Abstract
Sleep perturbations including fragmented sleep with frequent night-time awakenings and daytime naps are common in patients with Alzheimer’s disease (AD), and these daily disruptions are a major factor for institutionalization. The objective of this study was to investigate if sleep-wake patterns are altered in 5XFAD mice, a well-characterized double transgenic mouse model of AD which exhibits an early onset of robust AD pathology and memory deficits. These mice have five distinct human mutations in two genes, the amyloid precursor protein (APP) and Presenilin1 (PS1) engineered into two transgenes driven by a neuron specific promoter (Thy1), and thus develop severe amyloid deposition by 4 months of age. Age matched (4–6.5 months old) male and female 5XFAD mice were monitored and compared to wild-type littermate controls for multiple sleep traits using a non-invasive, high throughput, automated piezoelectric system which detects breathing and gross body movements to characterize sleep and wake. Sleep-wake patterns were recorded continuously under baseline conditions (undisturbed) for 3 days and after sleep deprivation of 4 hours, which in mice produces a significant sleep debt and challenge to sleep homeostasis. Under baseline conditions, 5XFAD mice exhibited shorter bout lengths (14% lower values for males and 26% for females) as compared to controls (p<0.001). In females, the 5XFAD mice also showed 12% less total sleep than WT (p<0.01). Bout length reductions were greater during the night (the active phase for mice) than during the day, which does not model the human condition of disrupted sleep at night (the inactive period). However, the overall decrease in bout length suggests increased fragmentation and disruption in sleep consolidation that may be relevant to human sleep. The 5XFAD mice may serve as a useful model for testing therapeutic strategies to improve sleep consolidation in AD patients.
Keywords: sleep, sleep homeostasis, amyloid beta, diurnal rhythm, sleep fragmentation
1. Introduction
Alzheimer’s disease (AD), which is characterized by accumulation of extracellular amyloid beta (Aβ) plaques and intra-neuronal hyperphosphorylated neurofibrillary tau tangles in the brain, is the most common form of dementia (Glenner and Wong, 1984). Aside from severe cognitive deficits, approximately 25 to 40% of AD patients also display profound circadian rhythm and sleep-wake disturbances, which may precede overt cognitive impairments (Carpenter et al., 1996, Moran et al., 2005). These disturbances include fragmented sleep, frequent nighttime awakenings, and excessive daytime sleepiness (Prinz et al., 1982, Bliwise, 2004, Bliwise et al., 2011). Altered sleep architecture in AD includes reduced rapid eye movement (REM) and slow wave (SWS) sleep in addition to increased latency to REM sleep (Prinz et al., 1982, Bliwise et al., 1989, Perry et al., 1999). Fragmented sleep, which is also common in many other pathological conditions including Parkinson’s Disease, Diffuse Lewy Body Disease (DLBD), sleep apnea, and neuromuscular disorders, has wide spread consequences ranging from excessive daytime sleepiness to impaired memory consolidation (Kimoff, 1996, Dauvilliers, 2007, Deschenes and McCurry, 2009, Rolls et al., 2011). Recent studies suggest that reduced slow wave sleep, which has been shown to have restorative functions, might be the contributing factor to this impaired memory consolidation (Walker, 2009). However, there is still much debate regarding the contribution of different sleep stages in the consolidation of different type of memories, with some data supporting a role for all stages of NREM in declarative memory and a greater role for REM in non-declarative memory (Tucker et al., 2006, Marshall and Born, 2007, Nishida et al., 2009, Diekelmann and Born, 2010).
In regard to circadian system dysfunction, Saitlin et al found that AD subjects have reduced locomotor activity and phase delays of approximately four hours in their activity rhythms and three hours for the core body temperature rhythm compared to healthy elderly subjects (Satlin et al., 1995). Often, AD patients also display “sundowning”; a behavioral state characterized by increased aggressiveness, restlessness and anxiety seen towards the afternoon and evening hours (Vitiello et al., 1992). These changes in sleep and circadian rhythms, which correlate positively with the degree of progression of AD, not only affect the quality of life of patients and their care givers but also constitute one of the major factors for institutionalization (Pollak and Perlick, 1991, Vitiello et al., 1991).
Aggregation of amyloid beta (Aβ) in the brain has been implicated in sleep perturbations as well as in the pathogenesis of AD (Hardy and Higgins, 1992, Hardy and Selkoe, 2002). Various findings suggest that Aβ aggregation, as indicated by reduced cerebrospinal fluid (CSF) Aβ42 levels, begins as early as 15 years prior to the appearance of clinical symptoms (i.e. the preclinical stage) (Morris and Price, 2001, Perrin et al., 2009, Sperling et al., 2011). Even in asymptomatic individuals, Aβ is associated with neural dysfunction of the brain networks subserving memory formation (Sheline et al., 2010). Among cognitively unimpaired individuals, those with higher levels of Aβ accumulation had poorer sleep quality and shorter sleep duration compared to controls without Aβ plaques (Ju et al., 2013, Malkki, 2013, Spira et al., 2013).
Various studies performed in mouse models of AD also indicate an association between sleep perturbations and AD pathogenesis. Using microdialysis, Kang et al demonstrated that young Tg2576 mice (a model of AD), and wild type mice (C57BL6) have diurnal oscillations of brain interstitial fluid (ISF) Aβ, with higher levels during the active phase (night-time) (Kang et al., 2009). In aged APPswe/PS1δE9 mice with prominent Aβ plaques, sleep is disrupted as well as ISF Aβ diurnal rhythm is lost (Roh et al., 2012). Further, sleep deprivation increases ISF Aβ, which decreases during sleep recovery (Kang et al., 2009, Roh et al., 2012). Similar diurnal oscillations were found for CSF (cerebrospinal fluid) Aβ in healthy human subjects, with higher Aβ levels in day and reduced levels at night (Kang et al., 2009). These studies suggest that sleep loss accelerates the Aβ deposition and therefore sleep alterations may serve not only as an early marker of AD but also raise the possibility that improved sleep could slow progression of the disease. The extent to which the changes in sleep-wake patterns contribute to or are the result of AD progression is poorly understood. Because studies in AD patients are difficult and expensive, an animal model displaying sleep alterations that mimic those found in AD is necessary.
Our present study aimed at investigating whether 5XFAD mice (MGI: 3693208), a well-characterized, double transgenic model of familial, early onset AD, show alterations in sleep-wake patterns. These mice have five distinct human mutations: three in the amyloid precursor protein (APP) namely Swedish, Florida and London mutations (K670N/M671L, I716V, V717I) and two in the Presenilin1 protein (PS1), i.e., mutations M146Ll and L286V engineered into two transgenes driven by a neuron specific promoter (Thy1). Each of these PS1 and APP mutations increase Aβ42 production but when present together act additively to bring about an excessive Aβ42 burden and hence early onset and aggressive AD pathology (Citron et al., 1997, Eckman et al., 1997, Citron et al., 1998, Oakley et al., 2006). These mice thus develop severe intraneuronal Aβ42 at an early age of 1.5 months, amyloid deposition at 2 months, and loss of synapses around 9 months of age (Oakley et al., 2006). As well as aggressive neuropathology, 5XFAD mice exhibit memory deficits as early as 4–6 months of age, in a range of behavioral assays such as Y maze, Morris water maze, contextual fear conditioning, auditory trace fear conditioning paradigm, and olfactory H maze (Oakley et al., 2006, Ohno et al., 2006, Ohno, 2009, Devi and Ohno, 2010, Girard et al., 2013). Since the 5XFAD mice exhibit well characterized, early onset AD-like neuropathological changes and cognitive impairments, the current study investigated whether these mice also exhibit sleep alterations similar to those reported in AD patients. Since AD affects men and women, we included both male and female 5XFAD mice in our study. More women are known to have AD compared to men, possibly because of longer life expectancy in women (Hebert et al., 2001) or due to hormonal alterations late in life (Morinaga et al., 2011, Barron and Pike, 2012, O'Hagan et al., 2012, Lan et al., 2014). In this study, we analyzed the following sleep traits: sleep during the day and night, sleep bouts during the day and night under baseline conditions, and then examined sleep behavior again after 4 hour sleep deprivation in an effort to find if 5XFAD mice model some aspects of the sleep alterations reported in human AD patients.
2. Experimental procedures
2.1. Animal and housing conditions
This study utilized individually housed 5XFAD mice (males: N=10; females: N=7) and wild type mice (males: N=7; females: N=11) for baseline recording and sleep deprivation protocol was applied on 5XFAD (males: N=9; females: N=6) and wild type mice (males: N=6; females: N=11) of 4–6.5 months of age (lost data for few mice because of system failure), obtained from a breeding colony maintained at University of Kentucky. The original 5XFAD stock was provided by The Jackson Laboratories. Originally, 5XFAD were generated on B6/SJL background as previously described (Oakley et al., 2006). This mouse model co-expresses three APP (Swedish: K670N/M671L, Florida: I716V and London mutation: V717I) and two PS1 human familial mutations (M146L, L286V) under the regulation of neuron-specific murine Thy1 promoter. These mice show intracellular Aβ accumulation at the age of 1.5 months. Plaque deposition can be detected since 2 months of age; first appearing in deep layers of cortex and subiculum and eventually spreading to most of the cortex, subiculum and hippocampus. Apart from neuroinflammation, these mice also present neuronal loss, a characteristic often missing in most of the other transgenic mouse models of AD (Oakley et al., 2006, Jawhar et al., 2012, Eimer and Vassar, 2013). In 5XFAD mice, synaptic degeneration as evident from reduced expression of synaptic markers is seen commencing at the age of 4 months, the same age at which various hippocampal- and cortical- dependent memory impairments have been observed (Oakley et al., 2006, Ohno et al., 2006, Ohno, 2009, Devi and Ohno, 2010, Girard et al., 2013). The 5XFAD mice have also been reported to have lower body weight (~10%) than wild-type controls at 6–7 months of age (Jawhar et al., 2012, Bhattacharya et al., 2014); whether this results from changes in food intake or metabolism has not been reported, to the best of the authors’ knowledge.
In the current study, all mice were exposed to an alternating light (L): dark cycle (D), with lights on from 7 AM to 7 PM. Food (pellets) and water were provided ad libitum. All experimental procedures (described below) were approved by the Institutional Animal Care and Use Committee at the University of Kentucky and are consistent with the Institute of Laboratory Animal Resources Guide for Care and Use of Laboratory Animals, 8th edition.
2.2. Sleep recording with piezoelectric system
Sleep and wake states were determined using a piezoelectric system, as described previously (Flores et al., 2007, Donohue et al., 2008). The system is comprised of plexiglass cages lined with piezoelectric films across the bottom that detect pressure variations. For all sleeping postures of the mouse, pressure variations from breathing are detected. Sleep states are characterized by quasi-periodic signals with low variations in amplitude, whereas wakefulness and rest states are characterized by irregular transient and high amplitude pressure variations corresponding to conscience body movements and weight shifting. Signal features sensitive to the differences between the sleep and wake states are extracted from the short-time pressure signal segments, and classification is automatically performed every 2 seconds. Data collected from the piezo system were binned over specified time periods (e.g. 5 minutes, 1 hour) using a rolling average of the percent sleep, as well as binned by length of individual bouts of sleep and the mean bout lengths were calculated. The sleep bouts were computed as the duration of contiguous sleep states. Sleep bouts were terminated by any arousal more than 2 seconds in duration. When counting all short arousals and short sleep bouts, average bouts in mice are typically less than 1 minute (Franken et al., 1999). The piezo system has been validated with EEG and human observations and demonstrates a classification accuracy of over 90% (Donohue et al., 2008, Mang et al., 2014).
Prior to sleep recording, the mice were acclimated in the plexiglass cages for 2–4 days. For the baseline measurements, mice were recorded for 3–5 days, during which time the mice were undisturbed except for monitoring once daily for food and water. The parameters that were analyzed under baseline conditions included total sleep time averaged over 24 h, average percentage of sleep across day (light phase), average percentage of sleep across night (dark phase), average sleep bout length (across 24 hours, day and night), activity onset defined as the time relative to dark onset when the first sharp increase in percent wake states computed over a 2-hour sliding window occurs between 3 hours before and 3 hours after dark onset, on each day. This is typically the largest increase in this period, increasing from below 40% wake time to over 80% wake time. Diurnal wake ratios are related to the differential wake percentages during the light and dark phases, and are defined as the ratio of maximum wake-state percent in the dark phase to the minimum wake-state percent in the light phase, where percentages are computed over 3-hours intervals. Activity onset, as defined above, was also used a phase marker for the daily rhythm of sleep and activity.
For the second part of the study, the mice were sleep deprived for 4 hours beginning either at 8 or 9 AM. Sleep deprivation was accomplished by transferring the mice to novel cages. To keep the mice awake, nestlets (squares of cotton fibers that mice shred to build nests) and other novel objects were introduced to the cages, and cages were tapped gently when mice appeared ready to sleep. As the four hours progressed, more action was needed to keep mice awake. First, cage lids were gently removed and then replaced, providing additional air flow and olfactory and visual stimulation. If this failed to arouse the mice, then they were gently manipulated to induce movement. At the end of the sleep deprivation protocol, the mice were transferred back to their piezoelectric cages to continue monitoring sleep bout lengths and total amount of sleep.
2.3. Data analysis
The data were analyzed using SPSS statistics software version 20.0. Group data was analyzed by a general linear model of analysis of variance (ANOVA) for the baseline studies. Initial assessment showed that males and females differed significantly from each other (not shown), consequently all the data were pooled and analyzed separately for the two sexes. Before conducting ANOVA, the data were tested for normal distribution and homogeneity of variance. For all of the sleep-wake parameters under consideration (listed above), P-value less than 0.05 were considered significant. Genotype was considered as an independent variable and the parameter under observation as the dependent variable. Hourly sleep percentage and bout length after 4 hour of sleep deprivation were analyzed with mixed ANOVA.
2.4. Post-mortem genotyping
After the sleep recording was complete, the mice were euthanized by CO2 inhalation and decapitation. From dissected brains, cortex was preserved at −70° C for genotyping. Genotyping was conducted using conventional PCR or/and three-step serial extractions of Aβ with sequentially increasing denaturing conditions followed by quantification with a two-site (sandwich) ELISA as described previously (Kukar et al., 2005, McGowan et al., 2005, Beckett et al., 2010, Bruce-Keller et al., 2011). The primers used are listed here:
PCR Primer Sequence (5' to 3')
APP Forward: AGAGTACCAACTATGACTACG
APP Reverse: ATGCTGGATAACTGCCTTCTTATC
PS1 Forward: ATGACAGAGTTACCTGCACCGTTG
PS1 Reverse: CTGACTTAATGGTAGCCACGACCA
3. Results
3.1. Sleep under baseline (undisturbed) conditions
Sleep-wake patterns were monitored in 5XFAD mice in order to determine the effects of Aβ42 overexpression. Statistical analyses (ANOVA) showed that 5XFAD male mice did not differ from control littermates in the average total amount of sleep [i.e., sleep across 24h (F(1,49)= 1.11, P=0.298)]; daytime sleep (F(1,49)=0.48, P=0.493), or nighttime sleep (F(1,49)=0.63, P=0.431) (Figure 1 and Table 1)]. In the case of females, 5XFAD mice showed a significant reduction in average total amount of sleep (F(1,52)=7.09, P=0.01) as well as nighttime sleep (F(1,52)=7.54, P=0.008), compared to wild-type mice (Figure 1). Additionally, average total sleep bout length (across 24h) was reduced in 5XFAD mice of both the sexes; 14% in males and 26% in females (Figure 1). The decreased bout length in 5XFAD mice was observed during both the light phase and the dark phase (male mice: average bout length across 24h, F(1,49)=12.12, P=0.001; daytime, F(1,49)=7.97, P=0.007; nighttime, F(1,49)=8.77, P=0.005) and for female mice: average bout length across 24h, F(1,52)=24.18, P<0.001; daytime, F(1,52)=10.48, P=0.002; nighttime, F(1,52)=34.48, P<0.001).
Fig. 1.
Sleep-wake patterns in 5XFAD and WT littermates under baseline conditions. Average percent sleep across 3 consecutive days analyzed over (A) 24 hours, (B) dark phase, and (C) light phase. Female but not male 5XFAD mice show reduction in sleep duration across 24 h and during the dark phase. (D to F) depicts average bout length in seconds (s) over (D) 24 h (E) dark phase, and (F) light phase. 5XFAD mice of both sexes had shorter average bout lengths across all phases in both the sexes. Values represent means ± SEM. *: P < 0.05; **P <0.01, ***P < 0.001
Table 1.
Effect of genotype on sleep wake traits under baseline conditions
| Parameter | Wild type | 5XFAD | Wild type | 5XFAD |
|---|---|---|---|---|
| Male | Male | Female | Female | |
| Percent sleep day | 56.5±0.8 | 55.7±0.8 | 55.1± 1.4 | 52.5± 0.8 |
| Percent sleep night | 27.1±1.5 | 25.4±1.4 | 23.2± 1.3 | 17.2± 1.8** |
| Percent sleep total-24h | 41.8±0.8 | 40.6±0.8 | 39.1± 1.1 | 34.8± 0.9* |
| Bout length day (sec) | 60.4±2.0 | 54.0±1.3** | 65.6± 2.1 | 54.0± 3.0** |
| Bout length night (sec) | 46.1±2.5 | 37.0± 1.9** | 33.7± 1.2 | 21.0± 1.9*** |
| Bout length total-24h (sec) | 54.5±1.9 | 46.9±1.2** | 50.4± 1.2 | 39.9± 2.0*** |
| Activity onset (hrs after dark onset) | 0.11±0.13 | 0.13±0.18 | 0.14±0.17 | 0.01±0.10 |
Values represent means ± SEM.
: P < 0.05;
P <0.01,
P < 0.001.
All comparisons are between WT and 5XFAD of the same sex.
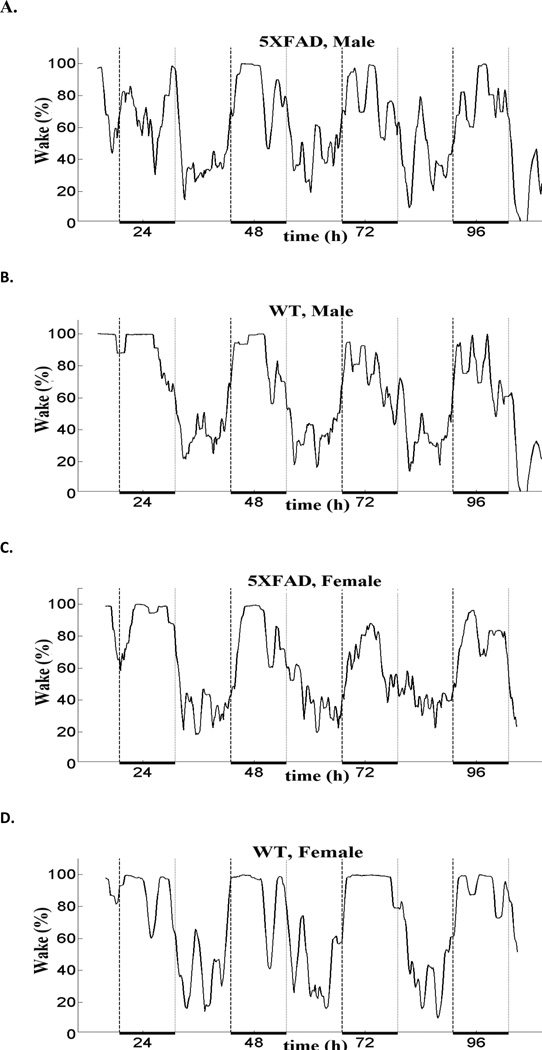
There was no apparent genotypic difference in the sleep wake profile for both the sexes compared to their control littermates (Figure 2). Both WT and 5XFAD mice had activity onsets closely coinciding with dark onset, suggesting that there was no apparent change in phase of the daily sleep-wake rhythms. Similarly, there was no change in the peak activity or diurnal wake ratio. For males, the diurnal wake ratio (mean ± S.E.M.) was: WT, 2.67±0.21, and 5XFAD, 2.93±0.34. For females, the diurnal wake ratio (mean ± S.E.M.) was: WT, 4.46±0.33 and 5XFAD, 4.38±0.58. The higher ratio in females was due to less sleep during the dark period, as is typical for female mice.
Fig. 2.
Representative sleep-wake profiles for 5XFAD mice (A and C) and control littermates (B and D). The percent wake plotted on the Y axis is represented as a sliding average over a 2 hour window. Hours of recording are plotted on the X-axis where 0 represents the midnight of day 1. Broken vertical lines demarcate the dark phase, which is also indicated by a heavy horizontal black line at the bottom.
3.2. Sleep after 4-h sleep deprivation
Percent of sleep and bout length (dependent variables) in the 6 hours immediately after sleep deprivation (4 hours) was compared between wild type and transgenic (5XFAD) groups using mixed ANOVA model with the genotype as the between-subjects variable and time (6 time points) as the repeated measure (or within subject variable). There was no interaction between genotype and time for bout length (males: F (5,65)=0.49, P=0.782; females: F (5,75)=0.580, P=0.715) and sleep duration (males: F (5,65)=1.55, P=0.186; females: F (5,75)=0.922, P=0.471) after sleep deprivation for either sex (Table 2 and Figure 3).
Table 2.
Effect of genotype on sleep wake traits after sleep deprivation (SD)
| Percent sleep post-SD | ||||||
| 1st h | 2nd h | 3rd h | 4th h | 5th h | 6th h | |
| WT Male | 24.4±6.2 | 65.9±7.3 | 46.9±8.5 | 30.0±7.5 | 41.4±10.3 | 10.4±8.2 |
| 5XFAD Male | 24. 9±5.1 | 49.1±5.9 | 49.8±7.0 | 39.9±6.1 | 35.3±8.4 | 23.7±6.7 |
| WT Female | 60.4±5.9 | 67.4±4.2 | 67.8±4.6 | 62.8±4.2 | 54.2±6.2 | 59.3±4.1 |
| 5XFAD Female | 50.3±8.1 | 57.4± 5.7 | 54.3±6.3 | 56.3±5.7 | 55.6±8.5 | 42.5±5.6 |
| Bout length (seconds) post-SD | ||||||
| 1st h | 2nd h | 3rd h | 4th h | 5th h | 6th h | |
| WT Male | 22.9±4.5 | 55.9±6.7 | 51.6±10.3 | 37.5±7.0 | 48.8±23.3 | 22.1±10.5 |
| 5XFAD Male | 20.5±3.7 | 36.7±5.5 | 43.5±8.4 | 34.9±5.7 | 50.6±19.0 | 25.9±8.6 |
| WT Female | 42.5±5.1 | 50.4±4.7 | 52.0±5.1 | 48.4±5.3 | 45.0±4.6 | 50.5±5.5 |
| 5XFAD Female | 45.4±6.9 | 44.2±6.4 | 46.6±6.9 | 43.7±7.1 | 37.7±6.3 | 39.6±7.4 |
Values represent mean ± SEM
Fig. 3.
Comparison of the sleep-wake patterns of WT and 5XFAD mice after a sleep deprivation of 4 h. Average percent sleep in males (A), females (B), and average bout length in male (C) and female mice (D) was analyzed for 6 h of the recovery period. No genotype difference was found using Mixed ANOVA.
4. Discussion
Sleep has become a key avenue of research in the quest to find mechanisms underlying Alzheimer’s disease and development of effective therapeutics. It plays a variety of roles crucial to maintaining optimal brain functions and has been found to be closely linked to AD pathology. One source of evidence comes from a study where improved sleep lowered the risk of AD in people with at least one APOE ε4 allele (Lim et al., 2013). This finding is consistent with other studies showing that AD patients frequently have poor quality of sleep, even before the onset of clear symptoms. Poor sleep may be one factor contributing to their compromised cognition since sleep plays a critical role in learning, memory, and other brain functions (Durmer and Dinges, 2005, Killgore et al., 2006, Killgore et al., 2008, Ker et al., 2010). In a recent study, Lim and colleagues found that loss of neurons in the intermediate nucleus, a proposed homologue of the rodent ventrolateral preoptic nucleus (VLPO), is a potential contributing factor for the fragmented sleep seen in older individuals including Alzheimer’s patients (Lim et al., 2014).
This study aimed at identifying sleep-wake alterations in the AD mouse model- 5XFAD, that recapitulates certain features of the human AD condition and may help in understanding the underlying mechanisms of this disease. Both male and females 5XFAD mice belonged to the age group, 4 to 6.5 months, which shows many of the pathological characteristics of AD including accumulation of intraneuronal Aβ, cerebral plaque deposition, gliosis, synaptic degeneration, neuronal loss, and memory deficits (Oakley et al., 2006, Ohno et al., 2006, Jawhar et al., 2012, Eimer and Vassar, 2013). Early onset of the robust pathology seen in these mice attributable to the incorporation of five additive mutations lead to increased total Aβ production which makes the 5XFAD mouse a useful experimental tool for investigating the effects of increased Aβ42 levels which is thought to be one of the key factors involved in disease progression. Also, only a handful of previous studies of AD mouse models have included both sexes or investigated sleep fragmentation.
Our findings show that under baseline conditions, average length of sleep bouts was reduced in both male and female 5XFAD mice. In addition, female mice also had significant reduction in total sleep time averaged over 3 days and sleep occurring during the dark periods. However, male mice did not differ from control littermates in their sleep duration. In contrast to initial expectations, reductions in bout length were found to be greater during the night (the active phase in mice), which does not necessarily model the human condition of disrupted sleep at night (the usual inactive phase for humans). However, the overall decrease in bout lengths in the 5XFAD mice suggests increased fragmentation and disruption in sleep consolidation throughout the day. This finding is likely to be relevant to human sleep disturbances, since mice (unlike humans) usually exhibit considerable amounts of sleep during both the day and night. Assessment of the sleep-wake parameters for the 6 h immediately after sleep deprivation (for 4 h) indicated that genotype did not affect bout length or sleep percentage in either males or females, although there was a general trend of reduced bout length in both sexes.
In general, our findings of decreased sleep bout lengths in 5XFAD mice support and extend previous findings of differences in sleep physiology in other AD mouse models. Reduced NREM duration has been reported in PLB1triple knock in mice (hAPP/hTau/hPS1), whereas lower REM sleep (during light period) was observed in PDAPP (overexpresses hβAPP) and Tg2576 mice (Huitron-Resendiz et al., 2002, Zhang et al., 2005, Platt et al., 2011). APPswe/PS1δE9 mice aged 9 months had reduced REM and NREM sleep stages across both light and dark phases (Roh et al., 2012). However, some AD mouse models, such as APP/PS1 knock-in mice, do not exhibit obvious changes in sleep (Duncan et al., 2012).
In the current study, we did not find any change in the phase of the rhythm in 5XFAD which replicates previous findings in APP/PS1 mice and other AD mouse models (Sterniczuk et al., 2010, Duncan et al., 2012). In this respect, the AD mice do not closely resemble the AD patients, which show large delays in the phase of their activity and temperature rhythms compared to those of normal elderly subjects. However, there were sex differences in the 5XFAD transgenic mice. The mechanisms causing the sex differences in sleep over 24 hours and sleep at night in the 5XFAD mice are unknown. It is possible that sex disparity in Aβ levels contributes to the sleep differences. Oakley et al in their studies on 5XFAD have reported that Aβ42 levels were higher in young females compared to age-matched males (until at least 9 months of age). This may explain the differences found between the two sexes in our study. Further, this observation indicates that the extent of sleep disruptions may be linked to the levels of Aβ as proposed by previous studies. In addition, some studies indicate that hormonal alterations in the later part of life in women may pose a higher risk of AD for them as compared to men, although some studies indicate otherwise (Morinaga et al., 2011, Barron and Pike, 2012, O'Hagan et al., 2012, Lan et al., 2014). It is possible that there may be other underlying causes present which require further investigation. In the 5XFAD mice, Devi et al illustrated that stressful conditions resulted in higher Aβ42 levels and plaque burden in hippocampus of females but not in males (Devi et al., 2010). Sex differences were also seen in a study of Tg2576 mice (Wisor et al., 2005). Post AD pathology (22 month old), females in addition to exhibiting sleepwake alterations common to males also showed increased REMS. However, Tg2576 mice (15–17 months) did not show any significant effect of sex or sex X genotype interaction on theta to delta ratio in the EEG (Wisor et al., 2005). In another study in 3XTg mice, males with AD pathology did not show genotypic differences in circadian phase shifts post AD pathology but females had a tendency towards large circadian phase shifts in response to light pulses presented in the early subjective night (Sterniczuk et al., 2010).
While the present findings indicate that the 5XFAD mice exhibits some sleep alterations that are relevant to AD, there were also some limitations to this study. One limitation was that the algorithms currently used by the piezoelectric system do not distinguish REM sleep from NREM sleep, although algorithms under development may be able to do this in the future. Also, it should be kept in mind that this mouse model represents the advanced stage of AD with its early onset and extensive pathology.
5XFAD mice show amyloid pathology- an important characteristic of Alzheimer’s disease - but fail to exhibit hyperphosphorylated tau. Those AD mouse models that do show tau pathology differ from human clinical presentation in important AD features like neuronal loss and intraneuronal Aβ (Wirths and Bayer, 2010, Li et al., 2011). In spite of their various limitations, the 5XFAD mice and other AD mouse models exhibit sleep alterations that resemble some aspects of the sleep disruptions reported in AD patients. As described above, the 5XFAD mouse model is especially useful because it exhibits neuronal loss, similar to AD patients, and the early onset pathology in the 5XFAD mice allows them to be studied at younger ages than other AD mouse models. Therefore, this mouse model is useful for investigations of the role of sleep loss in the progression of AD.
Recent studies show that sleep impacts Aβ levels in the brain. Diurnal oscillations of Aβ levels in human cerebrospinal fluid (CSF) and in mouse hippocampal interstitial fluid (ISF) exhibit lowest values during the rest phase. Furthermore, sleep deprivation during the normal rest phase elevates these Aβ levels (Kang et al., 2009). The diurnal rhythms of Aβ levels become attenuated and eventually lost as Aβ deposition in the brain progresses (Roh et al., 2012). These changes begin in parallel to onset of sleep disruptions in mice (Roh et al., 2012). Further, a recent study by Xie and colleagues demonstrated that sleep strongly increases clearance of Aβ, one of the metabolites generated by neuronal activity, which is greatest during wakefulness (Xie et al., 2013). These studies further support the concept that sleep disruption may be one of the causal factors involved in progression of the AD. A feedback loop might exist where Aβ accumulation might deteriorate sleep quality which could lead to further Aβ accumulation and increasing the susceptibility of the patients further to the pathophysiological changes associated with AD.
5. Conclusions
The 5XFAD mouse model of AD overexpresses amyloid β at an early stage and is therefore useful in studying the effect of Aβ on sleep. Our findings showed various sleep-wake alterations in both male and female 5XFAD mice under baseline conditions and also after sleep deprivation. The overall decrease in bout length suggests increased fragmentation and disruption in sleep consolidation that may be relevant to human sleep disturbances in AD and other neurological diseases. Because sleep disturbances precede overt AD symptoms by ten years or more, and experimental sleep disruption accelerates Aβ deposition, sleep enhancement may be a valuable therapeutic target for treatment of AD that can be investigated in 5XFAD mice.
AD patients have disturbed sleep, including increased sleep fragmentation.
Sleep and wake patterns in 5XFAD mice, a model of AD, were examined.
5XFAD mice of both sexes were found to have reduced sleep bout lengths.
Female 5XFAD were more severely affected, and had reduced total sleep as well.
Sleep alterations in 5XFAD mice may be relevant to human AD sleep disturbances.
Acknowledgments
This study was funded by NIH RO1AG13418 (MJD) and KSCHIRT award (Kentucky Spinal Cord and Head Injury Research Trust) grant 10-5A (BFH). Sleep monitoring was conducted at the University of Kentucky's Rodent Behavior Core: www.rodentbehaviorcore.uky.edu.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barron AM, Pike CJ. Sex hormones, aging, and Alzheimer's disease. Frontiers in bioscience. 2012;4:976–997. doi: 10.2741/e434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett TL, Niedowicz DM, Studzinski CM, Weidner AM, Webb RL, Holler CJ, Ahmed RR, LeVine H, Murphy MP. Effects of nonsteroidal anti-inflammatory drugs on amyloid-beta pathology in mouse skeletal muscle. Neurobiology of disease. 2010;39:449–456. doi: 10.1016/j.nbd.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S, Haertel C, Maelicke A, Montag D. Galantamine slows down plaque formation and behavioral decline in the 5XFAD mouse model of Alzheimer's disease. PloS one. 2014;9:e89454. doi: 10.1371/journal.pone.0089454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliwise DL. Sleep disorders in Alzheimer's disease and other dementias. Clin Cornerstone. 2004;6(Suppl 1A):S16–S28. doi: 10.1016/s1098-3597(04)90014-2. [DOI] [PubMed] [Google Scholar]
- Bliwise DL, Mercaldo ND, Avidan AY, Boeve BF, Greer SA, Kukull WA. Sleep disturbance in dementia with Lewy bodies and Alzheimer's disease: a multicenter analysis. Dementia and geriatric cognitive disorders. 2011;31:239–246. doi: 10.1159/000326238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliwise DL, Tinklenberg J, Yesavage JA, Davies H, Pursley AM, Petta DE, Widrow L, Guilleminault C, Zarcone VP, Dement WC. REM latency in Alzheimer's disease. Biological psychiatry. 1989;25:320–328. doi: 10.1016/0006-3223(89)90179-0. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Gupta S, Knight AG, Beckett TL, McMullen JM, Davis PR, Murphy MP, Van Eldik LJ, St Clair D, Keller JN. Cognitive impairment in humanized APP×PS1 mice is linked to A beta(1–42) and NOX activation. Neurobiology of disease. 2011;44:317–326. doi: 10.1016/j.nbd.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter BD, Strauss ME, Patterson ME. Sleep Disturbances in Community-Dwelling Patients with Alzheimer's Disease. Clin Gerontorl. 1996;16:35–49. [Google Scholar]
- Citron M, Eckman CB, Diehl TS, Corcoran C, Ostaszewski BL, Xia W, Levesque G, St George Hyslop P, Younkin SG, Selkoe DJ. Additive effects of PS1 and APP mutations on secretion of the 42-residue amyloid beta-protein. Neurobiology of disease. 1998;5:107–116. doi: 10.1006/nbdi.1998.0183. [DOI] [PubMed] [Google Scholar]
- Citron M, Westaway D, Xia W, Carlson G, Diehl T, Levesque G, Johnson-Wood K, Lee M, Seubert P, Davis A, Kholodenko D, Motter R, Sherrington R, Perry B, Yao H, Strome R, Lieberburg I, Rommens J, Kim S, Schenk D, Fraser P, St George Hyslop P, Selkoe DJ. Mutant presenilins of Alzheimer's disease increase production of 42-residue amyloid beta-protein in both transfected cells and transgenic mice. Nature medicine. 1997;3:67–72. doi: 10.1038/nm0197-67. [DOI] [PubMed] [Google Scholar]
- Dauvilliers Y. Insomnia in patients with neurodegenerative conditions. Sleep medicine. 2007;8(Suppl 4):S27–S34. doi: 10.1016/S1389-9457(08)70006-6. [DOI] [PubMed] [Google Scholar]
- Deschenes CL, McCurry SM. Current treatments for sleep disturbances in individuals with dementia. Current psychiatry reports. 2009;11:20–26. doi: 10.1007/s11920-009-0004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi L, Alldred MJ, Ginsberg SD, Ohno M. Sex- and brain region-specific acceleration of beta-amyloidogenesis following behavioral stress in a mouse model of Alzheimer's disease. Molecular brain. 2010;3:34. doi: 10.1186/1756-6606-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi L, Ohno M. Genetic reductions of beta-site amyloid precursor protein-cleaving enzyme 1 and amyloid-beta ameliorate impairment of conditioned taste aversion memory in 5XFAD Alzheimer's disease model mice. The European journal of neuroscience. 2010;31:110–118. doi: 10.1111/j.1460-9568.2009.07031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekelmann S, Born J. The memory function of sleep. Nature reviews Neuroscience. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Donohue KD, Medonza DC, Crane ER, O'Hara BF. Assessment of a non-invasive high-throughput classifier for behaviours associated with sleep and wake in mice. Biomed Eng Online. 2008;7:14. doi: 10.1186/1475-925X-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan MJ, Smith JT, Franklin KM, Beckett TL, Murphy MP, St Clair DK, Donohue KD, Striz M, O'Hara BF. Effects of aging and genotype on circadian rhythms, sleep, and clock gene expression in APPxPS1 knock-in mice, a model for Alzheimer's disease. Experimental neurology. 2012;236:249–258. doi: 10.1016/j.expneurol.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Seminars in neurology. 2005;25:117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- Eckman CB, Mehta ND, Crook R, Perez-tur J, Prihar G, Pfeiffer E, Graff-Radford N, Hinder P, Yager D, Zenk B, Refolo LM, Prada CM, Younkin SG, Hutton M, Hardy J. A new pathogenic mutation in the APP gene (I716V) increases the relative proportion of A beta 42(43) Human molecular genetics. 1997;6:2087–2089. doi: 10.1093/hmg/6.12.2087. [DOI] [PubMed] [Google Scholar]
- Eimer WA, Vassar R. Neuron loss in the 5XFAD mouse model of Alzheimer's disease correlates with intraneuronal Abeta42 accumulation and Caspase-3 activation. Molecular neurodegeneration. 2013;8:2. doi: 10.1186/1750-1326-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores AE, Flores JE, Deshpande H, Picazo JA, Xie XMS, Franken P, Heller HC, Grahn DA, O'Hara BF. Pattern recognition of sleep in rodents using piezoelectric signals generated by gross body movements. Ieee T Bio-Med Eng. 2007;54:225–233. doi: 10.1109/TBME.2006.886938. [DOI] [PubMed] [Google Scholar]
- Franken P, Malafosse A, Tafti M. Genetic determinants of sleep regulation in inbred mice. Sleep. 1999;22:155–169. [PubMed] [Google Scholar]
- Girard SD, Baranger K, Gauthier C, Jacquet M, Bernard A, Escoffier G, Marchetti E, Khrestchatisky M, Rivera S, Roman FS. Evidence for early cognitive impairment related to frontal cortex in the 5XFAD mouse model of Alzheimer's disease. Journal of Alzheimer's disease : JAD. 2013;33:781–796. doi: 10.3233/JAD-2012-120982. [DOI] [PubMed] [Google Scholar]
- Glenner GG, Wong CW. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochemical and biophysical research communications. 1984;120:885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science (New York, NY) 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science (New York, NY) 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, McCann JJ, Beckett LA, Evans DA. Is the risk of developing Alzheimer's disease greater for women than for men? Am J Epidemiol. 2001;153:132–136. doi: 10.1093/aje/153.2.132. [DOI] [PubMed] [Google Scholar]
- Huitron-Resendiz S, Sanchez-Alavez M, Gallegos R, Berg G, Crawford E, Giacchino JL, Games D, Henriksen SJ, Criado JR. Age-independent and age-related deficits in visuospatial learning, sleep-wake states, thermoregulation and motor activity in PDAPP mice. Brain research. 2002;928:126–137. doi: 10.1016/s0006-8993(01)03373-x. [DOI] [PubMed] [Google Scholar]
- Jawhar S, Trawicka A, Jenneckens C, Bayer TA, Wirths O. Motor deficits, neuron loss, and reduced anxiety coinciding with axonal degeneration and intraneuronal Abeta aggregation in the 5XFAD mouse model of Alzheimer's disease. Neurobiology of aging. 2012;33:196, e129–e140. doi: 10.1016/j.neurobiolaging.2010.05.027. [DOI] [PubMed] [Google Scholar]
- Ju YE, McLeland JS, Toedebusch CD, Xiong C, Fagan AM, Duntley SP, Morris JC, Holtzman DM. Sleep quality and preclinical Alzheimer disease. JAMA neurology. 2013;70:587–593. doi: 10.1001/jamaneurol.2013.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science (New York, NY) 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ker K, Edwards PJ, Felix LM, Blackhall K, Roberts I. Caffeine for the prevention of injuries and errors in shift workers. The Cochrane database of systematic reviews. 2010 doi: 10.1002/14651858.CD008508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WD, Balkin TJ, Wesensten NJ. Impaired decision making following 49 h of sleep deprivation. Journal of sleep research. 2006;15:7–13. doi: 10.1111/j.1365-2869.2006.00487.x. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Rupp TL, Grugle NL, Reichardt RM, Lipizzi EL, Balkin TJ. Effects of dextroamphetamine, caffeine and modafinil on psychomotor vigilance test performance after 44 h of continuous wakefulness. Journal of sleep research. 2008;17:309–321. doi: 10.1111/j.1365-2869.2008.00654.x. [DOI] [PubMed] [Google Scholar]
- Kimoff RJ. Sleep fragmentation in obstructive sleep apnea. Sleep. 1996;19:S61–S66. doi: 10.1093/sleep/19.suppl_9.s61. [DOI] [PubMed] [Google Scholar]
- Kukar T, Murphy MP, Eriksen JL, Sagi SA, Weggen S, Smith TE, Ladd T, Khan MA, Kache R, Beard J, Dodson M, Merit S, Ozols VV, Anastasiadis PZ, Das P, Fauq A, Koo EH, Golde TE. Diverse compounds mimic Alzheimer disease-causing mutations by augmenting Abeta42 production. Nature medicine. 2005;11:545–550. doi: 10.1038/nm1235. [DOI] [PubMed] [Google Scholar]
- Lan YL, Zhao J, Li S. Update on the Neuroprotective Effect of Estrogen Receptor Alpha Against Alzheimer's Disease. Journal of Alzheimer's disease : JAD. 2014 doi: 10.3233/JAD-141875. [DOI] [PubMed] [Google Scholar]
- Li L, Cheung T, Chen J, Herrup K. A comparative study of five mouse models of Alzheimer's disease: cell cycle events reveal new insights into neurons at risk for death. International journal of Alzheimer's disease. 2011;2011:171464. doi: 10.4061/2011/171464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim AS, Ellison BA, Wang JL, Yu L, Schneider JA, Buchman AS, Bennett DA, Saper CB. Sleep is related to neuron numbers in the ventrolateral preoptic/intermediate nucleus in older adults with and without Alzheimer's disease. Brain. 2014;137 doi: 10.1093/brain/awu222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim AS, Yu L, Kowgier M, Schneider JA, Buchman AS, Bennett DA. Modification of the relationship of the apolipoprotein E epsilon4 allele to the risk of Alzheimer disease and neurofibrillary tangle density by sleep. JAMA neurology. 2013;70:1544–1551. doi: 10.1001/jamaneurol.2013.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkki H. Alzheimer disease: Sleep alleviates AD-related neuropathological processes. Nature reviews Neurology. 2013;9:657. doi: 10.1038/nrneurol.2013.230. [DOI] [PubMed] [Google Scholar]
- Mang GM, Nicod J, Emmenegger Y, Donohue KD, O'Hara BF, Franken P. Evaluation of a piezoelectric system as an alternative to electroencephalogram/ electromyogram recordings in mouse sleep studies. Sleep. 2014;37:1383–1392. doi: 10.5665/sleep.3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Born J. The contribution of sleep to hippocampus-dependent memory consolidation. Trends in cognitive sciences. 2007;11:442–450. doi: 10.1016/j.tics.2007.09.001. [DOI] [PubMed] [Google Scholar]
- McGowan E, Pickford F, Kim J, Onstead L, Eriksen J, Yu C, Skipper L, Murphy MP, Beard J, Das P, Jansen K, DeLucia M, Lin WL, Dolios G, Wang R, Eckman CB, Dickson DW, Hutton M, Hardy J, Golde T. A beta 42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron. 2005;47:191–199. doi: 10.1016/j.neuron.2005.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran M, Lynch CA, Walsh C, Coen R, Coakley D, Lawlor BA. Sleep disturbance in mild to moderate Alzheimer's disease. Sleep medicine. 2005;6:347–352. doi: 10.1016/j.sleep.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Morinaga A, Ono K, Takasaki J, Ikeda T, Hirohata M, Yamada M. Effects of sex hormones on Alzheimer's disease-associated beta-amyloid oligomer formation in vitro. Experimental neurology. 2011;228:298–302. doi: 10.1016/j.expneurol.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Morris JC, Price JL. Pathologic correlates of nondemented aging, mild cognitive impairment, and early-stage Alzheimer's disease. Journal of molecular neuroscience : MN. 2001;17:101–118. doi: 10.1385/jmn:17:2:101. [DOI] [PubMed] [Google Scholar]
- Nishida M, Pearsall J, Buckner RL, Walker MP. REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cerebral cortex. 2009;19:1158–1166. doi: 10.1093/cercor/bhn155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hagan TS, Wharton W, Kehoe PG. Interactions between oestrogen and the renin angiotensin system - potential mechanisms for gender differences in Alzheimer's disease. American journal of neurodegenerative disease. 2012;1:266–279. [PMC free article] [PubMed] [Google Scholar]
- Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R, Vassar R. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M. Failures to reconsolidate memory in a mouse model of Alzheimer's disease. Neurobiology of learning and memory. 2009;92:455–459. doi: 10.1016/j.nlm.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M, Chang L, Tseng W, Oakley H, Citron M, Klein WL, Vassar R, Disterhoft JF. Temporal memory deficits in Alzheimer's mouse models: rescue by genetic deletion of BACE1. The European journal of neuroscience. 2006;23:251–260. doi: 10.1111/j.1460-9568.2005.04551.x. [DOI] [PubMed] [Google Scholar]
- Perrin RJ, Fagan AM, Holtzman DM. Multimodal techniques for diagnosis and prognosis of Alzheimer's disease. Nature. 2009;461:916–922. doi: 10.1038/nature08538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry E, Walker M, Grace J, Perry R. Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends in neurosciences. 1999;22:273–280. doi: 10.1016/s0166-2236(98)01361-7. [DOI] [PubMed] [Google Scholar]
- Platt B, Drever B, Koss D, Stoppelkamp S, Jyoti A, Plano A, Utan A, Merrick G, Ryan D, Melis V, Wan H, Mingarelli M, Porcu E, Scrocchi L, Welch A, Riedel G. Abnormal cognition, sleep, EEG and brain metabolism in a novel knock-in Alzheimer mouse, PLB1. PloS one. 2011;6:e27068. doi: 10.1371/journal.pone.0027068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak CP, Perlick D. Sleep problems and institutionalization of the elderly. Journal of geriatric psychiatry and neurology. 1991;4:204–210. doi: 10.1177/089198879100400405. [DOI] [PubMed] [Google Scholar]
- Prinz PN, Peskind ER, Vitaliano PP, Raskind MA, Eisdorfer C, Zemcuznikov N, Gerber CJ. Changes in the sleep and waking EEGs of nondemented and demented elderly subjects. Journal of the American Geriatrics Society. 1982;30:86–93. doi: 10.1111/j.1532-5415.1982.tb01279.x. [DOI] [PubMed] [Google Scholar]
- Roh JH, Huang Y, Bero AW, Kasten T, Stewart FR, Bateman RJ, Holtzman DM. Disruption of the sleep-wake cycle and diurnal fluctuation of beta-amyloid in mice with Alzheimer's disease pathology. Science translational medicine. 2012;4:150ra122. doi: 10.1126/scitranslmed.3004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls A, Colas D, Adamantidis A, Carter M, Lanre-Amos T, Heller HC, de Lecea L. Optogenetic disruption of sleep continuity impairs memory consolidation. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:13305–13310. doi: 10.1073/pnas.1015633108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satlin A, Volicer L, Stopa EG, Harper D. Circadian locomotor activity and core-body temperature rhythms in Alzheimer's disease. Neurobiology of aging. 1995;16:765–771. doi: 10.1016/0197-4580(95)00059-n. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, Mintun MA. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biological psychiatry. 2010;67:584–587. doi: 10.1016/j.biopsych.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, Zhou Y, Wong DF, Ferrucci L, Resnick SM. Self-reported sleep and beta-amyloid deposition in community-dwelling older adults. JAMA neurology. 2013;70:1537–1543. doi: 10.1001/jamaneurol.2013.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterniczuk R, Dyck RH, LaFerla FM, Antle MC. Characterization of the 3xTg-AD mouse model of Alzheimer's disease: Part 1. Circadian changes. Brain research. 2010;1348:139–148. doi: 10.1016/j.brainres.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Tucker MA, Hirota Y, Wamsley EJ, Lau H, Chaklader A, Fishbein W. A daytime nap containing solely non-REM sleep enhances declarative but not procedural memory. Neurobiology of learning and memory. 2006;86:241–247. doi: 10.1016/j.nlm.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Vitiello MV, Bliwise DL, Prinz PN. Sleep in Alzheimer's disease and the sundown syndrome. Neurology. 1992;42:83–93. discussion 93–84. [PubMed] [Google Scholar]
- Vitiello MV, Poceta JS, Prinz PN. Sleep in Alzheimer's disease and other dementing disorders. Canadian journal of psychology. 1991;45:221–239. doi: 10.1037/h0084283. [DOI] [PubMed] [Google Scholar]
- Walker MP. The role of slow wave sleep in memory processing. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2009;5:S20–S26. [PMC free article] [PubMed] [Google Scholar]
- Wirths O, Bayer TA. Neuron loss in transgenic mouse models of Alzheimer's disease. International journal of Alzheimer's disease 2010. 2010 doi: 10.4061/2010/723782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisor JP, Edgar DM, Yesavage J, Ryan HS, McCormick CM, Lapustea N, Murphy GM. Sleep and circadian abnormalities in a transgenic mouse model of Alzheimer's disease: A role for cholinergic transmission. Neuroscience. 2005;131:375–385. doi: 10.1016/j.neuroscience.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O'Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M. Sleep drives metabolite clearance from the adult brain. Science (New York, NY) 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Veasey SC, Wood MA, Leng LZ, Kaminski C, Leight S, Abel T, Lee VM, Trojanowski JQ. Impaired rapid eye movement sleep in the Tg2576 APP murine model of Alzheimer's disease with injury to pedunculopontine cholinergic neurons. The American journal of pathology. 2005;167:1361–1369. doi: 10.1016/S0002-9440(10)61223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]