Abstract
Temporal lobe epilepsy (TLE) is the most common epilepsy syndrome in adults. In particular, the hippocampus is highly susceptible to abnormal synchronization. Recent advances in the surgical treatment of patients with refractory TLE have shown that multiple hippocampal transections can effectively control seizures. It has been suggested that in TLE the synchrony in the longitudinal connections is required for seizure generation; however the physiological background for the increase in hippocampal synchronization along the longitudinal axis is not fully understood. The hippocampus varies in seizure susceptibility along its longitudinal axis with the ventral hippocampus (VH) region being more seizure-prone and susceptible to neuronal damage than the dorsal hippocampus (DH). In the present study we studied seizure susceptibility along the longitudinal axis of the hippocampus following pilocarpine-induced status epilepticus (SE). In control conditions the VH generates epileptiform activity (EA) more frequently than the DH when exposed to a low Mg2+/1Ca2+/5K+ solution. Following SE the probability of inducing epileptiform actvitiy (EA) is similar in the VH and DH slices. This SE-induced change is due to an increase in the proportion of DH slices responding to the low Mg2+/1Ca2+/5K+ solution with EA. Moreover, both the VH and DH show similar responses to a low Mg2+/1Ca2+/5K+ solution. These findings indicate that the hippocampus undergoes significant functional changes following SE, which may provide the necessary increase of synchrony along the longitudinal axis to generate seizures in TLE.
Keywords: ventral and dorsal hippocampus, temporal lobe epilepsy, seizure, surgery, lithium-pilocarpine model
1. INTRODUCTION
Although in many epilepsy-related studies the hippocampus is treated as a congruous structure, the hippocampal formation is not uniform along its longitudinal axis. Dorsal (septal) and ventral (temporal) regions are shown to be morphologically, biochemically and anatomically distinct, which determines their functional difference (Fanselow and Dong, 2010). Several in vitro and in vivo studies demonstrate the difference in the susceptibility of ventral hippocampus (VH) and dorsal hippocampus (DH) to generate seizures, with VH being more prone to epileptiform discharges (Bragdon et al., 1986; Derchansky et al., 2004; Papatheodoropoulos et al., 2005; Toyoda et al., 2013). In a chronic animal model of TLE it was shown that seizure onset most frequently occurs in the VH (Toyoda et al., 2013), which is homologous to the anterior hippocampus in humans, the most susceptible region for seizures in TLE and a common site for surgical resection for intractable epilepsy (Dam, 1980; King et al.,1997; Spencer et al., 1984). Although the role of the VH in the generation of epileptiform activity (EA) is significant, the importance of connections between DH and VH for full seizure development should not be underestimated. Recent clinical and basic research studies show that hippocampal longitudinal synchrony of EA is required for seizure generation and transection of the hippocampal longitudinal epileptic circuits is effective in suppressing seizures (Imamura et al., 2001; Shimizu et al., 2006; Umeoka et al., 2012). However the premise for the increase of hippocampal longitudinal synchronization in epileptic brain is not fully understood. Using the lithium-pilocarpine model of TLE we show that unlike control conditions where VH slices show a clear increase in seizure susceptibility compared to DH slices, in the SE group both the VH and DH slices show similar responses to low Mg2+/1Ca2+/5K+ solution due to an increase in seizure susceptibility of the DH. We suggest that such alignment of responses in both regions of the hippocampus can result in an increase in synchronization along the hippocampal longitudinal axis in TLE.
2. MATERIAL AND METHODS
All procedures were conducted in accordance with the guidelines set down by the National Institute of Health for the humane treatment of animals and approved by the University of Vermont Animal Care and Use Committee. Fifteen male Sprague-Dawley rats (postnatal day (P) 55-60) were IP injected with lithium chloride (127 mg/kg) 19-20 hr before IP administration of pilocarpine (30 mg/kg)(Isaev et al., 2011). SE was terminated after 2 hr with isoflurane anesthesia. Control animals (n=7) received 0.9% saline by IP administration. All pilocarpine-treated rats had one or more spontaneous behavioral seizure before being sacrificed. At P70-89 rats were decapitated under isoflurane anesthesia. The 4th to 6th slices from the dorsal and ventral ends of hippocampus (400 µm) were cut using a vibrating blade microtome and then submerged in oxygenated ACSF of the following composition (mM): NaCl 126, KCl 3.5, CaCl2 2.0, MgCl2 1.3, NaHCO3 25, NaH2PO4 1.2 and glucose 11 (pH7.25-7.30) at room temperature (22-240 C) for at least 2 hr before recording. One DH and one VH slices from the same animal (control or SE group) were transferred to the submerged recording chamber and perfused at 5 ml/min with oxygenated ACSF (31-33°C). All experiments were routinely started 10-15 min after transferring pair of slices into the recording chamber to allow equilibration of the temperature inside the slices.Extracellular field potential recordings were obtained from the CA1 pyramidal cell layer using glass microelectrodes filled with ACSF (1-2 MΩ) and differential amplifier (A-M Systems, Carlsborg, WA). EA was induced after 10-15 min of baseline recording by application of low Mg2+/1Ca2+/5K+ solution (in mM): NaCl 126, KCl 5, CaCl2 1.0, NaHCO3 25, NaH2PO4 1.2 and glucose 11 (pH 7.25-7.30). We did not observe EA in any slices before application of the low Mg2+/1Ca2+/5K+ solution.
Data were collected and analyzed using PClamp 9.0 (Axon Instruments), Origin 7.5 (Microcal Software, Northampton, MA) and Mini Analysis (version 6.0.3; Synaptosoft, Decatur, GA) software. The proportion of slices with and without EA was compared using the Fisher's exact test. Statistical differences were determined using the unpaired Student's t-test. Results were expressed as mean±SD.
3. RESULTS
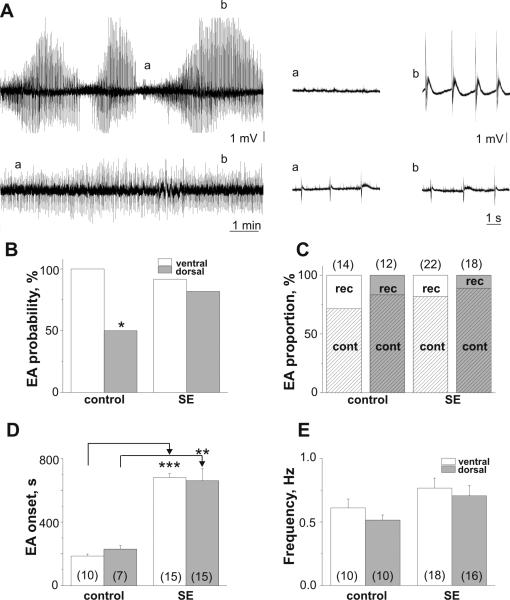
In the control group application of low Mg2+/1Ca2+/5K+ solution induced EA in 100% of VH (n=14) and 50% of DH (n=24) slices (p=0.006, Fig.1A,B). The probability of inducing EA in the SE group was similar to the VH (22/24; 91.7%) and DH (18/22; 81.8%) slices (Fig.1B) due to the significant increase in the proportion of DH slices responded on low Mg2+/1Ca2+/5K+ solution with EA (compared to control DH slices, p=0.03). Two types of EA were observed in response to application of low Mg2+/1Ca2+/5K+ solution: recurrent EA (rEA) and continuous EA (cEA) (Fig 1A)(Isaev et al., 2012). rEA was characterized by alternating short silent periods with periods of spontaneous high amplitude synchronous activity. cEA was characterized by a relatively homogeneous synchronous discharges. As the majority of slices regardless of the group responded to application of low Mg2+/1Ca2+/5K+ solution with cEA (Fig.1C), further analysis of difference in EA onset, frequency and time of washout was performed for cEA.
Figure 1.
Epileptiform activity induced by low Mg2+/1Ca2+/5K+ solution in VH and DH slices of control and pilocarpine-treated rats. (A) Examples of induced recurrent epileptiform activity (rEA, top trace, VH, control group, P75) and continuous EA (cEA, bottom trace, DH, SE, P77) with parts of traces (right) on an expanded time scale. Probability of EA induction (B) and proportion of rEA and cEA (C) in VH and DH slices in control and SE groups. (D) Delayed onset of EA in both VH and DH slices in SE group compare to controls. (E) EA frequency was unchanged in SE group. Number of slices shown in parentheses. ***p<0.001; **p<0.005.
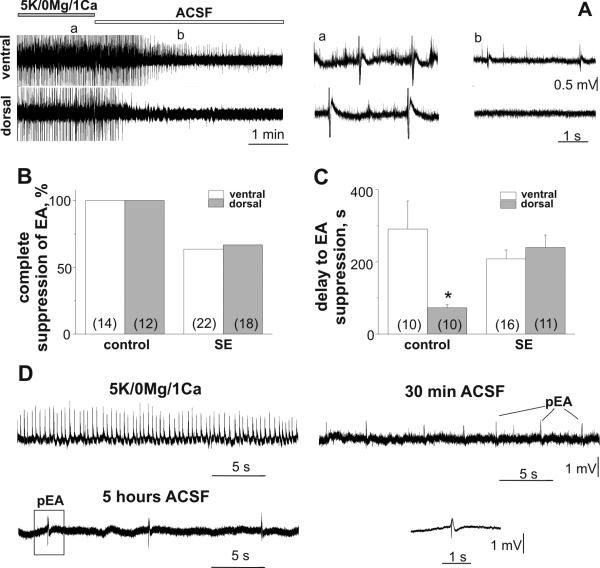
The EA onset in VH and DH slices in the control group was similar (Fig.1D, p=0.1). In the SE group EA onset was significantly delayed compared to controls (VH: p<0.001; DH: p=0.001, Fig.1D). The delayed EA onset in the SE rats could be explained by substantial neuronal loss during epileptogenesis and synaptic reorganization shown in studies of this model (Williams et al., 2002). Although the frequency of epileptiform discharges recorded in the VH and DH in the SE group were increased compared to controls, this difference was not significant (SE vs control, VH: p=0.2; DH: p=0.1). Figure 2A represents simultaneous recordings made from VH and DH slices in control group. Restoring normal ACSF after 1 hr of exposure of slices to low Mg2+/1Ca2+/5K+ solution resulted in rapid and complete suppression of EA in all DH slices and significantly delayed EA suppression in all VH slices in the control group (p=0.01, Fig.2A-C). In the SE group complete suppression of EA was observed in 63.6% of VH and 66.7% of DH slices. In the SE group the delay of EA washout in VH and DH slices with complete suppression of EA was not significantly different (p=0.5, Fig.2C). EA remained upon washout of low Mg2+/1Ca2+/5K+ solution in the SE group were much less frequent (at 30 min of washout - VH: 0.06±0.03 Hz (n=8); DH: 0.10±0.06 Hz (n=6); Fig. 2D). After recordings, several slices (2DH and 3VH) which expressed persistent EA upon washout were moved to the incubation chamber and 4-5 hours later field potential recordings were made again in normal ACSF. As shown in figure 2D, although less frequent, EA can still be recorded after five hours of washout with normal ACSF.
Figure 2.
Effect of restoration of normal perfusion solution on low Mg2+/1Ca2+/5K+-induced EA in VH and DH slices in control and SE groups. (A) Delayed EA suppression in the VH compared to the DH slice in control group (P70) upon low Mg2+/1Ca2+/5K+ solution washout. Simultaneous recordings from VH and DH slices with parts of traces (right) shown on an expanded time scale. The probability (B) and the delay (C) of EA suppression. (D) low Mg2+/1Ca2+/5K+ solution-induced EA and persistent EA (pEA) recorded in the VH of SE group at 30 min and 5 hr of washout with normal ACSF. Field recording outlined by box was low pass filtered at 100Hz and showed on an expended time scale. Number of slices used for analysis is shown in parenthesis. *p<0.05.
4. DISCUSSION
In agreement with previous studies, in the control group VH slices were more prone to generate seizures in response to a low Mg2+/1Ca2+/5K+ solution compared to DH slices (Derchansky et al., 2004; Papatheodoropoulos et al., 2005). In the chronic epileptic brain we obtained contrasting effects. Although we did not find an increase in VH seizure susceptibility, the DH region in the SE group becomes substantially more seizure-prone then in the control group. Moreover, VH and DH slices following SE have very similar characteristics to the response to low Mg2+/1Ca2+/5K+ solution, which suggest that DH undergoes significant functional changes after SE. Also we found that following SE more than 30% of both the VH and DH slices had EA that persisted despite restoring normal ACSF, indicating persistent EA in both hippocampal regions.
5. CONCLUSION
In summary, pilocarpine-induced SE results in chronic changes in functional properties of the hippocampus eliminating the difference in seizure susceptibility between VH and DH regions which occur in normal conditions. These changes can result in the increase in synchronization of the hippocampus along the longitudinal axis likely required to generate EA in TLE.
HIGHLIGHTS.
Epileptifom activity was induced in hippocampal slices by low Mg/1Ca/5K solution
In control conditions VH is more prone to generate epileptiform activity then DH
Adult Sprague-Dawley rats were subjected to lithium-pilocarpine SE
Dorsal and ventral hippocampus show similar seizure susceptibility after SE
This suggests the region-specific alteration in hippocampal seizure susceptibility
ACKNOWLEDGMENTS
This work was supported by the State Foundation of Fundamental Research of Ukraine F46.2/001, the National Institutes of Health, National Institute of Neurological Disorders and Stroke (R01NS073083) and the Emmory R. Shapses Research Fund and Michael J. Pietroniro Research Fund.
ABBREVIATIONS
- TLE
temporal lobe epilepsy
- EA
epileptiform activity
- pEA
persistent epileptiform activity
- rEA
recurrent epileptiform activinty
- cEA
continuous epileptiform activity
- DH
dorsal hippocampus
- VH
ventral hippocampus
- SE
status epilepticus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST.
None of the authors has a conflict of interest to declare.
REFERENCE
- Bragdon AC, Taylor DM, Wilson WA. Potassium-induced epileptiform activity in area CA3 varies markedly along the septotemporal axis of the rat hippocampus. Brain Res. 1986;378(1):169–173. doi: 10.1016/0006-8993(86)90300-8. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurkó A, Mamiya A, Buzsáki G. Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving rat. J. Neurosci. 1999;19(1):274–287. doi: 10.1523/JNEUROSCI.19-01-00274.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam AM. Epilepsy and neuron loss in the hippocampus. Epilepsia. 1980;21(6):617–629. doi: 10.1111/j.1528-1157.1980.tb04315.x. [DOI] [PubMed] [Google Scholar]
- Derchansky M, Shahar E, Wennberg RA, Samoilova M, Jahromi SS, Abdelmalik PA, Zhang L, Carlen PL. Model of frequent, recurrent, and spontaneous seizures in the intact mouse hippocampus. Hippocampus. 2004;14(8):935–947. doi: 10.1002/hipo.20007. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong H-W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65(1):7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura S, Tanaka S, Akaike K, Tojo H, Takigawa M, Kuratsu J. Hippocampal transection attenuates kainic acid-induced amygdalar seizures in rats. Brain Res. 2001;897(1-2):93–103. doi: 10.1016/s0006-8993(01)02098-4. [DOI] [PubMed] [Google Scholar]
- Isaev D, Ivanchick G, Khmyz V, Isaeva E, Savrasova A, Krishtal O, Holmes GL, Maximyuk O. Surface charge impact in low-magnesium model of seizure in rat hippocampus. J. Neurophysiol. 2012;107(1):417–423. doi: 10.1152/jn.00574.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaev D, Zhao Q, Kleen JK, Lenck-Santini PP, Adstamongkonkul D, Isaeva E, Holmes GL. Neuroaminidase reduces interictal spikes in a rat temporal lobe epilepsy model. Epilepsia. 2011;52(3):12–15. doi: 10.1111/j.1528-1167.2011.02988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King D, Bronen RA, Spencer DD, Spencer SS. Topographic distribution of seizure onset and hippocampal atrophy: Relationship between MRI and depth EEG. Electroencephalogr. Clin. Neurophysiol. 1997;103(6):692–697. doi: 10.1016/s0013-4694(97)00090-4. [DOI] [PubMed] [Google Scholar]
- Majores M, Schoch S, Lie A, Becker AJ. Molecular neuropathology of temporal lobe epilepsy: complementary approaches in animal models and human disease tissue. Epilepsia. 2007;48(2):4–12. doi: 10.1111/j.1528-1167.2007.01062.x. [DOI] [PubMed] [Google Scholar]
- Papatheodoropoulos C, Moschovos C, Kostopoulos G. Greater contribution of N-methyl-D-aspartic acid receptors in ventral compared to dorsal hippocampal slices in the expression and long-term maintenance of epileptiform activity. Neuroscience. 2005;135(3):765–779. doi: 10.1016/j.neuroscience.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin PA. Role of the hippocampus in epilepsy. Hippocampus. 1994;4(3):239–242. doi: 10.1002/hipo.450040302. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Kawai K, Sunaga S, Sugano H, Yamada T. Hippocampal transection for treatment of left temporal lobe epilepsy with preservation of verbal memory. J Clin Neurosci. 2006;13(3):322–328. doi: 10.1016/j.jocn.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Spencer DD, Spencer SS, Mattson RH, Williamson PD, Novelly RA. Access to the posterior medial temporal lobe structures in the surgical treatment of temporal lobe epilepsy. Neurosurgery. 1984;15(5):667–671. doi: 10.1227/00006123-198411000-00005. [DOI] [PubMed] [Google Scholar]
- Toyoda I, Bower MR, Leyva F, Buckmaster PS. Early activation of ventral hippocampus and subiculum during spontaneous seizures in a rat model of temporal lobe epilepsy. J. Neurosci. 2013;33(27):11100–15. doi: 10.1523/JNEUROSCI.0472-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeoka SC, Lüders HO, Turnbull JP, Koubeissi MZ, Maciunas RJ. Requirement of longitudinal synchrony of epileptiform discharges in the hippocampus for seizure generation: a pilot study. J. Neurosurg. 2012;116(3):513–24. doi: 10.3171/2011.10.JNS11261. [DOI] [PubMed] [Google Scholar]
- Williams PA, Wuarin J-P, Dou P, Ferraro DJ, Dudek FE. Reassessment of the effects of cycloheximide on mossy fiber sprouting and epileptogenesis in the pilocarpine model of temporal lobe epilepsy. J Neurophysiol. 2002;88(4):2075–2087. doi: 10.1152/jn.2002.88.4.2075. [DOI] [PubMed] [Google Scholar]