft tissue (LST) as an independent predictor of morbidity and mortality. It is clear from emerging studies that body composition health will be vital in treatment decisions, prognostic outcomes, and quality of life in several nonclinical and clinical states. This review explores the methodologies and the emerging value of imaging techniques in the assessment of body composition, focusing on the value of LST to predict nutrition status.
Keywords: body composition, lean body mass, lean soft tissue, imaging, nutrition status, DXA, CT, MRI, ultrasound, sarcopenia, sarcopenic obesity, osteosarcopenic obesity
Measurements of body composition are fundamental for an in-depth evaluation of nutrition status. Evaluating body weight or weight change (primarily weight loss) throughout the course of a clinical condition has been a paramount end point for the assessment of nutrition status and is, in fact, useful when drastic changes are observed. Nonetheless, in certain circumstances such as with aging or in certain patient cohorts, body weight (and hence body mass index [BMI]) may not accurately depict specific shifts between lean and adipose tissue compartments, and individuals may therefore present with weight stability while gaining fat and losing lean mass.1
This review explores the methodologies and the emerging value of imaging techniques used to assess body composition in research and clinical settings, focusing on the value of lean soft tissue (LST) to predict nutrition status and risk in clinical situations. This report also provides background information for clinical researchers, clinicians, and other healthcare professionals who are interested in exploring and using these imaging techniques in their practices to assess body composition.
Body Composition Terminology
There is often confusion in the literature regarding body composition terminology that can hinder our understanding and our ability to effectively compare results from individual studies without a careful evaluation of the methodological approach used. For instance, the terms lean body mass (LBM) and fat-free mass (FFM) are often used interchangeably, although they depict different body composition compartments (Figure 1).
Figure 1.
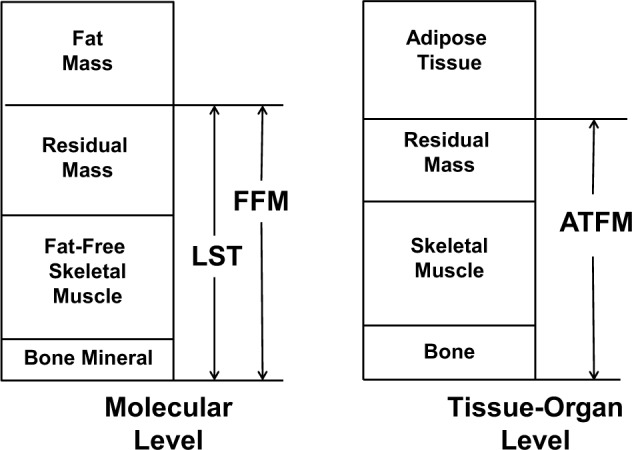
Selected molecular and tissue-organ body composition level components. ATFM, adipose-tissue free mass; FFM, fat-free mass; LST, lean soft tissue.
As listed in Figure 1, LBM, more correctly termed LST,2 is the sum of body water, total body protein, carbohydrates, nonfat lipids, and soft tissue mineral. Hence, the fat and bone mineral compartments are excluded. The sum of LST and the bone mineral compartments yields FFM, which is formed by skeletal and nonskeletal muscle, organs, connective tissue, and bone. On the tissue-level organizational system,2 skeletal muscle mass is a critical component of the LST and hence FFM compartments. While it is important for the reader to understand exactly which compartment (ie, muscle, LST, or FFM) is being measured in a specific study, losses or gains of these compartments occur in parallel of each.
Less confusion is observed among the definitions of fat mass and adipose tissue, which are respectively at the molecular and tissue-organ levels of body composition. Adipose tissue is a connective tissue formed by adipocytes, collagenous and elastic fibers, fibroblasts, and capillaries.3 Approximately 80% of adipose tissue is fat mass, the specific family of lipids consisting of triglycerides.2 The discussion of different body composition terminology is especially relevant for the understanding of each available imaging technique, as different methods assess different compartments of the human body. Advantages of assessing adipose tissue, for example, include the differentiation between subcutaneous, visceral, and intramuscular compartments.
Body Composition Measurement Methods
Body composition can be estimated using different modalities:
Anthropometry: lengths and breadths, circumferences, and skinfold thicknesses
Body volume/density: hydrodensitometry/underwater weighing and air displacement plethysmography, as well as 3-dimensional (3-D) body surface imaging
Total body water or hydrometry: tracer techniques using principles of dilution
Major body elements: whole-body counting and neutron activation analysis
Impedance: bioimpedance analysis (BIA)
Imaging/x-ray attenuation: dual-energy x-ray absorptiometry (DXA), computerized tomography (CT), magnetic resonance imaging (MRI), quantitative magnetic resonance (QMR), quantitative computed tomography (QCT) imaging, and ultrasound
Multicompartment models: combination of methods that can include total body water, body volume, and bone mineral content, for example
Each technique has its advantages and disadvantages, which have been summarized in Table 1. However, the focus of this article is the emerging use of imaging techniques (DXA, CT, MRI, and ultrasound) for the assessment of LST in the clinical setting.
Table 1.
Summary of Commonly Used Body Composition Techniques.
| Technique | Principle and Compartment Being Measured | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Anthropometry | ||||
| Lengths and breadths/circumferences /skinfold thickness | Body mass and geometry using scales, stadiometers, tape, skinfold calipers. Surrogate measures of relative fatness. Skinfold thickness may also be used to estimate FFM and muscle size. |
Easily obtained, inexpensive, noninvasive, and portable. | Methods are relatively insensitive. Body composition is not measured (lengths and breadths) or estimated (circumferences, skinfold thickness). Assumes a constant proportion of SAT thickness and that SAT will have a constant compressibility. |
90, 91 |
| Body volume/density | ||||
| Underwater weighing or hydrodensitometry | Body density is calculated by assessment of body volume measured by underwater weighing. Estimates FM and FFM (2-compartment model). |
Reliable and valid for measuring body density and percent body fat. | High cost and high patient burden. Limited application to highly specialized laboratories and/or research settings. Relies on assumed densities of FFM and FM compartments. |
92 |
| Air displacement plethysmography | Replacement for hydrodensitometry; used for the estimation of FM and FFM (2-compartment model). Relationship between pressure and volume is used to determine body’s volume, and the density is then calculated. |
Excellent precision and accuracy for measurement of volume. Can be safely used in all age groups (infants to elderly). Accommodates obese and very tall individuals. Body density can be used in multicompartment model calculations. |
Relies on assumed densities of FFM and FM compartments. Some studies reported poor validity compared with hydrodensitometry. Limited application to highly specialized laboratories and/or research settings. Relies on assumed densities of FFM and FM compartments. |
90 |
| 3-D body surface imaging | Digitalized optical method (densitometry) that measures body shape and dimensions generating a 3-D photonic image. Total and regional body volumes and dimensions are reported (circumferences, lengths, and percentage of body fat). |
Easy to use, low cost, safe, and provides fast and accurate measurement of the body. Additional anthropometric parameters can be calculated: waist and hip circumferences, sagittal abdominal diameter, segmental volumes, and body weight. Accommodates severely obese individuals Proposed as a novel approach for epidemiologic research. |
Limited availability of the technique. For accurate percentage body fat estimation, subjects must wear close-fitting minimal clothing and be able to stand motionless while holding their breath for 10 seconds, which may not be feasible in patients with certain clinical conditions. |
93–95 |
| Total body water or hydrometry | ||||
| Labeled water–isotope dilution techniques | Volume of the compartment is determined as administered tracer/tracer concentration. Used for the assessment of TBW with deuterated (2H), tritated (3H), or oxygen-labeled (18O) water. Estimates FM and FFM (2-compartment model). |
Safe and practical for field research. Easy to administer. Important for multicompartment model calculations. |
Several assumptions are made with regard to tracer distribution and effect in the body. Relies on assumed hydration of the FFM compartment (73.2%). Expensive, requires highly specialized equipment. |
90, 93 |
| Major body elements | ||||
| Whole-body counting (potassium) | Used to assess BCM and FFM. K is distributed mainly into the intracellular compartment with a relatively constant concentration within the BCM and FFM compartments. 40K is a naturally radioactive form of K, which can be quantified. |
Reference method for the assessment of BCM (high precision and accuracy). Safe and applicable across age groups. |
Limited to specialized research settings and requires advanced technical skills to operate. Expensive, requires highly specialized equipment and personnel. |
4 |
| Neutron activation analysis | Fast neutron source from a low radioactive field produces isotopic atoms that have unique emissions and decay paths and can, therefore, be measured. These atomic elements are components of specific compartments (protein, FM, TBW, and BMM). |
High precision and accuracy. Capable of quantifying all the main atomic elements found in vivo. |
High cost, including setup and maintenance; limited availability; and high technical skills are required to operate. Involves neutron radiation exposure, which poses potential danger during childhood and pregnancy. |
4, 90 |
| Impedance | ||||
| BIA | Measures resistance and reactance from an electrical signal sent to the body based on the fact that FFM has a higher water and electrolyte content; therefore, it is a good conductor. Measures TBW, which is then used to estimate FFM and FM. |
Portable, safe, reproducible, and low cost. | Relies on population-specific regression equations; some of these equations are not released by the manufacturer. Limited applicability in those with BMI >34 kg/m2 where BIA may overestimate FFM and underestimate FM. Some regression equations are not released by the manufacturer. |
89, 96, 97 |
| Imaging/x-ray attenuation | ||||
| DXA | Uses very low-radiation x-rays of 2 beams of energy. The generation of a high- and low-energy emission by an x-ray source is used to differentiate between soft tissue and bone. FM is then estimated from specific attenuation characteristics of soft tissues. | Differentiates fat, lean, and bone tissue. Regional measures of body composition can be obtained. Safe for repeated measures; fast and noninvasive. High precision and accuracy. |
There are differences within and between manufacturers and software versions. Inability to differentiate compartments within fat and lean tissues. Measurements are influenced by thickness of tissue and lean tissue hydration. Low radiation exposure but is not safe for pregnant women. |
4, 90 |
| CT scans | The x-ray attenuation through tissues is detected and an image is reconstructed. AT skeletal muscle, bone, visceral organs, and brain tissue can be identified by the different x-ray attenuation. |
Highly accurate quantitative and qualitative measure of body composition at the tissue-organ level, particularly total and regional AT and skeletal muscle tissue. High image resolution. Consistent image attenuation value within and between scans. Useful in clinical settings where these images are acquired for medical diagnosis/follow-up purposes. |
Limited to highly specialized settings, costly, and requires specialized skills to operate. Large radiation exposure. Cannot accommodate very large subjects (cannot fit in the scanner). |
4, 98 |
| MRI | When a strong magnetic field is generated, atomic protons become aligned in the magnetic field. These protons are then activated by a radio frequency wave, absorbing energy. The signal generated is used to develop regional and whole-body cross-sectional images. Quantifies adipose tissue, skeletal muscle, edema, and visceral organs. |
Excellent image resolutions. Most accurate method to determine body composition at the tissue-organ level, specifically whole-body and regional adipose tissue and skeletal muscle tissue (quantity and quality). Safe across age range and groups. |
Limited to highly specialized settings, costly, and requires specialized skills. Procedure requires individuals to hold their breath. Cannot accommodate very large subjects (cannot fit in the scanner). |
4, 92 |
| QMR | Detects different spin relaxation rates in different tissues through nuclear magnetic resonance relaxometry. Lean, fat, and free water tissues have different rates of relaxation rates that allow for differentiation. QMR differs from MRI in that the processed signal is obtained from the whole body at once (without spatial encoding). |
High measurement precision. Rapid, noninvasive. |
Relatively new technique, limited to highly specialized settings; costly and requires specialized skills to operate. Tendency to overestimate FM compared with a 4-compartment model. Not widely used/limited evidence. |
90, 93, 99, 100 |
| QCT | Measure volumetric BMD at the spine, hip, forearm, and tibia using a CT scanner. 3-D nonprojectional technique. |
High accuracy and reproducibility. Distinguish between trabecular and cortical bone components. Allows the measurement of density and 3-D geometric parameters of cortical bone. Trabecular volumes are largely independent of degenerative changes in the spine. |
High radiation exposure, high cost, and limited to highly specialized settings; costly and requires specialized skills to operate. Not widely used/limited evidence. |
93, 101 |
| Ultrasound | Acoustic waves (ultrasound) are reflected from tissue in the path of the ultrasound beam transmitted through the skin, which is partially reflected back to the transducer when in contact with a tissue. The amount of “sound” reflected is dependent on the changes in acoustic impedance, which is different among air, fat, muscle, and bone. Has mostly been used to measure SAT and VAT. Measurement of muscle and bone thickness is being studied. |
Safe, portable, fast, lower cost. Reliable, reproducible, and accurate measurement of AT. |
Lack of standardized measurement techniques; results affected by technical errors, protocol standardization, and anatomical irregularities. Provides more qualitative than quantitative results. Results are highly dependent on the skills of trained operators. |
37 |
| Multicompartment models | Combination of measurement techniques to assess whole-body composition. More commonly used in the 4-compartment models, including measurements of body volume (hydrodensitometry or air displacement plethysmography), TBW (dilution), and bone mass (DXA). |
Gold standard and criterion method (comprehensive analysis with minimal assumptions). | Limited to highly specialized settings; costly and requires specialized skills to operate. | 90, 93 |
AT, adipose tissue; BCM, body cell mass; BIA, bioimpedance analysis; BMD, bone mineral density; BMI, body mass index; BMM, bone mineral mass; CT, computerized tomography; DXA, dual-energy x-ray absorptiometry; FFM, fat-free mass; FM, fat mass; K, potassium; MRI, magnetic resonance imaging; QMR, quantitative magnetic resonance; QTC, quantitative computed tomography; SAT, subcutaneous adipose tissue; TBW, total body water; 3-D, 3-dimensional; VAT, visceral adipose tissue.
LST Assessment by Most Commonly Used Imaging Techniques
The most commonly used techniques to measure LST (or its related components) are DXA, CT, MRI, and ultrasound imaging.
Dual-Energy X-ray Absorptiometry
DXA is a widely used method that assesses body composition at the molecular level. Bone mineral mass and density, as well as fat and fat-free soft tissues at the whole-body and regional levels, can be assessed.4-6 One important advantage of using DXA is its ability to estimate appendicular skeletal muscle (ASM) mass by measuring the amount of LST in the arms and legs, which is mainly muscle (except for a small amount of connective tissue and skin).6,7 ASM has been widely used in the study of sarcopenia and the establishment of cut points for the definition of this syndrome.1,6,8,9
During a DXA scan, low-radiation x-rays of 2 different photon energy levels pass through the body and are identified by a photon detector that measures the amount of energy absorbed (attenuation) by soft tissue and bone at each pixel.5 Soft tissue is further subdivided into fat and LST based on the empiric attenuation of both pure fat and bone-free soft tissue.10
DXA is a quick, noninvasive, and safe method for body composition assessment, and the radiation exposure is considered small and safe for repeated measures. Moreover, DXA measures 3 body composition compartments and can provide regional estimates of these compartments.
DXA is also a very precise method for quantifying body composition; its overall precision exceeds that of any other body composition methodology.11 Although results of DXA scans, particularly fat and bone estimates, are highly correlated with cadaver analysis and in vivo neutron activation analysis,4 its accuracy depends on several factors such as patient’s body thickness and size, machine calibration procedures, software version used, and the definition of regions of interest, which is operator dependent.
The thickness of the body can affect DXA results.5 Increased tissue thickness (>25 cm) causes an increased attenuation of low-energy photons, causing a disproportional shift to the high-energy photons, which may lead to an underestimation of fat mass in obese patients.12 Moreover, the actual size of an individual presents a limitation for some DXA scans, as large persons are not able to easily fit inside the scan area, which is usually 60 × 197.5 cm. Nonetheless, recently developed DXA systems have expanded the limits of the DXA table so that now a patient who is morbidly obese can be accommodated.
Hydration status may affect DXA accuracy because of the programmed assumption of a constant and uniform FFM hydration. Large changes in hydration (higher than 5%) can change the attenuation of fat-free soft tissue,10 causing an overestimation of the LST compartment. Nevertheless, small changes in hydration levels do not greatly alter DXA estimates.5
Finally, the inability of DXA to decipher the different types of fat (visceral, subcutaneous, and intramuscular) and LST (muscle, organs) may also represent a practical limitation of this modality in clinical settings. In addition, orthopedic implants can create artifacts that affect DXA measurements, resulting in inaccurate identification of soft-tissue parameters,13 although little is known about the overall effect of such artifacts on whole-body composition measurements.14
A major limitation of DXA is the variability of the hardware and software packages between manufacturers. This inconstancy in technology limits the ability of healthcare professionals to compare measurements between different machines and to minimize residual calibration errors.1,14 A universal calibration of DXA machines is greatly needed to advance research in the field.
Despite these limitations, DXA scans have assumed an increasingly important role in clinical settings because of its decreasing cost and its availability for measuring bone density. As DXA machines are used worldwide in the assessment of osteoporosis, healthcare professional may seek its “secondary” use (ie, perform body composition assessments), provided that software and appropriate phantom calibration is available.14
Computerized Tomography Imaging
The use of CT imaging in the clinical setting has escalated in the past few years due to the accuracy, reliability, and availability of these images in certain clinical scenarios. As outlined in Table 1, CT is a gold-standard4 imaging method for body composition analysis at the tissue-organ level.15 For this method, x-ray attenuation is measured by a computer program that reconstructs cross-sectional images represented by a 2-D map of pixels.16 Pixels are then given a numerical value (Hounsfield unit), based on tissue attenuation (related to electron density) that are colored white (most dense [ie, water]) and black (least dense [ie, air]).15 Bone, skeletal muscle, and adipose tissue, as well as visceral organs, have specific Hounsfield unit ranges, allowing for their identification in the cross-sectional images.15,17 The tissue area (cm2) of the cross-sectional image is subsequently calculated by multiplying the number of pixels for a given tissue by the surface area of the individual tissues. In body composition research, CT imaging is most commonly used to assess the amount of adipose and skeletal muscle tissue and to determine the integrity of the latter. Adipose tissue found within skeletal muscle (intramuscular adipose tissue) causes a decrease in the pixels depicting skeletal muscle, affecting the quality of the assessment of this tissue; thus, the lower the mean Hounsfield units, the lower the density and, therefore, the greater the fat content.15
As reviewed previously, several studies have supported CT image analysis as a valid, accurate, and precise method to assess body composition.4,15 Moreover, single abdominal cross-sectional images can be used to estimate whole-body composition.18,19
The radiation dose generated by CT is high; therefore, it is not considered safe for repeated measurements. Exposing healthy individuals to this high radiation dose solely for the purpose of conducting body composition research may be considered unethical.15 In addition, the size of the patient may represent a limitation for CT imaging. Patients may not fit in the CT scan field of view, and tissues such as subcutaneous adipose tissue and even muscle may not appear in the cross-sectional image. Regardless of these limitations, CT images can be used opportunistically when they are obtained as part of the medical diagnosis and have been digitally stored in the patient’s medical record.
The third lumbar (L3) vertebra has been the landmark of interest for the study of body composition using CT scans. Shen and colleagues19 performed a study to determine the region at which tissue cross-sectional area in a single image was the best correlate of whole-body muscle volume. They started at L4/L5 and analyzed images at that point as well as +5 and +10 cm higher and −5 and −10 cm lower. The best correlation they found for skeletal muscle was 5 cm above L4/L5, which is around the L3 region. Once the L3 region is identified, analysis software (eg, SliceOmatic; TomoVision, Montreal, Quebec, Canada) is then used to identify specific tissue demarcation using Hounsfield unit thresholds established for skeletal muscle, visceral adipose tissue, subcutaneous adipose tissue, and intramuscular adipose tissue. Cross-sectional areas (cm2) are then computed for each tissue by summing tissue pixels and multiplying by the pixel surface area. Contrast-enhanced or unenhanced images can be used. It is important to understand that this technique is used to measure skeletal muscle cross-sectional area (cm2), which in turn can also be used to estimate whole-body skeletal muscle mass using a prediction equation developed by Shen et al.19 In addition, prediction equations have also been developed to estimate LST and FFM from CT-assessed muscle cross-sectional area in patients with cancer.20
Clinical conditions where CT images are available from the medical records of patient participants include but are not limited to cancer,21-24 respiratory failure,25 aortic stenosis (yet to be explored), and patients hospitalized in the intensive care unit (ICU) following trauma.26 The landmark of interest (ie, L3) may not always be available in the medical records of these patients. To our knowledge, the evidence of using other muscularity assessment techniques such as thoracic or psoas muscle cross-sectional area is limited. Likewise is the relationship of these surrogate measurements with total muscle cross-sectional area or whole-body muscle mass estimates.21
The establishment of a clinical or research program using CT scans to analyze body composition requires appropriate software, personnel trained to use the software with knowledge of anatomy (a radiologist is not essential), and image availability. In longitudinal studies, it is important that images of similar patients are analyzed by the same technician.
Although CT scans are typically used retrospectively, prospective studies can be strategically planned to assess body composition prospectively, according to expected clinical use. The MENAC study (Multimodal Exercise/Nutritional/Anti-inflammatory Treatment for Cachexia trial), a large, randomized phase 3 study comparing multimodal intervention vs standard of care for patients with cancer cachexia, has as its target outcome change in CT-assessed skeletal muscle mass from baseline to week 6—timelines where patients would normally have a CT scan for diagnostic/treatment follow-up purposes.27
Magnetic Resonance Imaging
An advantage of MRI over CT is that the images for MRI are not acquired using ionizing radiation. The data acquisition is based on a generated magnetic field that directs the alignment of hydrogen nuclei. Next, a radiofrequency pulse is applied, leading to energy absorption by the hydrogen protons, which release energy as the pulse is turned off and the protons are returned to their original position. The energy released is then detected by a receptor in the form of a radiofrequency signal used to create the whole-body or regional images.15 The difference between tissues and organs is related to the tissue-specific magnetic resonance properties, such as the density of hydrogen atoms and relaxation time.28
Using imaging analysis software, the generated gray-scale images can be determined based on the voxel (volume and pixel) information and the area (calculated based on cm2). Using anatomical knowledge and predetermined color codes for specific identified regions of interest, whole-body and/or regional volumes can be calculated based on a 3-D formula accounting for tissue area, slice thickness, distance between consecutive images, and number of images.15 Tissue mass (kg) can be calculated based on the assumed constant density values for skeletal muscle (1.04 g/cm3) and adipose tissue (0.92 g/cm3).
Because the MRI technique does not rely on ionizing radiation, it is safe across age range and groups and allows for serial assessments (longitudinal studies). Its excellent image resolution also contributes to the use of this technique as the most accurate method to determine body composition at the tissue-organ level at whole-body or regional levels. In addition, the lipid content within skeletal muscle may be determined using “chemical shift” imaging techniques,15 which are of emerging importance in the field of body composition prognostication in health and disease. With the advancement of the MRI technique, the time for reliable image acquisition has decreased significantly, reducing patient burden and making MRI a more useful technique in body composition research.15
The MRI technique has been used primarily to evaluate adipose tissue (quantity and distribution), followed by skeletal muscle mass. Although whole-body imaging provides state-of-the-art information,19 most of the studies to date have included single MRI technique protocols, especially at the mid-thigh level.
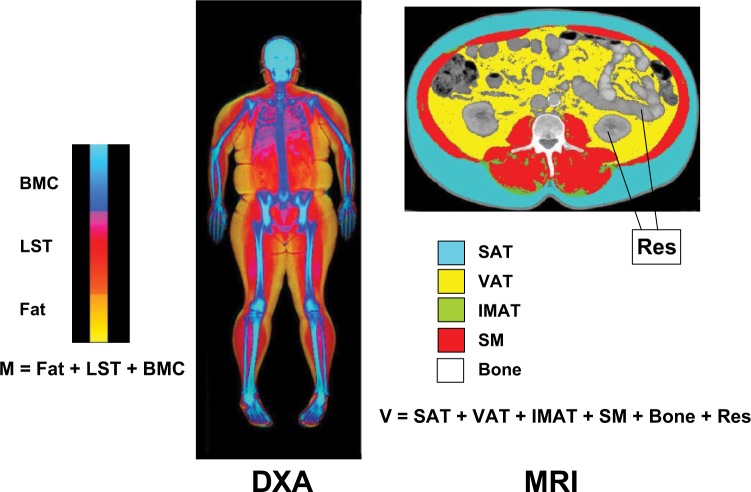
This technique has been useful in the evaluation of body composition changes in cross-sectional or interventional studies in obesity,29,30 sarcopenia,31 space travel,32 childhood obesity,33 hemodialysis,34 and human immunodeficiency virus (HIV).35 Limitations in the use of MRI in clinical and research settings are largely related to the high cost and technical expertise required for the analysis and the effect of respiratory motion on image quality for whole-body assessments. Although MRI has shown excellent accuracy in measuring skeletal muscle compared with cadaver validation studies,36 it is rather impractical to use in a clinical setting or in large epidemiological studies; therefore, DXA is the most commonly used imaging tool in the clinical setting. A comparison of body composition components obtained by MRI analysis vs DXA is provided in Figure 2.
Figure 2.
Selected body composition components measured by dual-energy x-ray absorptiometry (DXA; left) and magnetic resonance imaging (MRI; right). Body mass (M) and volume (V) represent the sum of these components for DXA and MRI, respectively. BMC, bone mineral content; IMAT, intermuscular adipose tissue; LST, lean soft tissue; Res, residual mass (organs and tissues remaining after subtracting skeletal muscle, bone, and adipose tissue volumes); SAT, subcutaneous adipose tissue; SM, skeletal muscle; VAT, visceral adipose tissue.
Ultrasound
Ultrasound is a commonly available technique used in clinical settings for diagnosis and follow-up purposes. The use of ultrasound in body composition research is possible due to its ability to quantify tissue thickness. For example, when the ultrasound beam is propagated through the skin, it is partially reflected back to the transducer as an echo when encountering underlying tissues such as subcutaneous adipose tissue and skeletal muscle.37,38 The degree of reflection of tissues varies according to its acoustic impedance (product of acoustic impedance and speed of sound)39 in a scale where air < adipose tissue < muscle < bone. The higher the acoustic impedance, the stronger the generated reflection will be and, therefore, the better the image quality. When the transducer receives the beam, it converts the echo into electric signals to form a 2-D image.37,38 Because the acoustic impedance of adipose tissue and muscle is somewhat similar, a weaker echo between the adipose tissue-muscle interface leads to a weaker echo compared with muscle and bone or adipose tissue and bone interfaces.37,38 The ability to interpret these interfaces represents 1 of the major drawbacks of the technique as it is somewhat subjective and requires specific technical expertise.
After the tissue boundaries have been determined on the ultrasound image, calipers are used to determine tissue thickness; the use of calipers also relies on practice and standardization of protocol.38 Nonetheless, ultrasound scanning is a simple, portable, safe, and a low-patient burden technique. It has been most extensively used in the assessment of subcutaneous adipose tissue, followed by visceral adipose tissue. As summarized by Bazzocchi et al,40 ultrasound has a higher correlation with areas or volumes of fat detected by CT or MRI, and, as assessed in the study, it provides an accurate, reproducible, and fast analysis of abdominal adiposity, discriminating between visceral and subcutaneous adiposity.
The measurement of muscle using ultrasonography is more prone to technical errors caused by muscle compressibility, selection of a reliable site, optimal transducer position management, ability to ensure a full relaxation or contraction state, and the patient’s resting state and hydration status.41 When potential technical issues are manageable, the technique can provide high test-retest reliability of muscle thickness and cross-sectional area.41 Tillquist et al42 recently demonstrated an excellent intra- and interrater reliability for ultrasound measurements of quadriceps muscle layer thickness, advocating for the use of the technique in hospital settings.
The use of ultrasound measurements of muscle size in clinical settings is promising because it is safe, noninvasive, and portable, and it allows for simplicity of measurements. Ultrasound has been used to measure muscle thickness as an index of LST in patients with multiple-organ failure prone to muscle wasting,43 patients with cystic fibrosis,44 elderly individuals,45-48 patients confined to bed rest,49 critically ill patients,50 patients with amyotrophic lateral sclerosis,51 and stroke survivors.52
The Value of Assessing LST: Clinical Considerations
As mentioned above, many features of body composition are masked by considering body weight as a whole and/or body weight change. Imaging-based examination of body composition is highly differentiated, which allows separate discrimination of many of its facets. As such, many abnormalities in body composition exist, including low bone mass/density (osteopenia/porosis), excess fat (obesity), low muscle mass (sarcopenia), and the combination of excess fat with low muscle (sarcopenic obesity), among others.53
Sarcopenia is a term denoting a reduced quantity of skeletal muscle.54 This definition is probably one of the most debated and the least agreed-upon topics on body composition research. Different cut points using different methodologies and rationale have been proposed, and the reader is referred to comprehensive reviews on the topic.8,55,56
The relevance of sarcopenia is due to the value of muscle mass and strength as critical components in maintaining physical function, mobility, and vitality. Sarcopenia is postulated to be a major factor in age-related strength decline and functional impairment57,58 and has been associated with physical disability, falls, fractures, and frailty.57,59 In fact, sarcopenia in the elderly and in patients with various diseases is associated with extended hospital stays, infectious and noninfectious complications, and overall mortality.60-62 Importantly, sarcopenia is not restricted to people who appear thin or underweight.1 Aging is often paralleled by increased muscle loss and increased fat, which may culminate in a condition termed sarcopenic obesity, a syndrome that entails the combined health risks of both sarcopenia and obesity. Sarcopenic obesity is increasing in prevalence around the world,1,53,63,64 and its consequences for all aspects of physical function and a variety of health outcomes have begun to be described.8 Nonetheless, there is considerable evidence to suggest that the double burden of sarcopenia and obesity may accentuate the risk of unfavorable health outcomes, leading to significantly greater morbidity and disability than either in isolation.65 As summarized in a literature review,8 sarcopenic obesity is associated with greater physical disability, increased risk of metabolic syndrome, increased length of hospital stay, and increased mortality.
Although most studies to date have been conducted in elderly individuals, sarcopenia and sarcopenic obesity are not limited only to the elderly. Individuals can be prone to muscle loss (with or without concurrent obesity) in several clinical disorders. These include diabetes, cancer, chronic obstructive pulmonary disease, cirrhosis, and rheumatoid arthritis, among others.
More recently, the term osteosarcopenic obesity has also been proposed53,66 to describe the concurrent appearance of obesity in individuals with low bone and muscle masses. As obesity masks both osteopenia/osteoporosis and sarcopenia, the detection and understanding of this phenotype is limited to the availability of sophisticated body composition techniques. The triple burden of osteosarcopenic obesity may accentuate the risk of developing chronic degenerative diseases, but more important, it could reduce disability-free life expectancy and increase the risk of mortality.53 Regardless of the terminology used to describe abnormal body composition phenotypes, it is paramount for healthcare professionals to understand they exist and are associated with poorer health outcomes.
As science evolves, so does the interest in exploring abnormal body composition phenotypes in the scientific community. The bottom line is that having an abnormal body composition, especially LST depletion, has been recently revealed as a predictor of unfavorable health outcomes (worst prognosis) in a variety of patient populations. Key examples in the literature include shorter survival (patients with cancer, cirrhosis, and kidney failure),24,67-69 longer length of hospital stay (elderly individuals and patients with cancer),62,70-72 higher prevalence of postoperative complications (elderly individuals and patients with cirrhosis and cancer),61,72-74 shorter time to tumor progression,75 and higher incidence of life-threatening toxicity to treatment (patients with cancer).21,22,76-79
Recent studies have also highlighted the use of imaging techniques to detect LST depletion in cohorts of individuals with respiratory failure,25,80 chronic obstructive pulmonary disease,81 HIV,82 Alzheimer disease,83 or traumatic injuries.26 Another emerging cohort of patients prone to body composition abnormalities are those with osteoarthritis, in whom body composition may have an important predictive value before and after hip or knee replacement surgery. Muscle atrophy has been demonstrated in patients with osteoarthritis of the hip,84 and a 20% reduction in muscle strength has been reported in patients scheduled for knee replacement surgery.85,86
The evolving importance of body composition assessment has been a paradigm shift in the assessment of nutrition status. Body composition is becoming a crucial component of clinical assessment, suggesting that commonly used subjective and superficial assessments such as body weight and BMI may neglect the health risks and status of patients.1 In a variety of clinical conditions, body composition assessment will be vital for making treatment decisions, predicting survival outcomes, and determining patient quality of life.
Notwithstanding, a major drawback for such advance is the understanding of the relationship between quantity and function/health or, in other words, the specific amount of LST related to poor prognosis (cut point) or the specific increase in LST, which would translate to change in a health outcome. As discussed by van Kan et al,87 regulatory agencies have to date failed to accept quantitative increases in LST as sufficient criteria for approval of therapeutic interventions to treat LST depletion.
Although not the focus of this article, muscle and adipose tissue and their whole-body–derived variables have been extensively used to evaluate other clinical outcomes such as determining time to tumor progression, outcomes of surgery (such as length of hospital stay and risk of infections), and survival.
Future Directions
Methodologies to assess body composition have emerged as reliable predictors of physiological reserves, suggesting that superficial measures of body weight may potentially neglect risk and status.1 As other medical fields have evolved to include sophisticated biomarker assessment methods in the clinical setting, we also advocate for the use of advanced body composition techniques for the assessment of health status of patients.1,88
Although further research is needed to support the appropriate use of body composition as an outcome measure in clinical trials,89 recognition of its importance is on the rise. Importantly, manipulating body composition changes by establishing adequate (or optimal) nutrient requirements for individuals across the categories of body composition phenotypes will lead to specific dietary recommendations reflecting different levels of nutrition status. Unfortunately, to date, dietary recommendations and medical nutrition products have been established as “one size fits all.” The identification of different body composition phenotypes suggests that individuals have different metabolism and hence utilization of fuel sources. This is an emerging area for future studies investigating the relationship between nutrition intake, energy expenditure, and overall metabolic regulation of the human body.
Supplementary Material
Acknowledgments
We thank Richard Porter (The JB Ashtin Group, Inc for assistance with formatting the references and paper and Dr Mark H. DeLegge (Baxter Healthcare Corporation) for his input and leadership throughout the development of this manuscript.
Footnotes
Financial disclosure:Baxter Healthcare Corporation provided financial support for The JB Ashtin Group, Inc.
Disclosures:Baxter Healthcare Corporation and the authors discussed the final content, the authors drafted and revised the manuscript, and Baxter provided a courtesy medical and legal review of the final version, including financial support for The JB Ashtin Group, Inc. The authors had final approval over the content.
This article originally appeared online on September 19, 2014.

Download a QR code reader on your smartphone, scan this image, and listen to the podcast for this article instantly. Or listen to this and other JPEN podcasts at http://pen.sagepub.com/site/misc/Index/Podcasts.xhtml.
Further Reading
- Baracos VE, Prado CM, Antoun S, Gioulbasanis I. Assessment of nutritional status. In: Del Fabbro E, Baracos V, Demark-Wahnefried W, Bowling T, Hopkinson J, Bruera E, eds. Nutrition and the Cancer Patient. New York, NY: Oxford University Press; 2010:19-34. [Google Scholar]
- Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci. 2000;904:437-448. [DOI] [PubMed] [Google Scholar]
- Heymsfield SB. Development of imaging methods to assess adiposity and metabolism. Int J Obes (Lond). 2008;32(suppl 7):S76-S82. [DOI] [PubMed] [Google Scholar]
- Heymsfield SB, Pietrobelli A, Wang Z, Saris WH. The end of body composition methodology research? Curr Opin Clin Nutr Metab Care. 2005; 8(6):591-594. [DOI] [PubMed] [Google Scholar]
- Lang T, Streeper T, Cawthon P, et al. Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporos Int. 2010;21(4):543-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siervo M, Prado CM. Nutritional assessment of the critically ill patient. In: Faber P, Siervo M, eds. Nutrition in Critical Care. Cambridge, UK: Cambridge University Press; 2014:13-19. [Google Scholar]
- Siervo M, Prado CM, Mire E, Wells JCK, Heymsfield S, Katzmarzyk P. Body composition indexes of a load-capacity model: gender and BMI-specific reference curves. Public Health Nutr. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternfeld B, Ngo L, Satariano WA, Tager IB. Associations of body composition with physical performance and self-reported functional limitation in elderly men and women. Am J Epidemiol. 2002;156(2):110-121. [DOI] [PubMed] [Google Scholar]
Glossary
Acoustic impedance: In ultrasound, acoustic impedance is the amount of sound pressure that is produced by the vibration of molecules at a given frequency.
Adipose tissue: A connective tissue formed by adipocytes, collagenous and elastic fibers, fibroblasts, and capillaries. There are 4 types of adipose tissue: subcutaneous, visceral, interstitial, and yellow marrow.
Appendicular skeletal muscle (ASM): The sum of skeletal muscle mass from arms and legs.
Body mass index (BMI): A measurement of body weight adjusted for height (body weight in kilograms/height in meters2).
Computerized tomography (CT) imaging: X-ray–based imaging method for body composition analysis at the tissue-organ level.
Dual-energy x-ray absorptiometry (DXA): Widely used x-ray based method that assesses body composition at the molecular level. DXA can assess the mass of bone mineral, fat, and lean soft tissue at the whole body and regional levels.
Fat mass (FM): The specific family of lipids consisting of triglycerides that makes up approximately 80% of adipose tissue.
Fat-free mass (FFM): The sum of lean soft tissue (LST) and bone mineral components.
Hounsfield unit: Measure of a CT’s x-ray attenuation expressed as the linear attenuation coefficient measurement in relation to air and water.
Intramuscular adipose tissue: Adipose tissue found within skeletal muscle.
Lean soft tissue: Also incorrectly referred to as lean body mass. LST is the sum of body water, total body protein, carbohydrates, nonfat lipids, and soft tissue mineral; all fat and bone mineral are excluded.
Magnetic resonance imaging (MRI): Method for determining body composition that is based on the interaction between hydrogen nuclei (protons) and the magnetic fields created and managed by the MRI system’s instrumentation.
Nutrition status: The current health status of the body in relation to the state of nourishment (eg, the consumption and utilization of nutrients).
Osteopenia/osteoporosis: Osteoporosis is a dramatic reduction in bone mass as a result of depleted calcium and bone protein. Osteopenia is a precursor to osteoporosis.
Osteosarcopenic obesity: Simultaneous appearance of low bone and muscle masses in obese individuals.
Pixel: Single rectangular area of an image in a CT image.
Sarcopenia: Low skeletal muscle mass associated with aging.
Sarcopenic obesity: The concurrent appearance of low muscle mass and excess adipose tissue.
Subcutaneous adipose tissue: Nonvisceral fat just below the skin.
Ultrasound: Widely available technique that can measure body composition by quantifying tissue thickness.
Visceral adipose tissue: Adipose tissue around internal organs.
Voxel: Volume and pixel information from an MRI scan that, along with image area, determines the generated gray-scale images.
References
- 1. Prado CM, Siervo M, Mire E, et al. A population-based approach to define body-composition phenotypes. Am J Clin Nutr. 2014;99(6):1369-1377. [DOI] [PubMed] [Google Scholar]
- 2. Shen W, St-Onge MP, Wang Z, Heymsfield SB. Study of body composition: an overview. In: Heymsfield SB, Lohman T, Wang Z, Going S. eds. Human Body Composition. 2nd ed. Champaign, IL: Human Kinetics; 2005;3-14. [Google Scholar]
- 3. Wang ZM, Pierson RN, Jr, Heymsfield SB. The five-level model: a new approach to organizing body-composition research. Am J Clin Nutr. 1992;56(1):19-28. [DOI] [PubMed] [Google Scholar]
- 4. Heymsfield SB, Wang Z, Baumgartner RN, Ross R. Human body composition: advances in models and methods. Annu Rev Nutr. 1997;17:527-558. [DOI] [PubMed] [Google Scholar]
- 5. Lohman TG, Chen Z. Dual-energy x-ray absorptiometry. In: Heymsfield SB, Lohman T, Wang Z, Going S, eds. Human Body Composition. 2nd ed. Champaign, IL: Human Kinetics; 2005;63-78. [Google Scholar]
- 6. Heymsfield SB, Adamek M, Gonzalez MC, Jia G, Thomas DM. Assessing skeletal muscle mass: historical overview and state of the art. J Cachexia Sarcopenia Muscle. 2014;5(1):9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heymsfield SB, Smith R, Aulet M, et al. Appendicular skeletal muscle mass: measurement by dual-photon absorptiometry. Am J Clin Nutr. 1990;52(2):214-218. [DOI] [PubMed] [Google Scholar]
- 8. Prado CM, Wells JC, Smith SR, Stephan BC, Siervo M. Sarcopenic obesity: a critical appraisal of the current evidence. Clin Nutr. 2012;31(5):583-601. [DOI] [PubMed] [Google Scholar]
- 9. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roubenoff R, Kehayias JJ, Dawson-Hughes B, Heymsfield SB. Use of dual-energy x-ray absorptiometry in body-composition studies: not yet a “gold standard.” Am J Clin Nutr. 1993;58(5):589-591. [DOI] [PubMed] [Google Scholar]
- 11. Pritchard JE, Nowson CA, Strauss BJ, Carlson JS, Kaymakci B, Wark JD. Evaluation of dual energy x-ray absorptiometry as a method of measurement of body fat. Eur J Clin Nutr. 1993;47(3):216-228. [PubMed] [Google Scholar]
- 12. Genton L, Hans D, Kyle UG, Pichard C. Dual-energy x-ray absorptiometry and body composition: differences between devices and comparison with reference methods. Nutrition. 2002;18(1):66-70. [DOI] [PubMed] [Google Scholar]
- 13. Madsen OR, Egsmose C, Lorentzen JS, Lauridsen UB, Sorensen OH. Influence of orthopaedic metal and high-density detection on body composition as assessed by dual-energy x-ray absorptiometry. Clin Physiol. 1999;19(3):238-245. [DOI] [PubMed] [Google Scholar]
- 14. Hangartner TN, Warner S, Braillon P, Jankowski L, Shepherd J. The official positions of the International Society for Clinical Densitometry: acquisition of dual-energy x-ray absorptiometry body composition and considerations regarding analysis and repeatability of measures. J Clin Densitom. 2013;16(4):520-536. [DOI] [PubMed] [Google Scholar]
- 15. Ross R, Janssen I. Computed tomography and magnetic resonance imaging. In: Heymsfield SB, Lohman T, Wang Z, Going S, eds. Human Body Composition. 2nd ed. Champaign, IL: Human Kinetics; 2005:89-108. [Google Scholar]
- 16. Goodpaster BH, Thaete FL, Kelley DE. Composition of skeletal muscle evaluated with computed tomography. In Vivo Body Composition Studies. 2000;904:18-24. [DOI] [PubMed] [Google Scholar]
- 17. Mattsson S, Thomas BJ. Development of methods for body composition studies. Phys Med Biol. 2006;51(13):R203-R228. [DOI] [PubMed] [Google Scholar]
- 18. Shen W, Punyanitya M, Wang Z, et al. Visceral adipose tissue: relations between single-slice areas and total volume. Am J Clin Nutr. 2004;80(2):271-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. 2004;97(6):2333-2338. [DOI] [PubMed] [Google Scholar]
- 20. Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33(5):997-1006. [DOI] [PubMed] [Google Scholar]
- 21. Prado CM. Body composition in chemotherapy: the promising role of CT scans. Curr Opin Clin Nutr Metab Care. 2013;16(5):525-533. [DOI] [PubMed] [Google Scholar]
- 22. Prado CM, Maia YL, Ormsbee M, Sawyer MB, Baracos VE. Assessment of nutritional status in cancer—the relationship between body composition and pharmacokinetics. Anticancer Agents Med Chem. 2013;13(8):1197-1203. [DOI] [PubMed] [Google Scholar]
- 23. Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31(12):1539-1547. [DOI] [PubMed] [Google Scholar]
- 24. Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629-635. [DOI] [PubMed] [Google Scholar]
- 25. Sheean PM, Peterson SJ, Gomez Perez S, et al. The prevalence of sarcopenia in patients with respiratory failure classified as normally nourished using computed tomography and subjective global assessment. JPEN J Parenter Enteral Nutr. 2014;38(7):873-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moisey LL, Mourtzakis M, Cotton BA, et al. Skeletal muscle predicts ventilator-free days, ICU-free days, and mortality in elderly ICU patients. Crit Care. 2013;17(5):R206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karra S, Fearon K. A Feasibility Study of Multimodal Exercise/Nutrition/Anti-Inflammatory Treatment for Cachexia—The Pre-MENAC Study. http://clinicaltrials.gov/show/NCT01419145. Accessed May 28, 2014.
- 28. Silver HJ, Welch EB, Avison MJ, Niswender KD. Imaging body composition in obesity and weight loss: challenges and opportunities. Diabetes Metab Syndr Obes. 2010;3:337-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Neeland IJ, Ayers CR, Rohatgi AK, et al. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity. 2013;21(9):E439-E447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Manini TM, Buford TW, Lott DJ, et al. Effect of dietary restriction and exercise on lower extremity tissue compartments in obese, older women: a pilot study. J Gerontol A Biol Sci Med Sci. 2014;69(1):101-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zoico E, Corzato F, Bambace C, et al. Myosteatosis and myofibrosis: relationship with aging, inflammation and insulin resistance. Arch Gerontol Geriatr. 2013;57(3):411-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. LeBlanc A, Lin C, Shackelford L, et al. Muscle volume, MRI relaxation times (T2), and body composition after spaceflight. J Appl Physiol (1985). 2000;89(6):2158-2164. [DOI] [PubMed] [Google Scholar]
- 33. Karlsson AK, Kullberg J, Stokland E, et al. Measurements of total and regional body composition in preschool children: a comparison of MRI, DXA, and anthropometric data. Obesity. 2013;21(5):1018-1024. [DOI] [PubMed] [Google Scholar]
- 34. Molfino A, Heymsfield SB, Zhu F, et al. Prealbumin is associated with visceral fat mass in patients receiving hemodialysis. J Ren Nutr. 2013;23(6):406-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shah K, Hilton TN, Myers L, Pinto JF, Luque AE, Hall WJ. A new frailty syndrome: central obesity and frailty in older adults with the human immunodeficiency virus. J Am Geriatr Soc. 2012;60(3):545-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985). 1998;85(1):115-122. [DOI] [PubMed] [Google Scholar]
- 37. Wagner DR. Ultrasound as a tool to assess body fat. J Obes. 2013;2013:280713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bellisari A, Roche AF. Anthropometry and ultrasound. In: Heymsfield SB, Lohman T, Wang Z, Going S, eds. Human Body Composition. 2nd ed. Champaign, IL: Human Kinetics; 2005:109-128. [Google Scholar]
- 39. Bushberg JT, Seibert JA, Leidholdt EM, Boone JM. The Essential Physics of Medical Imaging. 2nd ed. Philadelphia, PA: Williams & Wilkins; 2002. [Google Scholar]
- 40. Bazzocchi A, Filonzi G, Ponti F, et al. Accuracy, reproducibility and repeatability of ultrasonography in the assessment of abdominal adiposity. Acad Radiol. 2011;18(9):1133-1143. [DOI] [PubMed] [Google Scholar]
- 41. Mayans D, Cartwright MS, Walker FO. Neuromuscular ultrasonography: quantifying muscle and nerve measurements. Phys Med Rehabil Clin North Am. 2012;23(1):133-148, xii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tillquist M, Kutsogiannis DJ, Wischmeyer PE, et al. Bedside ultrasound is a practical and reliable measurement tool for assessing quadriceps muscle layer thickness. JPEN J Parenter Enteral Nutr. 2014;38(7):886-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Campbell IT, Watt T, Withers D, et al. Muscle thickness, measured with ultrasound, may be an indicator of lean tissue wasting in multiple organ failure in the presence of edema. Am J Clin Nutr. 1995;62(3):533-539. [DOI] [PubMed] [Google Scholar]
- 44. Enright SJ, Unnithan VB, Heward C, Withnall L, Davies DH. Effect of high-intensity inspiratory muscle training on lung volumes, diaphragm thickness, and exercise capacity in subjects who are healthy. Phys Ther. 2006;86(3):345-354. [PubMed] [Google Scholar]
- 45. Reimers CD, Harder T, Saxe H. Age-related muscle atrophy does not affect all muscles and can partly be compensated by physical activity: an ultrasound study. J Neurol Sci. 1998;159(1):60-66. [DOI] [PubMed] [Google Scholar]
- 46. Trappe TA, Lindquist DM, Carrithers JA. Muscle-specific atrophy of the quadriceps femoris with aging. J Appl Physiol (1985). 2001;90(6):2070-2074. [DOI] [PubMed] [Google Scholar]
- 47. Ikezoe T, Mori N, Nakamura M, Ichihashi N. Atrophy of the lower limbs in elderly women: is it related to walking ability? Eur J Appl Physiol. 2011;111(6):989-995. [DOI] [PubMed] [Google Scholar]
- 48. Fujiwara K, Toyama H, Asai H, et al. Effects of regular heel-raise training aimed at the soleus muscle on dynamic balance associated with arm movement in elderly women. J Strength Cond Res. 2011;25(9):2605-2615. [DOI] [PubMed] [Google Scholar]
- 49. Kawakami Y, Akima H, Kubo K, et al. Changes in muscle size, architecture, and neural activation after 20 days of bed rest with and without resistance exercise. Eur J Appl Physiol. 2001;84(1-2):7-12. [DOI] [PubMed] [Google Scholar]
- 50. Reid CL, Campbell IT, Little RA. Muscle wasting and energy balance in critical illness. Clin Nutr. 2004;23(2):273-280. [DOI] [PubMed] [Google Scholar]
- 51. Arts IM, Overeem S, Pillen S, Schelhaas HJ, Zwarts MJ. Muscle changes in amyotrophic lateral sclerosis: a longitudinal ultrasonography study. Clin Neurophysiol. 2011;122(3):623-628. [DOI] [PubMed] [Google Scholar]
- 52. Triandafilou KM, Kamper DG. Investigation of hand muscle atrophy in stroke survivors. Clin Biomech (Bristol, Avon). 2012;27(3):268-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ormsbee MJ, Prado CM, Ilich JZ, et al. Osteosarcopenic obesity: the role of bone, muscle, and fat on health [published online April 17, 2014]. J Cachexia Sarcopenia Muscle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rosenberg I. The epidemiologic and methodologic problems in determining nutritional status of older persons [summary comments]. Am J Clin Nutr. 1989;50:1231-1233. [PubMed] [Google Scholar]
- 55. Malafarina V, Uriz-Otano F, Iniesta R, Gil-Guerrero L. Sarcopenia in the elderly: diagnosis, physiopathology and treatment. Maturitas. 2012;71(2):109-114. [DOI] [PubMed] [Google Scholar]
- 56. Waters DL, Baumgartner RN. Sarcopenia and obesity. Clin Geriatr Med. 2011;27(3):401-421. [DOI] [PubMed] [Google Scholar]
- 57. Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50(5):889-896. [DOI] [PubMed] [Google Scholar]
- 58. Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755-763. [DOI] [PubMed] [Google Scholar]
- 59. Evans W. Functional and metabolic consequences of sarcopenia. J Nutr. 1997;127(suppl 5):998S-1003S. [DOI] [PubMed] [Google Scholar]
- 60. Roubenoff R, Kehayias JJ. The meaning and measurement of lean body mass. Nutr Rev. 1991;49(6):163-175. [DOI] [PubMed] [Google Scholar]
- 61. Cosqueric G, Sebag A, Ducolombier C, Thomas C, Piette F, Weill-Engerer S. Sarcopenia is predictive of nosocomial infection in care of the elderly. Br J Nutr. 2006;96(5):895-901. [DOI] [PubMed] [Google Scholar]
- 62. Pichard C, Kyle UG, Morabia A, Perrier A, Vermeulen B, Unger P. Nutritional assessment: lean body mass depletion at hospital admission is associated with an increased length of stay. Am J Clin Nutr. 2004;79(4):613-618. [DOI] [PubMed] [Google Scholar]
- 63. Rolland Y, Lauwers-Cances V, Cristini C, et al. Difficulties with physical function associated with obesity, sarcopenia, and sarcopenic-obesity in community-dwelling elderly women: the EPIDOS (EPIDemiologie de l’OSteoporose) Study. Am J Clin Nutr. 2009;89(6):1895-1900. [DOI] [PubMed] [Google Scholar]
- 64. Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res. 2004;12(12):1995-2004. [DOI] [PubMed] [Google Scholar]
- 65. Roubenoff R. Sarcopenic obesity: does muscle loss cause fat gain? Lessons from rheumatoid arthritis and osteoarthritis. Ann N Y Acad Sci. 2000;904:553-557. [DOI] [PubMed] [Google Scholar]
- 66. Ilich JZ, Kelly OJ, Inglis JE, Panton LB, Duque G, Ormsbee MJ. Interrelationship among muscle, fat, and bone: connecting the dots on cellular, hormonal, and whole body levels. Ageing Res Rev. 2014;15C:51-60. [DOI] [PubMed] [Google Scholar]
- 67. Honda H, Qureshi AR, Axelsson J, et al. Obese sarcopenia in patients with end-stage renal disease is associated with inflammation and increased mortality. Am J Clin Nutr. 2007;86(3):633-638. [DOI] [PubMed] [Google Scholar]
- 68. Montano-Loza AJ, Meza-Junco J, Prado CM, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10(2):166-173.e161. [DOI] [PubMed] [Google Scholar]
- 69. Vetrano DL, Landi F, Volpato S, et al. Association of sarcopenia with short- and long-term mortality in older adults admitted to acute care wards: results from the CRIME study. J Gerontol A Biol Sci Med Sci. 2014;69(9):1154-1161. [DOI] [PubMed] [Google Scholar]
- 70. Kyle UG, Pirlich M, Lochs H, Schuetz T, Pichard C. Increased length of hospital stay in underweight and overweight patients at hospital admission: a controlled population study. Clin Nutr. 2005;24(1):133-142. [DOI] [PubMed] [Google Scholar]
- 71. Lieffers JR, Bathe OF, Fassbender K, Winget M, Baracos VE. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer. 2012;107(6):931-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Peng PD, van Vledder MG, Tsai S, et al. Sarcopenia negatively impacts short-term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. HPB (Oxford). 2011;13(7):439-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Smith AB, Deal AM, Yu H, et al. Sarcopenia as a predictor of complications and survival following radical cystectomy [published online January 11, 2014]. J Urol. [DOI] [PubMed] [Google Scholar]
- 74. Montano-Loza AJ, Meza-Junco J, Baracos VE, et al. Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver Transpl. 2014;20(6):640-648. [DOI] [PubMed] [Google Scholar]
- 75. Prado CM, Baracos VE, McCargar LJ, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. 2009;15(8):2920-2926. [DOI] [PubMed] [Google Scholar]
- 76. Prado CM, Baracos VE, Xiao J, et al. The association between body composition and toxicities from the combination of Doxil and trabectedin in patients with advanced relapsed ovarian cancer. Appl Physiol Nutr Metab. 2014;39(6):693-698. [DOI] [PubMed] [Google Scholar]
- 77. Prado CM, Lima IS, Baracos VE, et al. An exploratory study of body composition as a determinant of epirubicin pharmacokinetics and toxicity. Cancer Chemother Pharmacol. 2011;67(1):93-101. [DOI] [PubMed] [Google Scholar]
- 78. Antoun S, Baracos VE, Birdsell L, Escudier B, Sawyer MB. Low body mass index and sarcopenia associated with dose-limiting toxicity of sorafenib in patients with renal cell carcinoma. Ann Oncol. 2010;21(8):1594-1598. [DOI] [PubMed] [Google Scholar]
- 79. Mir O, Coriat R, Blanchet B, et al. Sarcopenia predicts early dose-limiting toxicities and pharmacokinetics of sorafenib in patients with hepatocellular carcinoma. PLoS One. 2012;7(5):e37563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Braunschweig CA, Sheean PM, Peterson SJ, et al. Exploitation of diagnostic computed tomography scans to assess the impact of nutritional support on body composition changes in respiratory failure patients. JPEN J Parenter Enteral Nutr. 2014;38(7):880-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Schols AM, Broekhuizen R, Weling-Scheepers CA, Wouters EF. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr. 2005;82(1):53-59. [DOI] [PubMed] [Google Scholar]
- 82. Erlandson KM, Allshouse AA, Jankowski CM, MaWhinney S, Kohrt WM, Campbell TB. Functional impairment is associated with low bone and muscle mass among persons aging with HIV infection. J Acquir Immune Defic Syndr. 2013;63(2):209-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Burns JM, Johnson DK, Watts A, Swerdlow RH, Brooks WM. Reduced lean mass in early Alzheimer disease and its association with brain atrophy. Arch Neurol. 2010;67(4):428-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rasch A, Bystrom AH, Dalen N, Martinez-Carranza N, Berg HE. Persisting muscle atrophy two years after replacement of the hip. J Bone Joint Surg Br. 2009;91(5):583-588. [DOI] [PubMed] [Google Scholar]
- 85. Madsen OR, Brot C, Petersen MM, Sorensen OH. Body composition and muscle strength in women scheduled for a knee or hip replacement: a comparative study of two groups of osteoarthritic women. Clin Rheumatol. 1997;16(1):39-44. [DOI] [PubMed] [Google Scholar]
- 86. Sowers MF, Kshirsagar A, Crutchfield MM, Updike S. Joint influence of fat and lean body composition compartments on femoral bone mineral density in premenopausal women. Am J Epidemiol. 1992;136(3):257-265. [DOI] [PubMed] [Google Scholar]
- 87. van Kan GA, Cderbaum JM, Cesari M, et al. Sarcopenia: biomarkers and imaging (International Conference on Sarcopenia Research). J Nutr Health Aging. 2011;15(10):834-846. [DOI] [PubMed] [Google Scholar]
- 88. de van der Schueren M, Elia M, Gramlich L, Johnson MP, Lim SL, Philipson T, Prado CM. Clinical and economic outcomes of nutrition interventions across the continuum of care. Ann N Y Acad Sci. 2014;1321(1):20-40. [DOI] [PubMed] [Google Scholar]
- 89. Lukaski HC. Evolution of bioimpedance: a circuitous journey from estimation of physiological function to assessment of body composition and a return to clinical research. Eur J Clin Nutr. 2013;67(suppl 1):S2-S9. [DOI] [PubMed] [Google Scholar]
- 90. Siervo M, Jebb SA. Body composition assessment: theory into practice: introduction of multicompartment models. IEEE Eng Med Biol Mag. 2010;29(1):48-59. [DOI] [PubMed] [Google Scholar]
- 91. Garn SM. Anthropometry in clinical appraisal of nutritional status. Am J Clin Nutr. 1962;11(5):418-432. [DOI] [PubMed] [Google Scholar]
- 92. Baracos V, Prado CM, Antoun S, Gioulbasanis I. Assessment of nutritional status. In: Del Fabbro E, Baracos V, Demark-Wahnefried W, Bowling T, Hopkinson J, Bruera E, eds. Nutrition and the Cancer Patient. Oxford, England: Oxford University Press; 2010:19-36. [Google Scholar]
- 93. Lee SY, Gallagher D. Assessment methods in human body composition. Curr Opin Clin Nutr Metab Care. 2008;11(5):566-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wang J, Gallagher D, Thornton JC, Yu W, Horlick M, Pi-Sunyer FX. Validation of a 3-dimensional photonic scanner for the measurement of body volumes, dimensions, and percentage body fat. Am J Clin Nutr. 2006;83(4):809-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Xu B, Yu W, Yao M, Pepper MR, Freeland-Graves JH. Three-dimensional surface imaging system for assessing human obesity. Opt Eng. 2009;48(10):nihpa156427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Coppini LZ, Waitzberg DL, Campos AC. Limitations and validation of bioelectrical impedance analysis in morbidly obese patients. Curr Opin Clin Nutr Metab Care. 2005;8(3):329-332. [DOI] [PubMed] [Google Scholar]
- 97. Bohm A, Heitmann BL. The use of bioelectrical impedance analysis for body composition in epidemiological studies. Eur J Clin Nutr. 2013;67(suppl 1):S79-S85. [DOI] [PubMed] [Google Scholar]
- 98. Prado CM, Birdsell LA, Baracos VE. The emerging role of computerized tomography in assessing cancer cachexia. Curr Opin Support Palliat Care. 2009;3(4):269-275. [DOI] [PubMed] [Google Scholar]
- 99. McGuire LP, Guglielmo CG. Quantitative magnetic resonance: a rapid, noninvasive body composition analysis technique for live and salvaged bats. J Mamm. 2010;91(6):1375-1380. [Google Scholar]
- 100. Mitchell AD. Validation of quantitative magnetic resonance body composition analysis for infants using piglet model. Pediatr Res. 2011;69(4):330-335. [DOI] [PubMed] [Google Scholar]
- 101. Engelke K, Adams JE, Armbrecht G, et al. Clinical use of quantitative computed tomography and peripheral quantitative computed tomography in the management of osteoporosis in adults: the 2007 ISCD Official Positions. J Clin Densitom. 2008;11(1):123-162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.