Abstract
Purpose
Although adoptive cell therapy can be highly effective for the treatment of patients with melanoma, the application of this approach to the treatment of other solid tumors has been limited. The observation that the cancer germline (CG) antigen NY-ESO-1 is expressed in 70–80% and in approximately 25% of patients with synovial cell sarcoma and melanoma, respectively, prompted us to perform this first-in-man clinical trial employing the adoptive transfer of autologous PBMC that were retrovirally transduced with an NY-ESO-1 reactive TCR to heavily pretreated patients bearing these metastatic cancers.
Experimental Design
HLA-*0201 patients with metastatic synovial cell sarcoma or melanoma refractory to standard treatments and whose cancers expressed NY-ESO-1 received autologous TCR-transduced T cells following a lymphodepleting preparative chemotherapy. Response rates using Response Evaluation Criteria in Solid Tumors (RECIST), as well as immunologic correlates of response, are presented in this report.
Results
Eleven of 18 patients with NY-ESO-1+ synovial cell sarcomas (61%) and 11 of 20 patients with NY-ESO-1 positive melanomas (55%) who received autologous T cells transduced with an NY-ESO-1-reactive TCR demonstrated objective clinical responses. The estimated overall three and five year survival rates for patients with synovial cell sarcoma were 38 and 14%, respectively, while the corresponding estimated survival rates for patients with melanoma were both 33%.
Conclusions
The adoptive transfer of autologous T cells transduced with a retrovirus encoding a TCR against an HLA-A*0201 restricted NY-ESO-1 epitope can be an effective therapy for some patients bearing synovial cell sarcomas and melanomas that are refractory to other treatments.
INTRODUCTION
The in vitro expansion of tumor infiltrating lymphocytes (TIL) from fresh melanoma samples frequently leads to the generation of T cells reactive with autologous tumor cells. The administration of these TIL following lymphodepleting chemotherapy can mediate objective tumor regressions in 50–70% of patients with metastatic melanoma, with some patients achieving durable complete regressions.1 While there is evidence that T cells derived from additional tumor types can recognize autologous tumors2–4, tumor-reactive TIL are less frequently obtained from other tumors. One strategy for addressing the difficulty in generating tumor reactive T cells is the genetic modification of autologous T cells to express cloned T cell receptors (TCRs) directed against shared tumor antigens. Cancer germline (CG) antigens, molecules expressed in a wide variety of tumor types but often not expressed in any adult tissues with the exception of germline cells that lack HLA class I and class II expression, represent attractive targets for these therapies.5 The CG antigen NY-ESO-1 is expressed in 10 to 50% of metastatic melanomas, lung, breast, prostate, thyroid and ovarian cancers6–9 as well as between 70 and 80% of synovial cell sarcomas.10 In 2011 we reported preliminary results of this first-in-man clinical trial utilizing the adoptive transfer of autologous PBMC that were transduced with a high affinity TCR directed against an HLA-A*0201-restricted NY-ESO-1 epitope to six and 11 patients with metastatic synovial cell sarcoma and metastatic melanoma, respectively.11 In the current study, we present clinical response data for 12 additional synovial cell sarcoma patients and nine additional melanoma patients enrolled in this trial, updated response data for the 17 patients characterized in the first report, and analyses of the in vitro anti-tumor reactivity and in vivo persistence following adoptive transfer of the administered T cells.
Materials and Methods
Patients and Clinical Trial Design
Patients 18 years or older expressing HLA-A*0201 with either metastatic synovial cell sarcoma or metastatic melanoma refractory to standard chemotherapy and whose tumors expressed NY-ESO-1 as determined by immunohistochemical staining were enrolled in the current trial. All patients’ tumors stained strongly (2–4+ intensity in greater than 50% of cells) for NY-ESO-1 antigen expression using the specific anti-NY-ESO-1 monoclonal antibody E97812 (Invitrogen, Carlsbad, CA).
Patients 7–9 and 30–34 were immunized with a recombinant AVIPOX virus encoding the NY-ESO-1 HLA-A*0201 T cell epitope (AVIPOX-ESO) at the time of adoptive transfer as well as two weeks following transfer. There was no apparent immunologic or clinical impact of this vaccination and thus all synovial cell sarcoma and melanoma patients in this study are considered as individual cohorts.
This clinical trial (NCT00670748) was conducted in the Surgery Branch of the NCI and was reviewed and approved by the NIH Institutional Biosafety Committee, the NCI Institutional Review Board, the NIH Office of Biotechnology Activities, and the Food and Drug Administration (all Bethesda, MD). Patients were treated with a lymphodepleting chemotherapy regimen consisting of cyclophosphamide (60 mg/kg/day for two days) and fludarabine (25 mg/m2/day for five days)13 prior to the initial as well as any subsequent administration of TCR transduced cells. Gene transduced cells were administered intravenously one to three days later and IL-2 was administered at a dose of 720,000 IU/kg every eight hours to tolerance.
Retroviral vector and T cell transduction
The 1G4-α95:LY TCR that recognizes amino acids 157–165 of the NY-ESO-1 protein (NY-ESO-1:157–165) in the context of the HLA-A*0201 class I restriction element was cloned into the MSGV1 retroviral vector backbone as previously described14. The 1G4-α95:LY TCR contained two amino acid substitutions in the third complementarity determining region of the native 1G4 TCR α chain that specifically enhanced the ability of transduced CD8+ and CD4+ T cells to recognize HLA-A*0201+ and NY-ESO-1+ target cells.14 Clinical grade GMP retroviral supernatants were obtained from the National Gene Vector Laboratory at Indiana University. PBMC obtained from patient phereses were stimulated using 50 ng/ml of soluble anti-CD3 antibody (OKT3) (Ortho-Biotech, Bridgewater, NJ) in the presence of 300 IU of recombinant IL-2, transduced with a retrovirus encoding the modified 1G4-α95:LY anti-NY-ESO-1 TCR using retronectin, kindly provided by Takara Bio Inc (Otsu, Japan), and expanded further in vitro prior to adoptive transfer, as previously described.15
FACS Analysis
Samples of in vitro cultured T cells as well as PBMC obtained approximately one month after adoptive transfer were analyzed with an anti-Vβ13.1 antibody (Beckman Coulter, Miami, FL) that recognizes the β chain of the 1G4-α95:LY anti-NY-ESO-1 TCR, and an HLA-A*0201 tetramer prepared with the NY-ESO-1:157–165 peptide (Beckman Coulter), as previously indicated.11
Evaluation of IFN-γ Secretion, IFN-γ ELISPOT Responses
Co-cultures of in vitro cultured T cells and PBMC were carried out with target cells for 18 hours and secreted IFN-γ detected in a standard ELISA assay.14 ELISPOT assays were carried out by incubating TIL or PBMC overnight in the absence of exogenous cytokine and then co-cultured with target cells for 18 hours, after which the number of IFN-γ–secreting T cells were enumerated, as previously described.15
RESULTS
Patient and Treatment Characteristics
A total of 18 HLA-A*0201+ patients with NY-ESO-1+ metastatic synovial cell sarcomas including six patients described in a previous report, and 20 HLA-A*0201+ patients with NY-ESO-1+ metastatic melanoma, including 11 patients also described in the previous report11, received non-myeloablative chemotherapy followed by a median of 5.5×1010 T cells transduced with an anti-NY-ESO-1 TCR (range 0.9–13×1010) plus systemic IL-2. Characteristics of the patients and of the administered cells are shown in Table 1. With the exception of patients 4 and 17, more than 50% of the administered T cells were CD8+, and a median of 78% and 62% of the administered CD8+ and CD4+ T cells, respectively, bound to a tetramer prepared with HLA-A*0201 and the NY-ESO-1:157–165 peptide.
Table 1.
Characteristics of Patients and Administered T cells
| Synovial cell sarcoma | Melanoma | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| Patient | Age/ Sex |
Sites of Disease |
Prior Treatments |
Cells (×109) |
IL-2 doses |
CDS+/ CD4+(% of CD3+) |
Tetramer+ CDS+/CD4+ (% of CD3+) |
Patient | Age/ Sex |
Sites of Disease |
Prior Treatments |
Cells (×109) |
IL-2 doses |
CDS+/ CD4+(% of CD3+) |
Tetramer+ CDS+/CD4+ (% of CD3+) |
| 1 | 20/M | lu,bo | S,R,I,C(2)* | 83 | 5 | 82/8 | 63/5 | 19 | 52/M | ln | S,R,I | 130 | 6 | 97/2 | 83/1 |
| 2 | 37/F | lu | S,R,C(4) | 50 | 8 | 90/5 | 70/4 | 20 | 60/F | sc,lu | S,I | 71 | 6 | 82/17 | 62/9 |
| 3 | 47/F | lu, ln | S,R,C(2) | 56 | 8 | 89/11 | 72/8 | 21 | 30/F | bo,ln,panc,sb | S,R,I | 47 | 1 | 98/1 | 78/1 |
| 4 | 19/M | lu | S,R,C(1) | 16 | 5 | 46/40 | 31/25 | 22 | 56/M | lu,ki | S,R,I | 50 | 7 | 91/9 | 73/7 |
| 5 | 30/M | pl, hi | S,C(2) | 59 | 5 | 92/8 | 68/5 | 23 | 32/M | ln | S,I,C | 64 | 4 | 98/2 | 83/2 |
| 6 | 40/M | pl, hi | S,R,C(3) | 52 | 5 | 81/18 | 63/12 | 24 | 38/M | ln | S,I | 51 | 7 | 93/7 | 81/6 |
| 7 | 64/F | bo, lu | S,R,C(5) | 39 | 5 | 83/17 | 72/13 | 25 | 47/M | ln,lu | S,R,I | 23 | 7 | 96/4 | 67/2 |
| 8 | 37/M | lu | S,C(2) | 22 | 6 | 87/13 | 66/4 | 26 | 39/F | ln, br,lu | S,R,I,C | 38 | 8 | 68/32 | 53/22 |
| 9 | 21/F | lu | S,R,C(1) | 77 | 1 | 89/11 | 56/6 | 27 | 51/F | lu, ln,li | S,I,C | 31 | 10 | 94/6 | 78/4 |
| 10 | 31/F | lu | S,C(2) | 30 | 5 | 93/7 | 33/2 | 28 | 61/M | ln,li,spl,lu,bo | S,R,I,C | 16 | 8 | 84/16 | 66/9 |
| 11 | 65/F | lu | S,R,C(2) | 63 | 4 | 73/27 | 40/20 | 29 | 46/M | lu, li | S,R,I | 37 | 6 | 93/7 | 59/4 |
| 12 | 40/M | pane, bo | S,C(3) | 120 | 1 | −‡ | − | 30 | 39/M | lu, bo,li | S,R,I | 9 | 7 | 82/15 | 59/9 |
| 13 | 24/M | sc, lu | S,C(1) | 30 | 0 | 89/11 | 56/5 | 31 | 61/F | ln | S,I | 36 | 3 | 70/29 | 51/19 |
| 14 | 43/F | sc, pl | S,C(1) | 55 | 5 | 90/10 | 67/6 | 32 | 39/M | ln | S,I,C | 36 | 7 | 88/12 | 78/8 |
| 15 | 27/M | lu | S,C(1) | 69 | 4 | 83/17 | 52/11 | 33 | 51/M | ln, c | S,I | 49 | 6 | 89/11 | 70/7 |
| 16 | 40/M | lu | S,I,C(6) | 83 | 1 | 74/26 | 44/18 | 34 | 30/M | lu | S,I | 50 | 5 | 94/5 | 74/4 |
| 17 | 47/F | lu | S,C(2) | 33 | 4 | 45/55 | 33/39 | 35 | 55/M | ki,pl | S | 57 | 0 | 74/26 | 61/19 |
| 18 | 46/M | lu | S,C(1) | 57 | 7 | 62/38 | 52/31 | 36 | 65/M | lu, ln, ki | S,R,I | 55 | 3 | 93/7 | 76/6 |
| 37 | 64/M | ln, sp | S,I | 82 | 5 | 57/43 | 44/41 | ||||||||
| 38 | 51/M | ln | S,I,C | 78 | 4 | 92/8 | 74/7 | ||||||||
Abbreviations used: ln, lymph node; sc,subcutaneous; lu,lung; bo, bone; panc,pancreas; sb,small bowel; ki,kidney; br,brain; spl,spleen; pl,pleura; hi,hilum; S, Surgery; R, Radiation; I, Immunotherapy; C,Chemotherapy.
The number of chemotherapy regimens administered to synovial cell sarcoma patients prior to adoptive transfer is noted in parentheses.
The T cells administered to Patient 12, who developed sepsis and died three days following adoptive transfer, were not further characterized.
All of the synovial cell sarcoma patients on the trial had progressive metastatic disease following extensive pre-treatment consisting of surgery, often in combination with radiation and multiple chemotherapy regimens, and metastatic melanoma patients had often received multiple treatments including IL-2, radiation therapy, IFN-α, or GM-CSF.11
Clinical Results
Eleven of the 18 synovial cell sarcoma patients (61%) who received autologous TCR-transduced T cells experienced objective responses by RECIST criteria (Tables 2 and 3). Responses to a first treatment with the 1G4-αLY TCR are shown with the exception of patient 2, who exhibited a partial response lasting 6 months to an initial infusion of TCR-transduced T cells, as well as a partial response lasting 9 months following a second infusion of a similar T cell product, for a total combined response of 18 months following the initial treatment (Table 2). Five additional patients (1, 3, 5, 6, 8), received but failed to respond to a second infusion of 1G4-αLY transduced T cells, and patient 2 received but failed to respond to a third treatment with transduced T cells.
Table 2.
Clinical Responses and Characterization of Patient PBMC Approximately 1Month Following Adoptive Transfer
| Synovial Cell Sarcoma | Melanoma | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Patient | Response | CD8+/CD4 + T cells per ml |
Tetramer + CD8+/CD4+ T cells (% of CD3+) |
Tumor- reactive ELISPOTS/ 105 PBMC* |
Peptide- specific ELISPOTS/ 105 PBMC |
Patient | Response | CD8+/CD4 + T cells per ml |
Tetramer + CD8+/CD4+ T cells (% of CD3+) |
Tumor- reactive ELISPOTS/ 105 PBMC* |
Peptide- specific ELISPOTS/ 105 PBMC |
| 1# | PR(10) | 264/152 | 7/23 | 27 | 1,980 | 19 | PR(8) | 1462/218 | 19/3 | 15 | 1,650 |
| 2 | PR(18) | 327/194 | 2/1 | 845 | 5,015 | 20 | NR | 3095/1750 | 28/20 | 125 | 2,625 |
| 3 | PR(3) | 1,387/240 | 38/4 | 1,075 | 4,215 | 21 | NR | 1774/361 | <1/<1 | 0 | 110 |
| 4 | NR | 739/146 | 4/3 | 21 | 1,491 | 22 | CR(24) | 1104/108 | 3/<l | 0 | 4 |
| 5 | PR(8) | 729/140 | 5/1 | 0 | 25 | 23 | CR(58+) | 921/143 | 8/<l | 8 | 1,835 |
| 6 | NR | 62/166 | <1/<1 | 0 | 5 | 24 | PR(3) | 2693/153 | <1/<1 | 6 | 19 |
| 7 | PR(47+) | 144/65 | 12/33 | 75 | 3,125 | 25 | NR | 342/118 | 13/2 | 45 | 1,065 |
| 8 | PR(11) | 709/287 | 23/11 | 115 | 1,315 | 26 | NR | 1543/425 | 7/6 | 401 | 1,183 |
| 9 | PR(3) | 194/126 | 11/20 | 85 | 810 | 27 | NR | 371/134 | 16/13 | 312 | 880 |
| 10 | PR(5) | 947/124 | 4/5 | 5 | 0 | 28 | NR | 221/74 | 1/<1 | 0 | 40 |
| 11 | PR(7] | 772/245 | 34/23 | 80 | 10,305 | 29 | CR(54+) | 1002/257 | 8/2 | 25 | 620 |
| 13 | NR | 344/125 | NA | − | − | 30 | NR | 108/38 | 8/10 | 79 | 673 |
| 14 | NR | 2,311/346 | 12/6 | 0 | 825 | 31 | PR(10) | 265/510 | 5/32 | 60 | 1,018 |
| 15 | CR(20+) | 1,712/271 | 26/14 | 740 | 14,280 | 32 | NR | 347/113 | 4/18 | 80 | 2,480 |
| 15 | NR | 600/151 | 20/9 | 120 | 3,620 | 33 | NR | 666/197 | 14/17 | 850 | 8,223 |
| 17 | PR(4) | 359/132 | 9/7 | 0 | 35 | 34 | CR(40+) | 581/126 | 18/8 | 3,025 | 9,937 |
| 18 | NR | 143/152 | NA | − | − | 35 | PR(28) | 330/132 | 3/17 | 7 | 906 |
| 36 | PR(5) | 1483/36 | 24/9 | 469 | 5,110 | ||||||
| 37 | PR(6+) | 616/618 | 16/49 | 257 | 18,472 | ||||||
| 38 | PR(3) | 1462/193 | 72/6 | 13 | 430 | ||||||
Tumor-reactive ELISPOT responses were evaluated by stimulating with the HLA-A*02:01+ and NY-ESO-1+ melanoma cell line 624, and peptide-specific ELISPOT responses evaluated by stimulating with an HLA-A*02:01+ EBV-transformed lymphoblastoid cell line that was pulsed with the NY-ESO-1 peptide.
Patients 7,8, and 9, and 30–34 (first treatment), as well as patients 5, 6, 24 and 27 (second treatment) were immunized with a recombinant AVIPOX virus encoding the NY-ESO-1 HLA-A*0201 T cell epitope. Patients 1, 3 and 8 received a second infusion, and patient 2 received two additional infusions of TCR-transduced T cells but were not immunized with the recombinant AVIPOX virus. With the exception of patient 2, no patient responded to subsequent treatments with TCR-transduced T cells. Abbreviation used: NA – Sample not available.
Table 3.
Clinical response to adoptive transfer of T cells transduced with anti-NY-ESO-1 TCR
| Tumor type | n(%) of patients (duration in months)
|
|||
|---|---|---|---|---|
| Total | PR* | CR | OR | |
| Synovial cell sarcoma | 18 | 10(55) 47+,18,11,10, 8,7,5,4,3,3 |
1(6) 20+ |
11(61) |
| Melanoma | 20 | 7(35) 28,10,8,6+, 5,3,3 |
4(20) 58+,54+, 40+, 24 |
11(55) |
Response data updated as of December 1, 2014 or last follow-up date
PR – Partial response CR – Complete response OR – Objective response
Two synovial cell sarcoma patients have ongoing clinical responses: patient 7, who exhibited a nearly complete regression of multiple lung metastases, as well as substantial regression of a large pelvic bony lesion, both sustained nearly four years following treatment (Fig. 1A), and patient 15, who demonstrated a complete regression of all metastatic lesions, including multiple lung metastases maintained more than a year following treatment (Fig. 1B). Partial responses lasted from three to 18 months, and the estimated overall three and five year survival rates for patients with synovial cell sarcoma were 38 and 14%, respectively (Figure 2A)
Figure 1.
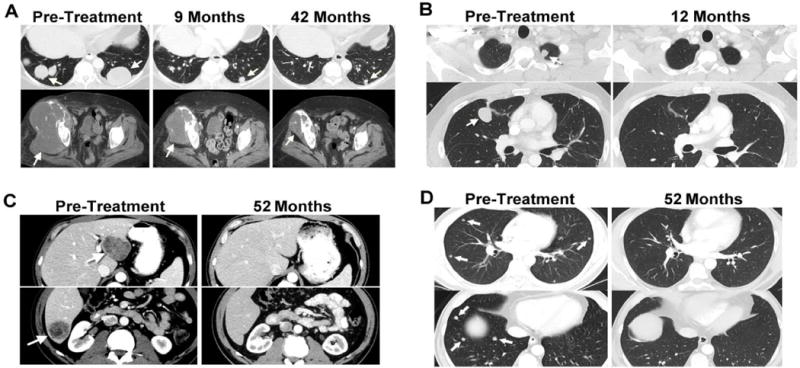
Computed tomography scans demonstrating tumor regression. Radiologic studies were carried out before and after treatment with the NY-ESO-1 TCR-transduced T cells. Tumors are indicated by arrows. (A) Continuing regression of lung metastases and a large pelvic lesion in a patient with synovial cell sarcoma (#7), with a partial response that is ongoing 44 months following adoptive T cell transfer. (B) Regression of representative lung metastases in a patient with synovial cell sarcoma (#15), who is exhibiting an ongoing complete response 17 months following adoptive T cell transfer. Regression of multiple liver (C) and lung lesions (D) in a patient with melanoma (#29) with a complete response that is ongoing 54 months after treatment.
Figure 2.
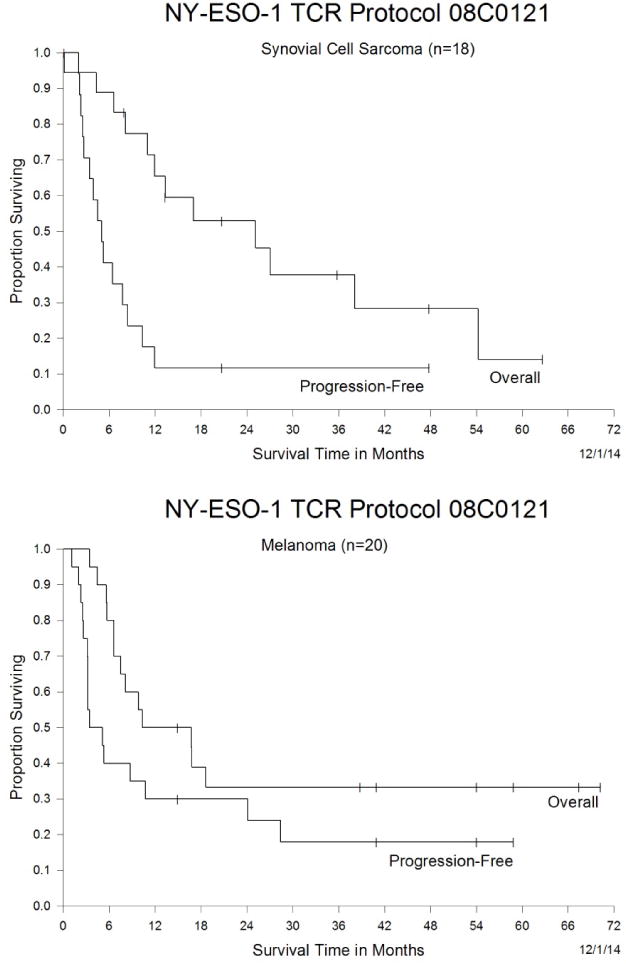
Kaplan-Meier curve of overall survival and progression-free survival. The overall and progression-free survival of heavily pretreated synovial cell sarcoma patients (A) and melanoma patients (B) in response to therapy with NY-ESO-1 TCR transduced T cells is presented.
Eleven of the 20 melanoma patients (55%) experienced objective responses to a first treatment with 1G4-αLY TCR transduced T cells. Two patients (24 and 27) failed to respond to a second infusion of 1G4-αLY transduced T cells. Four melanoma patients had complete responses, three ongoing at 40 to 58 months following treatment. One of the complete responders, patient 29, exhibited regression of multiple liver and lung metastases (Fig. 1C–E). Partial responses lasting three to 28 months were seen in seven melanoma patients (Tables 2 and 3) including patient 37, who was exhibiting an ongoing partial response six months after adoptive transfer, when he was censored due to the development of a second unrelated cancer. The estimated overall three and five year survival rates for patients with melanoma were both 33% (Figure 2B).
All patients experienced the transient neutropenia and thrombocytopenia induced by the preparative regimen and the transient toxicities associated with IL-2. Hematologic and chemical values returned to baseline in all but one patient who was the single treatment related death in this protocol. This patient, a 40 year old male (patient 12), developed septic shock from an E. coli bacteremia while he was neutropenic and died three days following adoptive T cell transfer. No toxicities were attributed to the transferred cells, in accord with the lack of expression of NY-ESO-1 on normal tissues with the exception of germline cells that do not express MHC molecules and thus are immunologically inert.
Clinical responses were observed in four of the five synovial cell sarcoma patients who were also vaccinated with the recombinant AVIPOX-ESO virus, one of which only lasted for three months, and in two of the six vaccinated melanoma patients (Table 2). These response rates did not appear to be significantly different from patients who did not receive the vaccine, but the small number of patients receiving this treatment made it impossible to draw any conclusions regarding the effect of immunization on clinical response to therapy with NY-ESO-1 TCR transduced cells.
Immunologic Correlates of Response
Attempts were then made to identify factors that are associated with response to therapy mediated by the NY-ESO-1 TCR. There was no significant difference between the number of T cells administered to synovial cell sarcoma and melanoma patients (Figure 3A) or between the interferon-gamma (IFN-γ) ELISPOT responses of T cells from synovial cell sarcoma and melanoma patients to either NY-ESO-1 peptide pulsed target cells (Figure 3B) or an HLA-A*0201+ and NY-ESO-1+ melanoma cell line (Fig. 3C). Given these findings and the relatively small number of patients in each cohort, we attempted to identify factors that are associated with response to therapy combining both synovial cell sarcoma and melanoma patients. The results indicated that clinical responses were associated with higher numbers of administered T cells (p<0.02) (Figure 3D) and higher numbers of IFN-γ ELISPOTs following stimulation with NY-ESO-1 peptide pulsed target cells (p<0.02) (Fig. 3E), but not an HLA-A*02:01+ and NY-ESO-1+ tumor cell line (Figure 3F).
Figure 3.
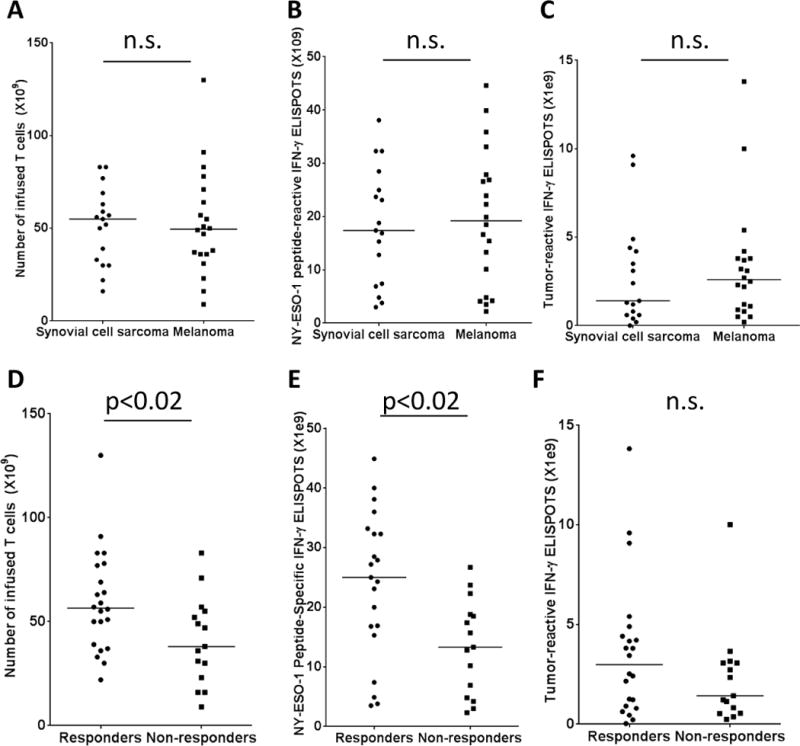
Association between number and function of administered T cells and response to therapy. Comparisons between the total number of T cells (A), the number of NY-ESO-1 peptide reactive IFN-γ ELISPOTS (B), and the number of tumor reactive IFN-γ ELISPOTS (C) administered to synovial cell sarcoma and melanoma patients are displayed. The associations between the number of administered T cells (D), the number of IFN-γ ELISPOTS generated by T cells in response an NY-ESO-1 peptide-pulsed, HLA-A*0201+ EBV transformed LCL (E) or number of IFN-γ ELISPOTS generated by TCR transduced T cells in response to the NY-ESO-1+ and HLA-A*0201+ tumor cell line 624 mel (C) were evaluated in responding as well as non-responding patients using Mann-Whitney non-parametric analysis. Median values are indicated by horizontal bars.
The percentages of CD8+ and NY-ESO-1 tetramer+ T cells in the peripheral blood of patients approximately one month following transfer were highly variable, ranging between less than one percent and 72% of CD3+ T cells, while the percentage of CD4+ and NY-ESO-1 tetramer+ T cells ranged between less than one percent and 49% of CD3+ T cells (Table 2). The median percentage of CD8+ NY-ESO-1 tetramer+ T cells detected in the peripheral blood of responders and non-responders at this time was 11 and 8.5%, respectively, while the median percentage of CD4+ NY-ESO-1 tetramer+ T cells at this time was 8 and 6.5%, respectively. Clinical responses were not associated with persistence at one month of CD8+ (Supplementary Figure 1A) or CD4+ (Supplementary Figure 1B) NY-ESO-1 tetramer+ T cells, results consistent with those seen in the prior study evaluating responses in a subset of these patients.11 One month following adoptive transfer, PBMC isolated from responders and non-responders generated a median of 1,650 and 973 IFN-γ ELISPOTS per 105 cells in response to HLA-A*02:01+ target cells pulsed with the NY-ESO-1:157-165 peptide (Supplementary Figure 1C), and a median of 27 and 62 IFN-γ ELISPOTS per 105 cells in response to HLA-A*0201+ and NY-ESO-1+ tumor cells, respectively (Supplementary Figure 1D), but these differences were not statistically significant. In addition, immunization with recombinant NY-ESO-1 AVIPOX did not significantly impact either the persistence of CD8+ or CD4+ T cells reactive with the NY-ESO-1 tetramer in peripheral blood approximately one month following adoptive transfer (Supplementary Figure 2A,B) or IFN-γ ELISPOT responses of peripheral T cells to either HLA-A*02:01+ cells pulsed with the NY-ESO-1 peptide or an HLA-A*02:01+ and NY-ESO-1+ tumor cell line (Supplementary Figure 2C,D).
DISCUSSION
The durable complete cancer regressions observed using cell transfer immunotherapy for melanoma in multiple clinical trials1,13,16 have stimulated efforts to genetically modify lymphocytes to improve their anti-tumor efficacy and to extend the range of tumors types that can be treated. In the first reported trial to examine the in vivo efficacy of TCR transduced T cells in cancer patients, the adoptive transfer of autologous T cells that were transduced with a MART-1 reactive TCR lead to tumor regression in two of 13 treated patients.17 In subsequent trials carried out with higher avidity TCRs, objective clinical responses were seen in six of 20 (30%) patients treated with autologous T cells that were transduced with a MART-1 reactive TCR17 and in three of 16 (19%) patients treated with a gp100-reactive TCR.15 Severe normal tissue toxicities resulting from expression of these antigens in normal melanocytes present in the skin, eye and ear were observed in these trials, emphasizing the need to target protein such as CG antigens that are not expressed in essential normal tissues.
The NY-ESO-1 protein represents a CG antigen expressed in between 70 and 80% of synovial cell sarcomas10 and in 10–50% of a variety of more common malignancies that include melanoma, bladder, lung, breast and ovarian cancer6,7,9, but not in adult normal tissues except for testes, which does not express HLA class I molecules and is thus immunologically protected. The adoptive transfer of an in vitro sensitized autologous CD4+ T cell clone that recognized an HLA-DP*04 restricted NY-ESO-1 peptide mediated regression of metastatic melanoma in one of nine patients.16,18 In 2010, we presented preliminary results of the first adoptive cellular immunotherapy trial for solid cancers to utilize the transfer of autologous PBMC transduced with a CG-reactive TCR. In that study as well as the current one, patients received autologous PBMC that were transduced with a TCR, termed 1G4-αLY, that possessed a high avidity for the HLA-A*0201-restricted NY-ESO-1:157-165 epitope.11 Response rates of 45% and 67% were observed in the initial cohorts of melanoma and synovial cell sarcoma patients, respectively, all of whom had progressive disease after extensive prior treatment. Clinical responses to therapy were not associated with the persistence of adoptively transferred T cells, as determined either using NY-ESO-1 tetramer binding or antigen-specific ELISPOT to evaluate T cell responses, although only six synovial sarcoma patients, as well as 11 melanoma patients, were evaluated in the previous study. The current study was carried out on 18 synovial cell sarcoma patients and 20 melanoma patients, including the six synovial cell sarcoma patients and 11 melanoma patients analyzed in the previous study. Objective responses were observed in 11 of the 18 patients (61%) with synovial cell sarcoma and 11 of the 20 patients with melanoma (55%) who received autologous TCR-transduced cells. The non-myeloablative chemotherapy regimen administered to all of the patients in this trial may have contributed to the transient partial responses lasting from three to eleven months seen in eight of the synovial cell sarcoma patients, and lasting from three to ten months in five of the treated melanoma patients. The 18 synovial cell sarcoma patients evaluated in the current trial, however, had progressed after receiving multiple rounds of chemotherapy. Thus, it is unlikely that the preparative chemotherapy regimen was responsible for the complete response seen in one of the synovial sarcoma patients that is ongoing at one year, and the substantial partial response observed in a second synovial cell sarcoma patient that is ongoing for over three years. Partial response rates of between 15 and 20% and complete response rates of 5–10% have been observed in melanoma and renal cancer patients who received high dose IL-2 alone (reviewed in19), and eighteen of the 20 melanoma patients treated in this trial received prior high dose IL-2 treatment. Thus, it is likely that the complete responses observed in four melanoma patients, three that are ongoing between three and nearly five years following treatment, resulted from the transfer of NY-ESO-1 reactive T cells and not the cytokine treatment alone.
As noted previously, the persistence of anti-NY-ESO-1 TCR-transduced T cells in peripheral blood approximately one month following transfer was not associated with response to therapy.11 This contrasts with observations in our trial involving treatment with MART-1 and gp100 TCR-transduced T cells indicating that response to therapy was associated with the levels of persistent peptide reactive and tumor reactive T cells.15 It is difficult to draw general conclusions as to the relationship between the persistence or anti-tumor activity of the TCR-transduced T cells and response to therapy, however, given the relatively small numbers of patients treated in these trials.
The objective response rates observed in previous clinical trials involving immunization with peptides derived from tumor antigens, whole proteins, as well as recombinant viral constructs encoding tumor antigens were generally 5% or lower (reviewed in20). Nevertheless, in an attempt to determine the effects of tumor antigen vaccination on responses to adoptively transferred T cells, 11 of the 38 patients treated in the current trial were immunized with a recombinant NY-ESO-1 AVIPOX vaccine. There was no significant difference, however, between clinical response rates in patients who either did or did not receive the NY-ESO-1 vaccine, and neither the persistence nor the function of peripheral NY-ESO-1 reactive T cells differed significantly between these two patient groups. Other factors such as differences between the levels of homeostatic cytokines or the rates of endogenous lymphocyte reconstitution in individual patients may play more significant roles in the persistence of antigen-reactive T cells following adoptive transfer.
In the current study, the total number of administered T cells and the number of T cells reactive with NY-ESO-1 peptide-pulsed target cells, but not the number of cells reactive with an HLA-A2+ NY-ESO-1+ melanoma cell line, were significantly associated with response to therapy with the anti-NY-ESO-1 TCR. A variety of factors that may be relevant to recognition of autologous tumor cells, such as the levels of expression of HLA-A*02:01, NY-ESO-1, and proteins that influence T cell recognition including adhesion, inhibitory and co-stimulatory molecules may have influenced in vitro recognition of the allogeneic tumor cell target and led to the discrepancy between peptide and tumor cell recognition. The number of administered T cells had not previously been associated in our studies with response to therapy with either autologous TIL1 or autologous PBMC transduced with TCRs directed against HLA-A*02:01-restricted epitopes of MART-1 or gp10015, although an association between the number of administered TIL and clinical response has been noted by others.21
The lack of a correlation between responsiveness and persistence seen in the current study was somewhat unexpected, as the persistence of bulk TIL populations was correlated with response to therapy22; however, the relatively small number of patients who were evaluated may in part been responsible for the discrepancies between these trials. The presence of persistent T cells in peripheral blood is not sufficient to mediate responses in all patients, as demonstrated by the relatively high levels of persistence observed in non-responding patients in this as well as the previous study22, and additional factors such as down-regulation of HLA and other gene products involved with antigen processing may have played a role in the lack of responses observed in these patients. The limited or undetectable levels of persistence of TCR transduced T cells observed in peripheral blood one month following therapy from three of the synovial cell sarcoma patients, patients 6, 13 and 18, and two of the melanoma patients, patients 21 and 28, may have been associated with the lack of response observed in these individuals. The relatively short durations of the objective responses seen in some patients who demonstrated relatively high levels of persistence at one month could potentially have resulted from a failure of the T cells to persist for longer time periods, but could not be evaluated due to the limited availability of samples at later time points. Finally, persistence in the periphery may not be strictly correlated with persistence in the tumor, but again, could not be evaluated in this study due to lack of tumor samples.
Expression of the NY-ESO-1 antigen is highly restricted to the tumor and not normal tissues; however, this does not appear to be the case for all CG antigens. The MAGEA3 gene product represents one of the most highly prevalent CG antigens and is significantly expressed in between 30 and 50% in a variety of common cancers. In a recent clinical trial, the adoptive transfer of autologous PBMC transduced with a MAGE-A3-reactive TCR resulted in the deaths of two patients due to severe neurological toxicity. The toxicity appeared to result from cross-reactivity of TCR-transduced T cells with a nearly identical epitope from MAGE-A12, a protein that was found to be expressed at low levels in normal brain tissue.23 In addition, the adoptive transfer of autologous PBMC transduced with an HLA-A1 restricted, MAGE-A3-reactive TCR containing four alpha chain amino acid substitutions that were introduced to enhance antigen recognition resulted in cardiac arrest and the deaths of the first two patients treated on this protocol24, which were attributed to cross-reactivity of TCR-transduced T cells with an epitope of titin, a protein that is highly expressed in cardiac tissue.25
Recent trials have demonstrated that intravenous administration of antibodies targeting inhibitory receptors such as CTLA-4 and PD-1 that are expressed on T cells, as well as inhibitory ligands such as PD-L1 that are expressed on a variety of cell types, can be effective at mediating tumor regressions in patients with some cancers. An overall clinical response rate of approximately 10% was observed in melanoma patients who received an antibody directed against the inhibitory receptor CTLA-426, and objective response rates of between 20 and 30% were seen in patients with melanoma, renal and non-small-cell lung cancer treated with BMS-936558, an antibody against the PD-1 checkpoint inhibitor.27 In addition, clinical responses were seen in between 5 and 15% of melanoma, renal and non-small-cell lung cancer patients who received an antibody directed against the PD-1 ligand PD-L128. In the current study, patient 37 had previously progressed following treatment with IL-2 and anti-PD-L1 antibody but demonstrated a partial response to transfer of NY-ESO-1 TCR transduced T cells. Combinations of checkpoint inhibitors with adoptive immunotherapies represents one strategy that may lead to enhanced anti-tumor responses, although a series of trials may be needed to determine the optimal dosage of checkpoint inhibitors and the appropriate sequencing of these treatments.
The factors that were responsible for the lack of response and the short duration of responses seen in the majority of patients evaluated in this protocol are unknown. Antigen loss does not appear to be a major contributor to the lack of responsiveness, as high levels of NY-ESO-1 expression were observed in tumor biopsies obtained from six of the synovial cell sarcoma patients and two of the melanoma patients prior to a second treatment with anti-NY-ESO-1 TCR transduced T cells.
Overall, these findings indicate that treatments employing TCRs directed against NY-ESO-1 are effective at safely mediating tumor regression in patients with metastatic, refractory synovial cell sarcoma and melanoma, and indicate that the total number of T cells and the number of antigen-reactive T cells administered to patients in this trial may represent important factors that influence response to therapy. Furthermore, these findings provide support for development of additional trials targeting NY-ESO-1 as well as other CT antigens expressed in common epithelial cancers.
Supplementary Material
Translational Relevance.
While extensive studies have demonstrated that the adoptive transfer of autologous tumor reactive T cells can effectively mediate cancer regression in metastatic melanoma patients, evidence suggesting that patients with other malignancies can benefit from this approach is limited. The identification of T cell receptors that recognize cancer germline antigens such as NY-ESO-1 and MAGEA gene family members has provided the means to evaluate the application of adoptive immunotherapies to the treatment of patients with a variety of prevalent cancers that include lung, breast and ovarian cancer. In the current study we provide evidence that the adoptive transfer of autologous T cells transduced with a high avidity anti-NY-ESO-1 TCR can lead to durable responses in patients with metastatic melanoma as well as synovial cell sarcoma. These findings suggest that adoptive therapies utilizing TCRs directed against cancer germline antigens may be effective at mediating tumor regression in patients with a broad array of cancers.
Acknowledgments
We would like to acknowledge Mr. Arnold Mixon and Mr. Shawn Farid for assistance with FACS analysis.
Grant Support
These studies were supported by NIH intramural funds along with funds from Kite Pharma and the Milstein Family Foundation.
Footnotes
The authors have no potential conflicts of interest to disclose
References
- 1.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–7. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–5. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanada K, Yewdell JW. Yang JC Immune recognition of a human renal cancer antigen through post-translational protein splicing. Nature. 2004;427:252–6. doi: 10.1038/nature02240. [DOI] [PubMed] [Google Scholar]
- 4.Wang QJ, Hanada K, Perry-Lalley D, Bettinotti MP, Karpova T, Khong HT, et al. Generating renal cancer-reactive T cells using dendritic cells (DCs) to present autologous tumor. J Immunother. 2005;28:551–9. doi: 10.1097/01.cji.0000175495.13476.1f. [DOI] [PubMed] [Google Scholar]
- 5.Caballero OL, Chen YT. Cancer/testis (CT) antigens: potential targets for immunotherapy. Cancer Sci. 2009;100:2014–21. doi: 10.1111/j.1349-7006.2009.01303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen YT, Scanlan MJ, Sahin U, Tureci O, Gure AO, Tsang S, et al. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci U S A. 1997;94:1914–8. doi: 10.1073/pnas.94.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrow C, Browning J, MacGregor D, Davis ID, Sturrock S, Jungbluth AA, et al. Tumor antigen expression in melanoma varies according to antigen and stage. Clin Cancer Res. 2006;12:764–71. doi: 10.1158/1078-0432.CCR-05-1544. [DOI] [PubMed] [Google Scholar]
- 8.Goydos JS, Patel M, Shih W. NY-ESO-1 and CTp11 expression may correlate with stage of progression in melanoma. J Surg Res. 2001;98:76–80. doi: 10.1006/jsre.2001.6148. [DOI] [PubMed] [Google Scholar]
- 9.Gure AO, Chua R, Williamson B, Gonen M, Ferrera CA, Gnjatic S, et al. Cancer-testis genes are coordinately expressed and are markers of poor outcome in non-small cell lung cancer. Clin Cancer Res. 2005;11:8055–62. doi: 10.1158/1078-0432.CCR-05-1203. [DOI] [PubMed] [Google Scholar]
- 10.Jungbluth AA, Antonescu CR, Busam KJ, Iversen K, Kolb D, Coplan K, et al. Monophasic and biphasic synovial sarcomas abundantly express cancer/testis antigen NY-ESO-1 but not MAGE-A1 or CT7. Int J Cancer. 2001;94:252–6. doi: 10.1002/ijc.1451. [DOI] [PubMed] [Google Scholar]
- 11.Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29:917–24. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaughan HA, Svobodova S, Macgregor D, Sturrock S, Jungbluth AA, Browning J, et al. Immunohistochemical and molecular analysis of human melanomas for expression of the human cancer-testis antigens NY-ESO-1 and LAGE-1. Clin Cancer Res. 2004;10:8396–404. doi: 10.1158/1078-0432.CCR-04-0809. [DOI] [PubMed] [Google Scholar]
- 13.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–9. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robbins PF, Li YF, El-Gamil M, Zhao Y, Wargo JA, Zheng Z, et al. Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. J Immunol. 2008;180:6116–31. doi: 10.4049/jimmunol.180.9.6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–46. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358:2698–703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–9. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forbes D. Patient’s own infection-fighting T cells put late-stage melanoma into long-term remission — without chemotherapy or radiation. 2008 http://www.fhcrc.org/about/ne/news/2008/06/18/T_cells.html.
- 19.Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192:5451–8. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nature Med. 2004;10:909–15. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radvanyi LG, Bernatchez C, Zhang M, Fox PS, Miller P, Chacon J, et al. Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2012;18:6758–70. doi: 10.1158/1078-0432.CCR-12-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robbins PF, Dudley ME, Wunderlich J, El-Gamil M, Li YF, Zhou J, et al. Cutting edge: Persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173:7125–30. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morgan RA, Chinnasamy N, Abate-Daga D, Gros A, Robbins PF, Zheng Z, et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother. 2013;36:133–51. doi: 10.1097/CJI.0b013e3182829903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linette GP, Stadtmauer EA, Maus MV, Rapoport AP, Levine BL, Emery L, et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. 2013;122:863–71. doi: 10.1182/blood-2013-03-490565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cameron BJ, Gerry AB, Dukes J, Harper JV, Kannan V, Bianchi FC, et al. Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci Transl Med. 2013;5:197ra103. doi: 10.1126/scitranslmed.3006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


