Abstract
During metastasis, circulating tumor cells migrate away from a primary tumor via the blood circulation to form secondary tumors in distant organs. Mounting evidence from clinical observations indicates that the number of circulating tumor cells (CTCs) in the blood correlates with the progression of solid tumors before and during chemotherapy. Beyond the well-established role of CTCs as a fluid biopsy, however, the field of targeting CTCs for the prevention or reduction of metastases has just emerged. Conventional cancer therapeutics have a relatively short circulation time in the blood which may render the killing of CTCs inefficient due to reduced exposure of CTCs to drugs. Nevertheless, over the past few decades, the development of nanoparticles and nanoformulations to improve the half-life and release profile of drugs in circulation has rejuvenated certain traditional medicines in the emerging field of CTC neutralization. This review focuses on how the principles of nanomedicine may be applied to target CTCs. Moreover, inspired by the interactions between CTCs and host cells in the blood circulation, novel biomimetic approaches for targeted drug delivery are presented.
Keywords: Circulating tumor cells, Metastasis, Nanomedicine
Introduction
Metastasis contributes to more than 90% of cancer-associated mortality.92 It is generally hypothesized that primary tumors shed circulating tumor cells (CTCs) via the lymphatics to neighboring lymph nodes or through hematogenous dissemination to distant organs. The presence of CTCs in the blood represents a poor prognosis in a variety of carcinomas.21,84,140 Nevertheless, the effective treatment for this deadly disease remains clinically challenging. In the case of hematogenous metastasis, CTCs must complete several sequential steps: (1) detachment from the primary tumor, (2) intravasation into the vascular system, (3) survival in the blood circulation, and (4) extravasation into the target tissue.41 The finding that very few metastases develop despite the release of millions of CTCs into the vasculature daily by large primary tumors suggests that the process of metastasis is very inefficient.76 This is consistent with a recent experimental demonstration that only a small subpopulation of metastasis-initiating cells (MICs) among human luminal breast cancer CTCs gave rise to distant metastases in mice and the existence of MICs correlated with overall metastatic incidence in patients.11 Therefore, early metastasis intervention procedures, such as neutralization of CTCs and particularly MICs in circulation, may offer new therapeutic opportunities.
The majority of existing cancer therapies including nanomedicine- and nanoformulation-based therapeutics target solid tumors (primary and metastatic).23 The underlying principle of most current nanotechnology-based drug delivery platforms is based on the observation that tumor-associated vasculatures are more leaky than normal vessels and thus are more permeable to nanoparticles and macromolecules. Additionally, solid tumors retain large molecules due to inefficient lymphatic drainage.16,27 While the enhanced permeability and retention (EPR) effect has proven to be a key pharmacokinetic feature for existing nanomedicines, this mechanism is not applicable to potential nanomedicines that target CTCs in circulation. The physical and biological environments surrounding CTCs are drastically different from those of solid tumors. CTCs are exposed to a broad range of fluid shear stresses when transiting in different vascular compartments (arteries, veins, and capillaries). Compared to non-transformed epithelial cells, transformed cells are remarkably resistant to varied fluid shear stress (FSS).13 In contrast, cancer cells in solid tumors are subjected to high interstitial fluid pressure caused by the stiff extracellular matrix.59,129 Additionally, certain CTCs gain the advantage of metastasis via interactions with different blood cells (neutrophils, macrophages, platelets, etc.) whereas solid tumors become aggressive by benefiting from hypoxia or certain tumor-promoting cells such as tumor-associated macrophages.40,62,84,147 These differences suggest that existing nanomedicines dependent on the EPR effect for targeted drug delivery must be tailored to the specific requirements for the neutralization of CTCs in circulation (Fig. 1).
Figure 1.
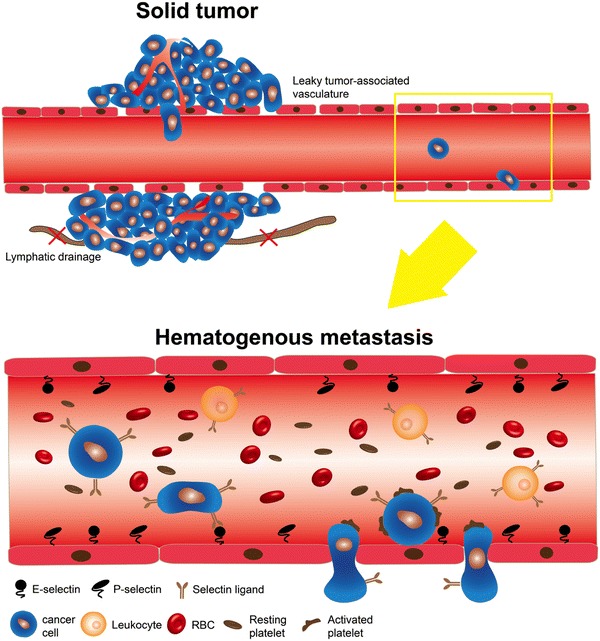
Two different contexts for the delivery of nanomedicines: solid tumor vs. the blood circulation. In a solid tumor, nanomedicine migrates to the tumor through leaky tumor-associated vasculature where dysfunctional lymphatic drainage enables accumulation of nanomedicine in the tumor. In contrast, once cancer cells are shed into the blood circulation to form CTCs, they are subjected to environmental changes such as shear stress, abundant RBCs and plasma proteins, and interactions with platelets, endothelial cells and other vascular components
In this review, we summarize the interactions of CTCs with different host cells during their hematogenous transit and how blocking CTC–host cell interactions via drug molecules have reduced metastases in animal models of cancer. Since the majority of these drugs suffer from fast clearance from circulation, methods to improve their circulation time through existing nanotechnologies will be discussed. Particularly, novel biomimetic CTC-targeting nanotechnologies are highlighted. Finally, potential CTC neutralization strategies that bridge conventional nanomedicine with technologies that are being utilized for CTC isolation and enumeration are discussed.
Biology of Circulating Tumor Cells
Aberrant Biological Events in CTCs
Solid tumors, at either primary or metastatic locations, can be accessed surgically in sufficient quantity for diagnostic tests and studies involving genomic sequencing, gene expression microarrays, immunohistology and mass spectrometry. In contrast, it remains a challenge to study the biology of rare CTCs in blood with conventional techniques. Efficient CTC isolation together with single-cell RNA-sequencing, exosome sequencing and immunofluorescence staining have enabled the discovery of several intracellular biological events underlying CTC-mediated metastasis. These events include but are not limited to: upregulation of Wnt2 in pancreatic cancer-derived CTCs,155 p53 mutation within CTCs of prostate cancer,88 activation of TGF-β and BMP signaling in CTCs from melanoma,89 and EGFR mutation in lung cancer CTCs.34 More generally, in nearly all epithelial cancers, a subpopulation of CTCs exist that are characterized by epithelial-to-mesenchymal transition (EMT) markers.10,12,85,152,156 During EMT, representative epithelial markers such as EpCAM and E-cadherin are downregulated, keratin expression pattern is altered, and mesenchymal markers such as N-cadherin, vimentin, and Snail are upregulated in turn.63,115 Entry to the mesenchymal state confers specific properties to CTCs including invasiveness, resistance to anoikis, chemo-resistance, and cancer stemness.2,36,90,107 Regardless of changes in signaling pathways and genomic integrity identified in CTCs, it remains to be answered whether these alterations merely mirror tumor progression and evolution occurring at primary and secondary tumors where CTCs are being shed. It has been postulated that subpopulations of metastasis-initiating cells (MICs) and cancer stem cells (CSCs) contribute to metastases in distant organs.3,11 It is of clinical significance if targeting of the aberrant signaling pathways could reduce the frequency of MICs and CSCs and thereby lead to better prognosis.
Selectin-Mediated Hematogenous Metastasis
Selectins (L-, E-, and P-selectin) are integral membrane glycoproteins. They share several structurally similar domains: an N-terminal C-type lectin domain, an epidermal growth factor (EGF)-like domain, a variable number of short consensus repeats (2, 6, and 9 for L-, E-, and P-selectin, respectively), a single-pass transmembrane domain, and a short intracellular C-terminal tail.83 L-selectin is constitutively expressed on the surface of leukocytes whereas E- and P-selectin are restricted to inflamed endothelial cells, and P-selectin on activated platelets.44 It has been well accepted that during inflammation, the presence of E- and P-selectin on the endothelium causes the rolling and migration of neutrophils and monocytes which express selectin ligands.123,158
Selectin ligands, however, are not limited to leukocytes and they have been identified on the surface of certain cancer cells. Several lines of evidence support that both E- and P-selectin facilitate the rolling and adhesion of CTCs in hematogenous metastasis. Köhler et al. provided the first in vivo finding that E- and P-selectin are essential for colorectal cancer metastasis. In their study, E- and P-selectin double knockout mice had a 84% reduction of lung metastatic nodules by number compared to wild-type mice after they were subcutaneously implanted with HT29 colon cancer cells.68 Lung endothelial cells, however, do not constitutively express E- and P-selectin unless they receive inflammatory signals. It is not clear how selectins mediated the adhesion of HT29 cells to the lung endothelium. In contrast to the lungs, endothelial cells in the bone marrow constitutively express E-selectin.49,146 Studies have shown that E-selectin ligands on both human and mouse prostate cancer (PCa) cells facilitate bone metastasis in an E-selectin dependent manner.15,86,150 Instead of utilizing E-selectin knockout mice, these two studies overexpressed α-1,3 fucosyltransferases (FTs) in E-selectin ligand (ESL)-negative human and mouse PCa cell lines based on the findings that ESL-positive PCa cells highly express FT3, 6 or FT7. Consequently, these engineered PCa cells produced increased incidence of bone metastasis in mice. The role of specific selectin ligands in mediating the hematogenous metastasis of CTCs has been reviewed extensively elsewhere.41,69,84 This review, however, focuses on how nanobiotechnology can be utilized to inhibit the interaction between cancer cells and the endothelium for the prevention of metastasis.
Given that selectins recognize sialylated fucosylated glycans on selectin ligands such as sLex, sLex analogs have been explored as competitive inhibitors for the binding of CTC selectin ligands to E- and P-selectin. For instance, a sLex analog, GSC-150, was tested for its effect on hepatic metastasis of human colon carcinoma in nude mice. It was found that liver metastases were significantly attenuated when cancer cells were co-administered with GSC-150.130 In addition to sLex analogs, compounds that can interfere with the synthesis of sLex represent another class of selectin inhibitors. We previously showed that a fluorinated fucose mimetic (2F-Peracetyl-Fucose) could be used to reduce E-selectin-dependent bone metastasis in mice by inhibiting the activity of FT6.86
Contribution of Platelets to CTC-Mediated Metastasis
The involvement of platelets in cancer was first reported in the mid-nineteenth century by the French clinician Armand Trousseau.141 He diagnosed patients with migratory thrombophlebitis caused by an occult visceral carcinoma. Mounting evidence has shown that the interaction of platelets with tumor cells can promote metastasis through several mechanisms. For instance, interactions with platelets protect CTCs from immune-mediated clearance,77,102,106 because such adhesion events affect the recognition of CTCs by natural killer cells. Platelet aggregation can result in the grafting of MHC class I ligands onto CTCs, which are typically absent. The newly acquired ligands prevent natural killer cells from identifying the CTCs as “non-self” and spare them from attack.114 Additionally, activated platelets can induce EMT as well as pro-survival and pro-metastatic signaling in tumor cells, which are associated with enhanced invasiveness and metastatic potential.35,74,75
It has been demonstrated that therapies targeting the interactions between platelets and CTCs can reduce the formation of secondary metastases. One approach to minimizing these interactions has focused on utilizing anti-coagulation agents. Agents such as recombinant mouse tissue factor pathway inhibitor (TFPI) and Cilostazol have been found to reduce the formation of secondary metastases.8,9,145 Unfortunately, the use of anticoagulants may also adversely affect the normal hemostatic function of platelets in the case of bleeding. A more focused approach aims to block the signaling between platelets and CTCs. Invasive behavior can be induced by transforming growth factor-β1 (TGF-β1), which is secreted by activated platelets. Temporary contact with platelets is sufficient to induce invasive behavior in CTCs through TGF-β1.74 Blockage of TGF-β1 receptor I (TβR1) kinase activity through the use of SD-208, a small molecule inhibitor, was shown to prevent the development of TGF-β induced bone metastases in a melanoma mouse model.100 Thus, blocking platelet-CTC signaling is a potentially viable targeted therapy to prevent the formation of additional metastases.
Challenges of Targeting CTCs by Nanomedicine
Although nanomedicine is a very general concept and represents a broad range of nanoformulations for existing drugs, it manifests itself largely in targeted drug delivery and controlled release. The biological characteristics of CTCs and the physical environment where they reside may pose certain challenges and problems when targeted by nanomedicine (Table 1). Searching for a rare number of CTCs in circulation is likely analogous to the problem of finding a needle in a haystack.5,47 This challenge may be solved by adopting the strategies utilized for CTC capture and enrichment. For example, targeting moieties for CTC isolation, such as anti-EpCAM monoclonal antibody, can be functionalized onto nanoparticles for the recognition of CTCs with epithelial origin in the circulation.28
Table 1.
Challenges of targeting CTCs by nanomedicine.
| CTC properties | Practical consequences | Refs. |
|---|---|---|
| Rarity of CTCs: a needle in a haystack problem | Low efficiency of targeting CTCs | 5,47 |
| Heterogeneous subpopulations | Mesenchymal CTCs are not recognized by nanomedicine targeting epithelial cell markers such as EpCAM; Necessity of killing all CTCs vs. MICs or CSCs in the circulation; Differential drug resistance among different subpopulations | 73,78,149,161 |
| Formation of CTC clusters | Increased invasiveness, resistance to anoikis and trapping in microvessels | 1,24,46,66,163 |
| Short circulation time of CTCs | Limited exposure time to therapeutics against CTCs in circulation | 93,125,135 |
| Shielding of CTCs by platelets | Physical barrier to penetration of nanomedicine into CTCs; pro-metastatic role via induction of EMT, establishment of early metastatic niches, pro-survival signaling etc. | 35,74,75 |
| Off-target effects associated with systemic drug delivery | Systemic cytotoxicity | 136,162 |
The heterogeneity of CTCs, however, dictates that there is no universal antigen for comprehensive targeting. CTC phenotypes can be categorized into epithelial (epithelial+/mesenchymal−), complete EMT (epithelial−/mesenchymal+), and intermediate EMT (epithelial+/mesenchymal+).73,78 It is possible that all three phenotypes exist in the circulation simultaneously and thus targeting CTCs with epithelial features may become ineffective against those with mesenchymal characteristics.161 Moreover, CTCs can also be divided into CSCs and non-CSCs according to their tumor-initiating capability, which is not necessarily coupled to the EMT status.149 This raises the question of whether it is necessary to neutralize all CTCs in circulation to achieve a net reduction in metastasis.
CTCs have been detected both as individual cells and as cellular clusters in blood.24,66 Although rare in circulation compared to individual CTCs, CTC clusters show increased invasiveness, are more resistant to anoikis and have a higher likelihood of becoming trapped in microvessels, thereby favoring their survival and extravasation into distant organs.46,163 In a study using breast cancer CTCs, it was been found that CTC clusters display a 23- to 50-fold increase in metastatic potential. Moreover, high expression of the cell junction protein plakoglobin was identified as responsible for this intercellular adhesion.1 In addition to promoting invasiveness, it is reasonable that, in large cell aggregates, the cells within the core may be less accessible to nanomedicine approaches compared to those at the periphery.
Recently, ex vivo culture of breast cancer CTCs has enabled individualized testing of drug susceptibility.157 Although such a strategy is mainly utilized for the treatment of solid tumors, which are the origin of the CTCs expanded ex vivo, it remains unclear whether the drugs selected from such screening would be effective against CTCs in blood compared to the CTC cell lines expanded in culture medium. In light of several protective effects associated with the adhesion of platelets to CTCs in blood as discussed in the “Contribution of Platelets to CTC-Mediated Metastasis” section, platelet shielding may not only provide a physical barrier to nanomedicine-mediated drug delivery to CTCs, but also potentially confer drug resistance.
The systemic dissemination of CTCs defines a requirement that nanomedicine vehicles must exist in the circulation for an extended period of time to patrol for metastatic CTCs released from solid tumors. When conventional cancer drugs with systemic cytotoxicity such as doxorubicin and paclitaxel are utilized for CTC neutralization, they should ideally be released only after nanocarriers encapsulating the drugs have been internalized into CTCs to avoid off-target effects on normal tissues.136,162 In contrast, when more cancer-specific therapeutics such as monoclonal antibodies and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) are applied to target CTCs, such systemic toxicity may be less of a concern.70,126,143 In addition to their systemic nature, CTCs are reported to have a relatively short half-life (<24 h) in circulation.93,125,135 This provides a narrow time window for efficient killing of CTCs via nanomedicine approaches. A rational solution to this challenge is to design nanocarriers with extended circulation times. Recently, our group demonstrated for the first time that leukocytes coated with nanoscale TRAIL-liposomes were able to efficiently neutralize CTCs in an experimental mouse model of metastasis. This approach utilizes the long circulation time of leukocytes to deliver an apoptosis signal to cancer cells with minimal side effects on normal cells such as leukocytes and endothelium.95 An alternative strategy, however, is to deliver drugs that inhibit intravasation of cancer cells via enhanced tumor cell–matrix interactions.164
Potential Paradigms of Conventional Nanomedicine in CTC Neutralization
Despite the promise of utilizing synthetic compounds for blocking selectin-mediated CTC metastasis or inhibitors for blocking platelet–CTC interactions and signaling, these compounds are likely to be cleared from the body within a short time period via renal filtration due to their relatively low molecular weight (LMW).87 Therefore, frequent administration of these molecules is necessary and may become prohibitively expensive for clinical implementation. Nevertheless, the paradigms that have been exploited for nanoparticles and nanoformulations over the past few decades are potentially applicable to extend the circulation time of these LMW compounds. To date, two nanoformulations for treating solid tumors have been approved for clinical use, specifically liposomal doxorubicin (Doxil) and protein-bound paclitaxel (Abraxane).37 One major obstacle to using nanoparticles in vivo is rapid clearance by the mononuclear phagocyte system (MPS). The main strategy for extending the circulation time of nanoparticles is by grafting uncharged hydrophilic polymers onto the surface of particles to create so-called “stealth” particles, with the most commonly used polymer being polyethylene glycol (PEG).110,116,127 By encapsulating sLex analogs or fucosyltransferase inhibitors into nanoparticles with PEG-coated surfaces, this is likely to achieve both controlled drug release and extended circulation time. In addition to the intervention of selectin-mediated adhesion of CTCs to endothelium via ESL inhibitor-encapsulated nanoparticles, a coexisting or alternative adhesion event mediated by ICAM-1 (expressed on vascular endothelium) and MUC1 (expressed on some CTCs) has been shown as a potential target for the prevention of metastasis.117,121 The Decuzzi lab recently demonstrated that long circulating lipid–polymer nanoparticles encapsulating curcumin were able to substantially reduce the adhesion of highly metastatic human breast cancer cells MDA-MB-231 to TNF-α-treated HUVEC cells in an ICAM-1- and MUC1-dependent manner.111 It remains to be determined, however, whether such curcumin-encapsulated nanoparticles would be effective in mouse models with experimental and/or spontaneous metastases.
Alternatively, sLex or synthetic sLex analogs can be functionalized onto the surface of nanoparticles for targeting tumor-associated endothelium. For instance, sLex-conjugated liposomes loaded with cisplatin have been shown to accumulate on an E-selectin-expressing endothelium in the vicinity of tumor cells. It was found that sLex-conjugated liposomes enabled sixfold higher cisplatin accumulation than non-conjugated liposomes in tumors.45 Although this nanoformulation was intended to target solid tumors as opposed to CTCs, an additional benefit may have been to help neutralize CTCs that reseed into solid tumors.65 Bone-metastatic prostate cancer (PCa) cells bearing ESLs are known to attach more avidly to bone marrow (BM) endothelial cells which constitutively express E-selectin.14,86 Therefore, sLex-conjugated nanoparticles encapsulating cancer therapeutics can potentially deliver conventional cancer drugs to the BM niche of CTCs for the prevention and treatment of BM metastasis in prostate cancer.
Advanced Nanotechnologies in CTC Targeting
Effect of Nanoparticle Morphology on Their Fate in Blood Circulation
Many nanoparticle designs have been proposed for cancer therapy and diagnosis, with varied biodistribution in the blood circulation depending on their administration route, particle size, composition, and surface charge.18 In general, polymeric nanoparticles less than 10 nm in diameter may be easily cleared by the kidneys as blood carries them through the renal system, and particles greater than 100 nm may be easily cleared by phagocytic uptake and hepatic filtration.4 For the targeting of CTCs that extravasate from the vessel wall of the microvasculature, nanoparticles that exhibit the capability to adhere to the vascular wall may be more effective at delivering therapeutic cargo to CTCs.122 Gentile et al. 43 have shown that for spherical particles, large particles will have better margination compared to small particles that are less than 200 nm in diameter. Margination is a biorheological process in which semi-rigid cells and particles are displaced toward the vessel wall in circulation, providing more opportunities for interactions with the endothelium43 such as CTC intravasation and extravasation and blockade of these processes. The shape of nanoparticles is an important factor in determining their behavior in the blood circulation, where the adhesive interactions between particles and cells have to counteract the hemodynamic forces exerted by the flowing blood.29 These two counteracting forces will affect the particles’ targeting and attachment abilities within the microvasculature. Various shapes of nanoparticles, such as spherical, hemispherical, discoidal, cylindrical, conical, vase- and rod-shaped, have all been manufactured with emerging nanofabrication technologies and have demonstrated differential behaviors in flow.22,30
In flowing blood, a spherical morphology is suited for rotational motion,58 which is important for leukocytes that roll on and interact with the endothelium. In contrast to symmetric spherical particles (including cells), non-spherical particles may align or tumble under flow, with surprising transport properties. For example, red blood cells (RBCs), having a flexible biconcave disc shape with an average diameter of 8 μm,108 routinely pass through the reticular meshwork filtering units in the sinusoidal spleen in which the cell slit size rarely exceeds 200–500 nm in width, whereas spherical nanoparticles must be less than 200 nm in diameter to do so.22,99 In contrast to rigid spheres, biconcave discs are found to deform to other shapes such as parachute and slipper-like morphologies in response to changes in local flow velocity and shear stress, yet they retain the ability to recover their discoidal shape at reduced velocity.108 Shape flexibility and deformability allow RBCs to pass through vessels of different dimensions and narrow constrictions, making them an excellent vehicle for traveling in blood. Another important type of blood cell, platelets, normally exhibiting an oblate spheroidal shape, have been shown to assume different morphologies in vitro after prolonged exposure to adhesive surfaces.58 Activated platelets resemble spheres with a rough surface. The activated platelet shape greatly influences platelet collisions, including the frequency, contact time and available area of collision, as well as the magnitude of shear and normal forces acting on the cells.98
Inspired by nature’s adaptation of non-spherical particles for unique transport properties and cellular interactions in flowing blood, synthetic particles of various shapes have been developed and evaluated for drug delivery in recent years.29 For instance, Decuzzi et al. 30 injected silicon-based particles of quasi-hemispherical, cylindrical, and discoidal shapes into tumor-bearing mice and observed different distribution profiles among these particles. They further showed that discoidal particles can maximize accumulation in target organs while reducing sequestration by the liver. Geng et al. 42 prepared filomicelles, which are flexible filamentous vehicles that have been shown to effectively and efficiently deliver the anticancer drug paclitaxel to tumors in mice,20 that persist in circulation 10 times longer than their spherical counterpart. Gandra et al. 38 modified a filamentous bacteriophage and demonstrated its potential use as a biological nanowire to convey cargos of cancer-targeting peptides and photosensitizing agents. Bruckman et al. 19 prepared viral nanoparticles in the forms of rods and spheres, and observed that the nanorods circulated longer in the bloodstream of mice and were cleared from tissues more slowly compared to nanospheres. Theoretical and experimental calculations further confirmed the distinctive diffusion profiles of these two shapes in the tumor microenvironment.82 Carbon nanotubes (CNTs) have attracted much attention in drug delivery research due to their long circulation time and the efficient methodologies for chemical modification.17 For example, Yinghuai et al. 151 constructed water-soluble functionalized CNTs as delivery vehicles of cancer therapeutics—BNCT agents—which were found to be concentrated in tumor cells in mice.
These bio-inspired approaches exploiting non-spherical particles for tumor homing are directly applicable for the targeting of CTCs in the bloodstream. Elongated, rod-shaped and filamentous materials possess distinctive transport properties compared to spheres due to their enhanced flexibility and permeability. They also show improved margination toward the vessel wall,79,82 and are thus potentially more effective at accessing diseased vessels and CTCs.82 Moreover, the extended circulation lifetime of these particles can increase their probability of interaction with CTCs. Although non-spherical nanoformulations are promising anticancer drug delivery vehicles, they represent an emerging field that requires more extensive evaluations, including focus on mechanical properties, polydispersity, and stability of the carriers. In addition, other design parameters can have significant effects on vascular transport properties as well. For example, size and density are important in particle design. A fine balance among these three factors—shape, size and density—may allow for the design of particles with enhanced vascular interactions80 that are able to mimic biomolecules and cells, sensing and interacting with endothelial cells and CTCs in the blood circulation.
Biomimetic Strategies for Drug Delivery to CTCs
Red blood cells (RBCs), or erythrocytes, have been exploited as drug delivery vehicles since Inler et al.54 first created enzyme-loaded RBC ghosts in the early 1970s.104 Their work continues to inspire the design and engineering of biomimetic delivery systems today. Drug delivery vehicles derived from natural RBCs can be divided into four major classes:50 (1) carrier RBCs, which are natural RBC ghosts carrying therapeutic cargos; (2) synthetic RBC-mimicking particles, which are made of polymers that aim to simulate the mechanical and chemical properties of RBCs; (3) RBC membrane-derived liposomes, which are synthesized from native RBC membranes; and (4) RBC-membrane camouflaged nanoparticles (RBC-NPs), which are nanoparticles coated with native RBC membranes.
Although RBC derivatives have not been extensively evaluated in the context of CTCs, their unique biomimetic features suggest they are an excellent type of delivery vehicle for drugs that are intended to act in the bloodstream,104 and exhibit the potential to effectively target CTCs. Both RBCs and CTCs reside in the circulatory system, and can travel to different organs through blood vessels. Therefore, if bioengineered RBCs are capable of recognizing and eliminating CTCs in the vasculature before CTCs are able to extravasate, they could prevent cancer cells from colonizing secondary organs and reduce metastasis. In addition, human erythrocytes have a life span of 100–120 days,104 a circulation time much longer than that of nanoparticle drug carriers at present. Many types of synthetic nanoparticles sub-100 nm in diameter have a circulation half-life on the scale of hours, even after PEGylation.60 The longer blood circulation time of RBC derivatives enhances drug retention in the body and allows for sustained drug release,51 as well as increasing the vehicles’ interactions with CTCs.
Another property that makes RBC derivatives an excellent tool for targeting CTCs is their superior biocompatibility. The biocompatibility of nanoparticles is dictated by particle size, surface charge, hydrophobicity-hydrophilicity, as well as the steric effects of their outer coating.166 Many polymers used for nanoparticle stealth coating, such as PEG, create a hydrophilic shell on the particle surface, thus shielding the nanoparticles from immune recognition and decreasing their rate of elimination.55 In the early 2000s, however, different groups reported a phenomenon called “accelerated blood clearance”,26 in which repeated injections of PEGylated liposomes resulted in immune rejection in animal studies.26,55–57 This phenomenon raised concerns in the repeated administration of sterically stabilized nanoparticles for drug delivery. In contrast to PEGylation, which provides nanoparticles with an outer shell that attenuates immune recognition, RBC derivatives adopt a different mechanism by disguising as the “self”, which can potentially be more compatible with the immune system and avoid accelerated blood clearance. Residing in the same environment as macrophages and lymphocytes, RBCs evade the immune system by displaying self-antigens on their outer membrane.50,64,109 RBC derivatives that incorporate self-antigens, such as CD47,48 or a complete RBC membrane,39,50,51 have shown reduced immunogenicity compared to naked particles. For instance, Gao et al. 106 demonstrated a fourfold reduction in the uptake of gold nanoparticles (AuNP) coated with RBC membrane by macrophages in vitro.
Early studies have shown enhanced therapeutic efficacy and reduced immunogenicity in animal cancer models treated with erythrocyte-encapsulated antitumor drugs. In a study by Zocchi et al.,165 murine RBCs were subjected to hypotonic dialysis followed by doxorubicin encapsulation during membrane resealing. Compared to the non-encapsulated drug, mice treated with erythrocyte-encapsulated doxorubicin showed significant inhibition of metastatic growth in liver and lung at a much lower dosage. Doxorubicin encapsulated in RBCs was also administered to dogs with lymphosarcoma.91 This treatment achieved sustained drug release and induced complete and partial remissions of lymphosarcoma in dogs. Skorokhod et al. 133 administered doxorubicin-loaded erythrocytes to 15 lymphoma patients and reported improved pharmacokinetics compared to those of free doxorubicin, as well as good tolerance in cancer patients. The same research group has also studied the pharmacokinetics of daunorubicin-loaded erythrocytes in patients with acute leukemia132 and similar findings were reported, thus demonstrating the promising clinical applications of RBC delivery vehicles in treating blood cancers. Another drug used to treat acute leukemia, L-asparaginase, is one of the most widely studied enzymes for RBC encapsulation.94 Different groups have developed various methods for preparing asparaginase-loaded RBCs, which have been evaluated for their pharmacokinetics and antitumor activities in mice,7,71 dogs,31 monkeys139 and humans.72 These studies have demonstrated the advantages of using RBC derivatives for targeting CTCs in vivo.
To date, RBC derivatives have not been reported in the literature for targeting CTCs that are not of blood cancer origin. Nevertheless, studies involving leukemia or lymphoma animal models and human patients have corroborated the potential use of RBC carriers in targeting non-blood CTCs. Despite the attractive features of erythrocyte derivatives in targeted drug delivery, there are still many clinical challenges. Unlike animals, humans have many different blood groups. To make the technology more versatile for patients, the removal of immunogenic antigens is essential during drug synthesis.51 In addition, because of their biological origin, RBC derivatives are difficult and expensive to store. They also present great variability, which makes standardization and scale-up challenging.94 At present, there is no known receptor or ligand on the native RBC membrane that would allow RBC vehicles to interact with CTCs in the circulation. A potential solution is to create RBC derivatives carrying antibodies that would specifically recognize CTC surface antigens such as EpCAM, which is present in many types of CTCs. EpCAM expression on CTCs is also found to correlate with metastatic cancer prognosis.120,124 Conjugation of various molecules, including antibodies, to RBC membranes can be accomplished through a biotin-avidin linkage.50,103
In contrast to RBC biomimetic platforms that primarily seek to improve drug circulation time and biocompatibility, nanoparticles that mimic behaviors of platelets may not only extend the half-life of particles in blood but also bind CTCs with mechanisms similar to natural platelets. A platelet-mimetic approach for metastasis-targeted nanomedicine has been recently developed. In a study from the Gupta group,97 highly metastatic human breast cancer cells MDA-MB-231 were examined for surface expression of platelet-interactive receptors, which were then compared to a weakly metastatic human breast cancer cell line, MCF-7. Interestingly, certain platelet-interactive receptors were found to be significantly overexpressed on the surface of MDA-MB-231 cells such as GPIIb-IIIa-like receptors (which can bind to platelets mediated by fibrinogen), P-selectin (which can bind to platelets mediated by sialoprotein ligands), GPIa-IIa-like receptors (which can bind to platelets mediated by collagen-like molecules), E-selectin (which can bind to sialyl Lewis moieties), integrin αVβ3 (which can bind to fibronectin, vitronectin, etc.), and GPIbα-like proteins (which can bind to von Willebrand factor). In contrast, these receptors were weakly expressed in MCF-7 cells. More importantly, MDA-MB-231 cells showed significantly enhanced binding interactions with active platelets compared to MCF-7 cells. In light of these differences, two specific receptors were selected (GPIIb-IIIa-like integrin and P-selectin), and their corresponding ligands were engineered onto the surface of liposomes to enable platelet-mimetic binding to the cancer cells under physiological flow conditions. Nevertheless, it remains to be answered whether such a platelet-mimetic approach could target real CTCs in a patient’s blood. To address this question, both a mouse metastatic cancer model and patient-derived blood containing CTCs will need to be tested. In addition, the in vivo biodistribution and circulation time of such nanomedicines must be measured. Despite the uncertainties that remain to be addressed, this represents the first paradigm for targeting metastatic CTCs though interactions with platelets.
Integrating CTC Isolation Technologies with Nanomedicine
Enumeration of CTCs in the peripheral blood of cancer patients has shown promise for the diagnosis and monitoring of cancer progression, and it serves as an alternative to conventional imaging methods.61,96,154 CTCs of epithelial origin are defined as being positive for epithelial cell adhesion molecule (EpCAM+) and cytokeratin 8, 18, or 19 (CK+), and negative for CD45 (CD45−).6 Therefore, approaches for isolating such CTCs are largely based on the positive selection for epithelial markers.25,138 The existence of EpCAM-negative CTCs (i.e., CTCs with EMT signature), necessitates approaches such as negative depletion to select for CD45− cells.33 Alternatively, label-independent enrichment methods that are based on size and/or density differences between cancer cells and blood cells have also been developed for CTC enrichment.112,142 In addition to cell heterogeneity, the rarity of CTCs in blood relative to white blood cells has made their detection and isolation even more challenging.5,10,47 To solve this problem, nanomaterials have been developed to enable high-density coatings of different capture molecules such as antibodies for improved sensitivity of CTC detection.148 Recently, silicon nanopillars,144 quartz nanowires81 and TiO2 nanofibers159 have been used to trap CTCs, with enhanced capture efficiency due to the higher aspect ratio of the nanomaterials. Moreover, when such nanomaterial-based platforms have been integrated with flow-based systems such as microfluidic devices, a significant increase in capture yield is observed via continuous flow of patient blood through the devices.32,105,153
In contrast to the rapid development of CTC isolation technologies, few studies have been performed to enable drug delivery through CTC enrichment devices. It is conceivable that anti-EpCAM-conjugated nanoparticles loaded with cancer drugs could be utilized for targeting CTCs of epithelial state in blood. Moreover, by combining high capture efficiency of 3D nanotopographic features such as silicon nanowires with enhanced drug encapsulation for nanoporous materials, novel nanomedicines can possibly target a subpopulation of CTCs with CSC properties or multi-drug resistance.67,113,128 In addition, since mouse models and clinical observations have provided evidence that CTC count correlates with disease progression in cancer patients,28,65,154,160 it is intriguing that an implanted shunt system with similar nanostructure and surface functionalization could be utilized to filter out rare CTCs from the blood circulation. As a proof-of-concept, our group invented a biomimetic approach to capture and kill CTCs in vitro. In this system, a microfluidic device was functionalized with E-selectin, which interacts with CTCs during extravasation through the endothelium. Additionally, the surface was coated with a tumor-specific cytokine, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), for inducing apoptosis when CTCs were captured from the flow.118,119 More recently, the device was further functionalized with naturally occurring nontoxic halloysite nanotubes for the enhanced capture of CTCs.52,53 Nevertheless, several issues need to be addressed to demonstrate the efficacy of this approach in vivo. First, the device must be compatible with the body. As E-selectin and TRAIL are expressed in host cells, it is unlikely that they will induce an immune response. It requires, however, further examination on whether the material that comprises the device and additional nanostructured surface would cause any side effects when interfacing with blood.131 Secondly, it is necessary to test the isolation efficiency of CTCs in the presence of abundant plasma proteins in the blood. It has been found that owing to high surface free energy, certain nanomaterials adsorb biomolecules upon contact with biological fluids. In particular, plasma proteins may bind to the nanostructured surface to form a biological coating, known as the protein corona.101,137 This corona may affect the interaction of the device with the host system.134 Lastly, it is crucial to evaluate when the device surface becomes saturated with CTCs and requires replacement. Nevertheless, such biomimetic approaches for the delivery of apoptotic signals represents an intriguing proof of concept for future integration of CTC isolation technologies with nanomedicines.
Conclusion
In the past decade, the presence of CTCs has been utilized as an indicator of poor prognosis in several carcinomas. The difficulty of identifying these rare cells in the blood has driven the development of numerous devices for the isolation and characterization of CTCs in clinical settings. It is not until recently, however, that the presence of metastasis-initiating cells (MICs) among CTCs has been experimentally demonstrated. Therefore, neutralizing MICs or CTCs in the blood may represent a new paradigm for the intervention of metastases in distant organs. In contrast to conventional nanomedicines, which extend the half life of chemotherapeutics in the blood, novel approaches that are inspired by the context of CTCs in circulation have led to a variety of biomimetic nanoparticle or nanoformulation platforms. Integration of nanotechnologies with a deeper understanding of diverse CTC–host cell interactions may offer exciting and promising directions for novel therapeutic interventions in the future.
Acknowledgments
This work was supported by NIH Grant No. CA143876 to M.R.K.
Conflict of interest
Jiahe Li, Charles Sharkey, Dantong Huang and Michael King declare that they have no conflict of interest.
Ethical Standards
No human subjects research was carried out by the authors for this article. No animal studies were carried out by the authors for this article.
References
- 1.Aceto N, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158(5):1110–1122. doi: 10.1016/j.cell.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed N, et al. Epithelial mesenchymal transition and cancer stem cell-like phenotypes facilitate chemoresistance in recurrent ovarian cancer. Curr. Cancer Drug Targets. 2010;10(3):268–278. doi: 10.2174/156800910791190175. [DOI] [PubMed] [Google Scholar]
- 3.Aktas B, et al. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res. 2009;11(4):R46. doi: 10.1186/bcr2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexis F, et al. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008;5(4):505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alix-Panabieres C, Schwarzenbach H, Pantel K. Circulating tumor cells and circulating tumor DNA. Annu. Rev. Med. 2012;63:199–215. doi: 10.1146/annurev-med-062310-094219. [DOI] [PubMed] [Google Scholar]
- 6.Allard WJ, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin. Cancer Res. 2004;10(20):6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 7.Alpar H, Lewis D. Therapeutic efficacy of asparaginase encapsulated in intact erythrocytes. Biochem. Pharmacol. 1985;34(2):257–261. doi: 10.1016/0006-2952(85)90133-9. [DOI] [PubMed] [Google Scholar]
- 8.Amirkhosravi A, et al. Tissue factor pathway inhibitor reduces experimental lung metastasis of B16 melanoma. Thromb. Haemost. 2002;87(6):930–936. [PubMed] [Google Scholar]
- 9.Amirkhosravi A, et al. The role of tissue factor pathway inhibitor in tumor growth and metastasis. Semin. Thromb. Hemost. 2007;33(7):643–652. doi: 10.1055/s-2007-991531. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong AJ, et al. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol. Cancer Res. 2011;9(8):997–1007. doi: 10.1158/1541-7786.MCR-10-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baccelli I, et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat. Biotechnol. 2013;31(6):539–544. doi: 10.1038/nbt.2576. [DOI] [PubMed] [Google Scholar]
- 12.Balasubramanian P, et al. Multiparameter analysis, including EMT markers, on negatively enriched blood samples from patients with squamous cell carcinoma of the head and neck. PLoS One. 2012;7(7):e42048. doi: 10.1371/journal.pone.0042048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnes JM, Nauseef JT, Henry MD. Resistance to fluid shear stress is a conserved biophysical property of malignant cells. PLoS One. 2012;7(12):e50973. doi: 10.1371/journal.pone.0050973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barthel SR, et al. Analysis of glycosyltransferase expression in metastatic prostate cancer cells capable of rolling activity on microvascular endothelial (E)-selectin. Glycobiology. 2008;18(10):806–817. doi: 10.1093/glycob/cwn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barthel SR, et al. Alpha 1,3 fucosyltransferases are master regulators of prostate cancer cell trafficking. Proc. Natl. Acad. Sci. U.S.A. 2009;106(46):19491–19496. doi: 10.1073/pnas.0906074106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertrand N, et al. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014;66:2–25. doi: 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bianco A, Kostarelos K, Prato M. Applications of carbon nanotubes in drug delivery. Curr. Opin. Chem. Biol. 2005;9(6):674–679. doi: 10.1016/j.cbpa.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 2002;54(5):631–651. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 19.Bruckman MA, et al. Biodistribution, pharmacokinetics, and blood compatibility of native and PEGylated tobacco mosaic virus nano-rods and -spheres in mice. Virology. 2014;449:163–173. doi: 10.1016/j.virol.2013.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai S, et al. Micelles of different morphologies—advantages of worm-like filomicelles of PEO-PCL in paclitaxel delivery. Pharm. Res. 2007;24(11):2099–2109. doi: 10.1007/s11095-007-9335-z. [DOI] [PubMed] [Google Scholar]
- 21.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer. 2002;2(8):563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 22.Champion JA, Katare YK, Mitragotri S. Particle shape: a new design parameter for micro- and nanoscale drug delivery carriers. J. Control Release. 2007;121(1–2):3–9. doi: 10.1016/j.jconrel.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chauhan VP, Jain RK. Strategies for advancing cancer nanomedicine. Nat. Mater. 2013;12(11):958–962. doi: 10.1038/nmat3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho EH, et al. Characterization of circulating tumor cell aggregates identified in patients with epithelial tumors. Phys. Biol. 2012;9(1):016001. doi: 10.1088/1478-3975/9/1/016001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cristofanilli M, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 2004;351(8):781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 26.Dams E, et al. Accelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomes. JPET. 2000;292(3):1071–1079. [PubMed] [Google Scholar]
- 27.Dawidczyk CM, et al. State-of-the-art in design rules for drug delivery platforms: lessons learned from FDA-approved nanomedicines. J. Control Release. 2014;187C:133–144. doi: 10.1016/j.jconrel.2014.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Bono JS, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2008;14(19):6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 29.Decuzzi P, Ferrari M. The adhesive strength of non-spherical particles mediated by specific interactions. Biomaterials. 2006;27(30):5307–5314. doi: 10.1016/j.biomaterials.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 30.Decuzzi P, et al. Size and shape effects in the biodistribution of intravascularly injected particles. J. Control Release. 2010;141(3):320–327. doi: 10.1016/j.jconrel.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 31.DeLoach JR, et al. Intraperitoneal administration of carrier erythrocytes in dogs: an improved method for delivery of L-asparaginase. Biotechnol. Appl. Biochem. 1990;12(3):331–335. [PubMed] [Google Scholar]
- 32.den Toonder J. Circulating tumor cells: the grand challenge. Lab Chip. 2011;11(3):375–377. doi: 10.1039/c0lc90100h. [DOI] [PubMed] [Google Scholar]
- 33.Deneve E, et al. Capture of viable circulating tumor cells in the liver of colorectal cancer patients. Clin. Chem. 2013;59(9):1384–1392. doi: 10.1373/clinchem.2013.202846. [DOI] [PubMed] [Google Scholar]
- 34.Earhart CM, et al. Isolation and mutational analysis of circulating tumor cells from lung cancer patients with magnetic sifters and biochips. Lab Chip. 2014;14(1):78–88. doi: 10.1039/c3lc50580d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Egan K, et al. Platelet adhesion and degranulation induce pro-survival and pro-angiogenic signalling in ovarian cancer cells. PLoS One. 2011;6(10):e26125. doi: 10.1371/journal.pone.0026125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frisch SM, Schaller M, Cieply B. Mechanisms that link the oncogenic epithelial-mesenchymal transition to suppression of anoikis. J. Cell Sci. 2013;126(Pt 1):21–29. doi: 10.1242/jcs.120907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaitanis A, Staal S. Liposomal doxorubicin and nab-paclitaxel: nanoparticle cancer chemotherapy in current clinical use. Cancer Nanotechnol. Methods Protoc. 2010;624:385–392. doi: 10.1007/978-1-60761-609-2_26. [DOI] [PubMed] [Google Scholar]
- 38.Gandra N, et al. Bacteriophage bionanowire as a carrier for both cancer-targeting peptides and photosensitizers and its use in selective cancer cell killing by photodynamic therapy. Small. 2013;9(2):215–221. doi: 10.1002/smll.201202090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao W, et al. Surface functionalization of gold nanoparticles with red blood cell membranes. Adv. Mater. 2013;25(26):3549–3553. doi: 10.1002/adma.201300638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat. Rev. Cancer. 2011;11(2):123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geng Y, Marshall JR, King MR. Glycomechanics of the metastatic cascade: tumor cell-endothelial cell interactions in the circulation. Ann. Biomed. Eng. 2012;40(4):790–805. doi: 10.1007/s10439-011-0463-6. [DOI] [PubMed] [Google Scholar]
- 42.Geng Y, et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat. Nanotechnol. 2007;2(4):249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gentile F, et al. The margination propensity of spherical particles for vascular targeting in the microcirculation. J. Nanobiotechnol. 2008;6:9. doi: 10.1186/1477-3155-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grailer JJ, Kodera M, Steeber DA. L-selectin: role in regulating homeostasis and cutaneous inflammation. J. Dermatol. Sci. 2009;56(3):141–147. doi: 10.1016/j.jdermsci.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirai M, et al. Novel and simple loading procedure of cisplatin into liposomes and targeting tumor endothelial cells. Int. J. Pharm. 2010;391(1–2):274–283. doi: 10.1016/j.ijpharm.2010.02.030. [DOI] [PubMed] [Google Scholar]
- 46.Hou JM, et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J. Clin. Oncol. 2012;30(5):525–532. doi: 10.1200/JCO.2010.33.3716. [DOI] [PubMed] [Google Scholar]
- 47.Hou HW, et al. Isolation and retrieval of circulating tumor cells using centrifugal forces. Sci. Rep. 2013;3:1259. doi: 10.1038/srep01259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hsu Y, et al. Reduced phagocytosis of colloidal carriers using soluble CD47. Pharm. Res. 2003;20(10):1539–1542. doi: 10.1023/a:1026114713035. [DOI] [PubMed] [Google Scholar]
- 49.Hsu JW, et al. Suppression of prostate cancer cell rolling and adhesion to endothelium by 1 alpha, 25-dihydroxyvitamin D-3. Am. J. Pathol. 2011;178(2):872–880. doi: 10.1016/j.ajpath.2010.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu CM, Fang RH, Zhang L. Erythrocyte-inspired delivery systems. Adv. Healthc. Mater. 2012;1(5):537–547. doi: 10.1002/adhm.201200138. [DOI] [PubMed] [Google Scholar]
- 51.Hu CM, et al. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. USA. 2011;108(27):10980–10985. doi: 10.1073/pnas.1106634108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hughes AD, King MR. Use of naturally occurring halloysite nanotubes for enhanced capture of flowing cells. Langmuir. 2010;26(14):12155–12164. doi: 10.1021/la101179y. [DOI] [PubMed] [Google Scholar]
- 53.Hughes AD, et al. Microtube device for selectin-mediated capture of viable circulating tumor cells from blood. Clin. Chem. 2012;58(5):846–853. doi: 10.1373/clinchem.2011.176669. [DOI] [PubMed] [Google Scholar]
- 54.Ihler G, Glew R, Schnure F. Enzyme loading of erythrocytes. Proc. Nat. Acad. Sci. 1973;70(9):2663–2666. doi: 10.1073/pnas.70.9.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ishida T, Kiwada H. Accelerated blood clearance (ABC) phenomenon upon repeated injection of PEGylated liposomes. Int. J. Pharm. 2008;354(1–2):56–62. doi: 10.1016/j.ijpharm.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 56.Ishida T, et al. Accelerated clearance of PEGylated liposomes in rats after repeated injections. J. Controlled Release. 2003;88(1):35–42. doi: 10.1016/s0168-3659(02)00462-5. [DOI] [PubMed] [Google Scholar]
- 57.Ishida T, et al. PEGylated liposomes elicit an anti-PEG IgM response in a T cell-independent manner. J. Control Release. 2007;122(3):349–355. doi: 10.1016/j.jconrel.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 58.Jackson SP. The growing complexity of platelet aggregation. Blood. 2007;109:5087–5095. doi: 10.1182/blood-2006-12-027698. [DOI] [PubMed] [Google Scholar]
- 59.Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J. Clin. Oncol. 2013;31(17):2205–2218. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jokerst J, et al. Nanoparticle PEGylation for imaging and therapy. Nanomedicine. 2011;6(4):715–728. doi: 10.2217/nnm.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Joosse SA, Gorges TM, Pantel K. Biology, detection, and clinical implications of circulating tumor cells. EMBO Mol. Med. 2014;7(1):1–11. doi: 10.15252/emmm.201303698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat. Rev. Cancer. 2009;9(4):239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009;119(6):1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim DD, Song WC. Membrane complement regulatory proteins. Clin. Immunol. 2006;118(2–3):127–136. doi: 10.1016/j.clim.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 65.Kim MY, et al. Tumor self-seeding by circulating cancer cells. Cell. 2009;139(7):1315–1326. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim H, et al. Development of on-chip multi-imaging flow cytometry for identification of imaging biomarkers of clustered circulating tumor cells. PLoS One. 2014;9(8):e104372. doi: 10.1371/journal.pone.0104372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim DJ, et al. Drug response of captured BT20 cells and evaluation of circulating tumor cells on a silicon nanowire platform. Biosens. Bioelectron. 2014;67:370–378. doi: 10.1016/j.bios.2014.08.057. [DOI] [PubMed] [Google Scholar]
- 68.Kohler S, et al. E-/P-selectins and colon carcinoma metastasis: first in vivo evidence for their crucial role in a clinically relevant model of spontaneous metastasis formation in the lung. Br. J. Cancer. 2010;102(3):602–609. doi: 10.1038/sj.bjc.6605492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Konstantopoulos K, Thomas SN. Cancer cells in transit: the vascular interactions of tumor cells. Annu. Rev. Biomed. Eng. 2009;11:177–202. doi: 10.1146/annurev-bioeng-061008-124949. [DOI] [PubMed] [Google Scholar]
- 70.Koschny R, Walczak H, Ganten TM. The promise of TRAIL—potential and risks of a novel anticancer therapy. J. Mol. Med. (Berl.) 2007;85(9):923–935. doi: 10.1007/s00109-007-0194-1. [DOI] [PubMed] [Google Scholar]
- 71.Kravtzoff R, et al. Erythrocytes as carriers for L-asparaginase. Methodological and mouse in vivo studies. J. Pharm. Pharmacol. 1990;42:473–476. doi: 10.1111/j.2042-7158.1990.tb06598.x. [DOI] [PubMed] [Google Scholar]
- 72.Kravtzoff R, et al. Improved pharmacodynamics of L-asparaginase-loaded in human red blood cells. Eur. J. Clin. Pharmacol. 1996;49(6):465–470. doi: 10.1007/BF00195932. [DOI] [PubMed] [Google Scholar]
- 73.Ksiazkiewicz M, Markiewicz A, Zaczek AJ. Epithelial-mesenchymal transition: a hallmark in metastasis formation linking circulating tumor cells and cancer stem cells. Pathobiology. 2012;79(4):195–208. doi: 10.1159/000337106. [DOI] [PubMed] [Google Scholar]
- 74.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20(5):576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Labelle M, Begum S, Hynes RO. Platelets guide the formation of early metastatic niches. Proc. Natl. Acad. Sci. U.S.A. 2014;111(30):E3053–E3061. doi: 10.1073/pnas.1411082111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Labelle M, Hynes RO. The initial hours of metastasis: the importance of cooperative host-tumor cell interactions during hematogenous dissemination. Cancer Discov. 2012;2(12):1091–1099. doi: 10.1158/2159-8290.CD-12-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lanier LL. NK cell recognition. Annu. Rev. Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 78.Lecharpentier A, et al. Detection of circulating tumour cells with a hybrid (epithelial/mesenchymal) phenotype in patients with metastatic non-small cell lung cancer. Br. J. Cancer. 2011;105(9):1338–1341. doi: 10.1038/bjc.2011.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee SY, Ferrari M, Decuzzi P. Shaping nano-/micro-particles for enhanced vascular interaction in laminar flows. Nanotechnology. 2009;20(49):495101. doi: 10.1088/0957-4484/20/49/495101. [DOI] [PubMed] [Google Scholar]
- 80.Lee SY, Ferrari M, Decuzzi P. Design of bio-mimetic particles with enhanced vascular interaction. J. Biomech. 2009;42(12):1885–1890. doi: 10.1016/j.jbiomech.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 81.Lee SK, et al. Nanowire substrate-based laser scanning cytometry for quantitation of circulating tumor cells. Nano Lett. 2012;12(6):2697–2704. doi: 10.1021/nl2041707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee KL, et al. Shape matters: the diffusion rates of TMV rods and CPMV icosahedrons in a spheroid model of extracellular matrix are distinct. Biomater. Sci. 2013;1(6):581–588. doi: 10.1039/C3BM00191A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ley K. The role of selectins in inflammation and disease. Trends Mol. Med. 2003;9(6):263–268. doi: 10.1016/s1471-4914(03)00071-6. [DOI] [PubMed] [Google Scholar]
- 84.Li J, King MR. Adhesion receptors as therapeutic targets for circulating tumor cells. Front. Oncol. 2012;2:79. doi: 10.3389/fonc.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li YM, et al. Epithelial-mesenchymal transition markers expressed in circulating tumor cells in hepatocellular carcinoma patients with different stages of disease. Cell Death Dis. 2013;4:e831. doi: 10.1038/cddis.2013.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li J, et al. Human fucosyltransferase 6 enables prostate cancer metastasis to bone. Br. J. Cancer. 2013;109(12):3014–3022. doi: 10.1038/bjc.2013.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Livney YD, Assaraf YG. Rationally designed nanovehicles to overcome cancer chemoresistance. Adv. Drug Deliv. Rev. 2013;65(13–14):1716–1730. doi: 10.1016/j.addr.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 88.Lohr JG, et al. Whole-exome sequencing of circulating tumor cells provides a window into metastatic prostate cancer. Nat. Biotechnol. 2014;32(5):479–484. doi: 10.1038/nbt.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Luo X, et al. Isolation and molecular characterization of circulating melanoma cells. Cell Rep. 2014;7(3):645–653. doi: 10.1016/j.celrep.2014.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Matherne CM, et al. Clinical efficacy and toxicity of doxorubicin encapsulated in glutaraldehyde-treated erythrocytes administered to dogs with lymphosarcoma. Am. J. Vet. Res. 1994;55(6):847–853. [PubMed] [Google Scholar]
- 92.Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat. Rev. Cancer. 2006;6(6):449–458. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- 93.Meng S, et al. Circulating tumor cells in patients with breast cancer dormancy. Clin. Cancer Res. 2004;10(24):8152–8162. doi: 10.1158/1078-0432.CCR-04-1110. [DOI] [PubMed] [Google Scholar]
- 94.Millan CG, et al. Drug, enzyme and peptide delivery using erythrocytes as carriers. J. Control Release. 2004;95(1):27–49. doi: 10.1016/j.jconrel.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 95.Mitchell MJ, et al. TRAIL-coated leukocytes that kill cancer cells in the circulation. Proc. Natl. Acad. Sci. USA. 2014;111(3):930–935. doi: 10.1073/pnas.1316312111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miyamoto DT, Sequist LV, Lee RJ. Circulating tumour cells-monitoring treatment response in prostate cancer. Nat. Rev. Clin. Oncol. 2014;11(7):401–412. doi: 10.1038/nrclinonc.2014.82. [DOI] [PubMed] [Google Scholar]
- 97.Modery-Pawlowski CL, et al. A platelet-mimetic paradigm for metastasis-targeted nanomedicine platforms. Biomacromolecules. 2013;14(3):910–919. doi: 10.1021/bm301996p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mody NA, King MR. Platelet adhesive dynamics. Part I: characterization of platelet hydrodynamic collisions and wall effects. Biophys. J . 2008;95(5):2539–2555. doi: 10.1529/biophysj.107.127670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol. Rev. 2001;53(2):283–318. [PubMed] [Google Scholar]
- 100.Mohammad KS, et al. TGF-beta-RI kinase inhibitor SD-208 reduces the development and progression of melanoma bone metastases. Cancer Res. 2011;71(1):175–184. doi: 10.1158/0008-5472.CAN-10-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Monopoli MP, et al. Physical-chemical aspects of protein corona: relevance to in vitro and in vivo biological impacts of nanoparticles. J. Am. Chem. Soc. 2011;133(8):2525–2534. doi: 10.1021/ja107583h. [DOI] [PubMed] [Google Scholar]
- 102.Moretta L, Moretta A. Unravelling natural killer cell function: triggering and inhibitory human NK receptors. EMBO J. 2004;23(2):255–259. doi: 10.1038/sj.emboj.7600019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Muzykantov V. Avidin/biotin-mediated conjugation of antibodies to erythrocytes: an approach for in vivo immunoerythrocyte exploration. In: Meier T, Fahrenholz F, editors. A laboratory guide to biotin-labeling in biomolecule analysis. Basel: Birkhäuser; 1996. pp. 167–182. [Google Scholar]
- 104.Muzykantov VR. Drug delivery by red blood cells: vascular carriers designed by mother nature. Expert Opin. Drug Deliv. 2010;7(4):403–427. doi: 10.1517/17425241003610633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nagrath S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450(7173):1235–1239. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nieswandt B, et al. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59(6):1295–1300. [PubMed] [Google Scholar]
- 107.Nieto MA. Epithelial plasticity: a common theme in embryonic and cancer cells. Science. 2013;342(6159):1234850. doi: 10.1126/science.1234850. [DOI] [PubMed] [Google Scholar]
- 108.Noguchi H, Gompper G. Shape transitions of fluid vesicles and red blood cells in capillary flows. Proc. Natl. Acad. Sci. USA. 2005;102(40):14159–14164. doi: 10.1073/pnas.0504243102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Oldenborg PA. Role of CD47 as a marker of self on red blood cells. Science. 2000;288(5473):2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 110.Onyskiw PJ, Eniola-Adefeso O. Effect of PEGylation on ligand-based targeting of drug carriers to the vascular wall in blood flow. Langmuir. 2013;29(35):11127–11134. doi: 10.1021/la402182j. [DOI] [PubMed] [Google Scholar]
- 111.Palange AL, et al. Lipid-polymer nanoparticles encapsulating curcumin for modulating the vascular deposition of breast cancer cells. Nanomed. Nanotechnol. Biol. Med. 2014;10(5):991–1002. doi: 10.1016/j.nano.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Park JM, et al. Highly efficient assay of circulating tumor cells by selective sedimentation with a density gradient medium and microfiltration from whole blood. Anal. Chem. 2012;84(17):7400–7407. doi: 10.1021/ac3011704. [DOI] [PubMed] [Google Scholar]
- 113.Peng F, et al. Doxorubicin-loaded silicon nanowires for the treatment of drug-resistant cancer cells. Biomaterials. 2014;35(19):5188–5195. doi: 10.1016/j.biomaterials.2014.03.032. [DOI] [PubMed] [Google Scholar]
- 114.Placke T, et al. Platelet-derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumor reactivity of natural killer immune cells. Cancer Res. 2012;72(2):440–448. doi: 10.1158/0008-5472.CAN-11-1872. [DOI] [PubMed] [Google Scholar]
- 115.Radisky DC. Epithelial-mesenchymal transition. J. Cell Sci. 2005;118(Pt 19):4325–4326. doi: 10.1242/jcs.02552. [DOI] [PubMed] [Google Scholar]
- 116.Raemdonck K, et al. Merging the best of both worlds: hybrid lipid-enveloped matrix nanocomposites in drug delivery. Chem. Soc. Rev. 2014;43(1):444–472. doi: 10.1039/c3cs60299k. [DOI] [PubMed] [Google Scholar]
- 117.Rahn J, et al. MUC1 mediates transendothelial migration in vitro by ligating endothelial cell ICAM-1. Clin. Exp. Metastasis. 2005;22(6):475–483. doi: 10.1007/s10585-005-3098-x. [DOI] [PubMed] [Google Scholar]
- 118.Rana K, Liesveld JL, King MR. Delivery of apoptotic signal to rolling cancer cells: a novel biomimetic technique using immobilized TRAIL and E-selectin. Biotechnol. Bioeng. 2009;102(6):1692–1702. doi: 10.1002/bit.22204. [DOI] [PubMed] [Google Scholar]
- 119.Rana K, Reinhart-King CA, King MR. Inducing apoptosis in rolling cancer cells: a combined therapy with aspirin and immobilized TRAIL and E-selectin. Mol. Pharm. 2012;9(8):2219–2227. doi: 10.1021/mp300073j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rao CG, et al. Expression of epithelial cell adhesion molecule in carcinoma cells present in blood and primary and metastatic tumors. Int. J. Oncol. 2005;27(1):49–57. [PubMed] [Google Scholar]
- 121.Regimbald LH, et al. The breast mucin MUC1 as a novel adhesion ligand for endothelial intercellular adhesion molecule 1 in breast cancer. Cancer Res. 1996;56(18):4244–4249. [PubMed] [Google Scholar]
- 122.Ruenraroengsak P, Cook JM, Florence AT. Nanosystem drug targeting: facing up to complex realities. J Control Release. 2010;141(3):265–276. doi: 10.1016/j.jconrel.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 123.Schmidt S, Moser M, Sperandio M. The molecular basis of leukocyte recruitment and its deficiencies. Mol. Immunol. 2013;55(1):49–58. doi: 10.1016/j.molimm.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 124.Schulze K, et al. Presence of EpCAM-positive circulating tumor cells as biomarker for systemic disease strongly correlates to survival in patients with hepatocellular carcinoma. Int. J. Cancer. 2013;133(9):2165–2171. doi: 10.1002/ijc.28230. [DOI] [PubMed] [Google Scholar]
- 125.Schwarzenbach H, et al. Comparative evaluation of cell-free tumor DNA in blood and disseminated tumor cells in bone marrow of patients with primary breast cancer. Breast Cancer Res. 2009;11(5):R71. doi: 10.1186/bcr2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat. Rev. Cancer. 2012;12(4):278–287. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 127.Shapira A, et al. Nanomedicine for targeted cancer therapy: towards the overcoming of drug resistance. Drug Resist. Updates. 2011;14(3):150–163. doi: 10.1016/j.drup.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 128.Shen J, et al. High capacity nanoporous silicon carrier for systemic delivery of gene silencing therapeutics. ACS Nano. 2013;7(11):9867–9880. doi: 10.1021/nn4035316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sheth RA, et al. Barriers to drug delivery in interventional oncology. J. Vasc. Interv. Radiol. 2013;24(8):1201–1207. doi: 10.1016/j.jvir.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 130.Shirota K, et al. Anti-metastatic effect of the sialyl Lewis-X analog GSC-150 on the human colon carcinoma derived cell line KM12-HX in the mouse. Biol. Pharm. Bull. 2001;24(3):316–319. doi: 10.1248/bpb.24.316. [DOI] [PubMed] [Google Scholar]
- 131.Singh S, Nalwa HS. Nanotechnology and health safety–toxicity and risk assessments of nanostructured materials on human health. J. Nanosci. Nanotechnol. 2007;7(9):3048–3070. doi: 10.1166/jnn.2007.922. [DOI] [PubMed] [Google Scholar]
- 132.Skorokhod O, et al. Pharmacokinetics or erythrocyte-bound daunorubicin in patients with acute leukemia. Med. Sci. Monit. 2004;10(4):PI55–PI64. [PubMed] [Google Scholar]
- 133.Skorokhod O, et al. Doxorubicin pharmacokinetics in lymphoma patients treated with doxorubicin-loaded erythrocytes. Hematologica. 2007;92:570–571. doi: 10.3324/haematol.10770. [DOI] [PubMed] [Google Scholar]
- 134.Sobczynski DJ, et al. Plasma protein corona modulates the vascular wall interaction of drug carriers in a material and donor specific manner. PLoS One. 2014;9(9):e107408. doi: 10.1371/journal.pone.0107408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stott SL, et al. Isolation and characterization of circulating tumor cells from patients with localized and metastatic prostate cancer. Sci. Transl. Med. 2010;2(25):25ra23. doi: 10.1126/scitranslmed.3000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013;65(2):157–170. doi: 10.1111/j.2042-7158.2012.01567.x. [DOI] [PubMed] [Google Scholar]
- 137.Tenzer S, et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013;8(10):772–781. doi: 10.1038/nnano.2013.181. [DOI] [PubMed] [Google Scholar]
- 138.Tewes M, et al. Molecular profiling and predictive value of circulating tumor cells in patients with metastatic breast cancer: an option for monitoring response to breast cancer related therapies. Breast Cancer Res. Treat. 2009;115(3):581–590. doi: 10.1007/s10549-008-0143-x. [DOI] [PubMed] [Google Scholar]
- 139.Updike SJ, Wakamiya RT. Infusion of red blood cell-loaded asparaginase in monkey. Immunologic, metabolic, and toxicologic consequences. J. Lab. Clin. Med. 1983;101(5):679–691. [PubMed] [Google Scholar]
- 140.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147(2):275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Varki A. Trousseau’s syndrome: multiple definitions and multiple mechanisms. Blood. 2007;110(6):1723–1729. doi: 10.1182/blood-2006-10-053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vona G, et al. Isolation by size of epithelial tumor cells: a new method for the immunomorphological and molecular characterization of circulatingtumor cells. Am. J. Pathol. 2000;156(1):57–63. doi: 10.1016/S0002-9440(10)64706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang S. The promise of cancer therapeutics targeting the TNF-related apoptosis-inducing ligand and TRAIL receptor pathway. Oncogene. 2008;27(48):6207–6215. doi: 10.1038/onc.2008.298. [DOI] [PubMed] [Google Scholar]
- 144.Wang S, et al. Three-dimensional nanostructured substrates toward efficient capture of circulating tumor cells. Angew. Chem. 2009;48(47):8970–8973. doi: 10.1002/anie.200901668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wenzel J, Zeisig R, Fichtner I. Inhibition of metastasis in a murine 4T1 breast cancer model by liposomes preventing tumor cell–platelet interactions. Clin. Exp. Metastasis. 2010;27(1):25–34. doi: 10.1007/s10585-009-9299-y. [DOI] [PubMed] [Google Scholar]
- 146.Winkler IG, et al. Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat. Med. 2012;18(11):1651–1657. doi: 10.1038/nm.2969. [DOI] [PubMed] [Google Scholar]
- 147.Wu QD, et al. Human neutrophils facilitate tumor cell transendothelial migration. Am. J. Physiol. Cell Physiol. 2001;280(4):C814–C822. doi: 10.1152/ajpcell.2001.280.4.C814. [DOI] [PubMed] [Google Scholar]
- 148.Xia XR, Monteiro-Riviere NA, Riviere JE. An index for characterization of nanomaterials in biological systems. Nat. Nanotechnol. 2010;5(9):671–675. doi: 10.1038/nnano.2010.164. [DOI] [PubMed] [Google Scholar]
- 149.Yao X, et al. Functional analysis of single cells identifies a rare subset of circulating tumor cells with malignant traits. Integr. Biol. 2014;6(4):388–398. doi: 10.1039/c3ib40264a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yin X, et al. Knockdown of fucosyltransferase III disrupts the adhesion of circulating cancer cells to E-selectin without affecting hematopoietic cell adhesion. Carbohydr. Res. 2010;345(16):2334–2342. doi: 10.1016/j.carres.2010.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yinghuai Z, et al. Substituted carborane-appended water-soluble single-wall carbon nanotubes: new approach to boron neutron capture therapy drug delivery. J. Am. Chem. Soc. 2005;127(27):9875–9880. doi: 10.1021/ja0517116. [DOI] [PubMed] [Google Scholar]
- 152.Yokobori T, et al. Plastin3 is a novel marker for circulating tumor cells undergoing the epithelial-mesenchymal transition and is associated with colorectal cancer prognosis. Cancer Res. 2013;73(7):2059–2069. doi: 10.1158/0008-5472.CAN-12-0326. [DOI] [PubMed] [Google Scholar]
- 153.Yoon HJ, et al. Sensitive capture of circulating tumour cells by functionalized graphene oxide nanosheets. Nat. Nanotechnol. 2013;8(10):735–741. doi: 10.1038/nnano.2013.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yu M, et al. Circulating tumor cells: approaches to isolation and characterization. J. Cell Biol. 2011;192(3):373–382. doi: 10.1083/jcb.201010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yu M, et al. RNA sequencing of pancreatic circulating tumour cells implicates WNT signalling in metastasis. Nature. 2012;487(7408):510–513. doi: 10.1038/nature11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yu M, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339(6119):580–584. doi: 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yu M, et al. Cancer therapy. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science. 2014;345(6193):216–220. doi: 10.1126/science.1253533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zarbock A, et al. Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood. 2011;118(26):6743–6751. doi: 10.1182/blood-2011-07-343566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang N, et al. Electrospun TiO2 nanofiber-based cell capture assay for detecting circulating tumor cells from colorectal and gastric cancer patients. Adv. Mater. 2012;24(20):2756–2760. doi: 10.1002/adma.201200155. [DOI] [PubMed] [Google Scholar]
- 160.Zhang L, et al. Meta-analysis of the prognostic value of circulating tumor cells in breast cancer. Clin. Cancer Res. 2012;18(20):5701–5710. doi: 10.1158/1078-0432.CCR-12-1587. [DOI] [PubMed] [Google Scholar]
- 161.Zhang L, et al. The identification and characterization of breast cancer CTCs competent for brain metastasis. Sci. Transl. Med. 2013;5(180):180ra48. doi: 10.1126/scitranslmed.3005109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhao P, Astruc D. Docetaxel nanotechnology in anticancer therapy. ChemMedChem. 2012;7(6):952–972. doi: 10.1002/cmdc.201200052. [DOI] [PubMed] [Google Scholar]
- 163.Zhao Q, et al. Interaction between circulating galectin-3 and cancer-associated MUC1 enhances tumour cell homotypic aggregation and prevents anoikis. Mol. Cancer. 2010;9:154. doi: 10.1186/1476-4598-9-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zijlstra A, et al. The inhibition of tumor cell intravasation and subsequent metastasis via regulation of in vivo tumor cell motility by the tetraspanin CD151. Cancer Cell. 2008;13(3):221–234. doi: 10.1016/j.ccr.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zocchi E, et al. Encapsulation of doxorubicin in liver-targeted erythrocytes increases the therapeutic index of the drug in a murine metastatic model. Proc. Natl. Acad. Sci. 1988;86:2040–2044. doi: 10.1073/pnas.86.6.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zolnik BS, et al. Nanoparticles and the immune system. Endocrinology. 2010;151(2):458–465. doi: 10.1210/en.2009-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]


