Abstract
Purpose
Hypsarrhythmia, the pathognomonic EEG pattern of West syndrome, is typically characterized by a high amplitude, arrhythmic, and asynchronous pattern. While this severely aberrant pattern would suggest severe abnormalities in connectivity, coherence has not yet been systematically assessed in hypsarrhythmia.
Methods
We evaluated the EEGs of 28 infants, 12 with infantile spasms with hypsarrhythmia and 16 similarly age control infants for coherence and spectral power.
Results
Children with infantile spasms and hypsarrhythmia EEGs had marked abnormalities in coherence and spectral power compared to normal children of similar ages. During sleep increases in delta, theta, alpha and beta coherences were seen, particularly at long inter-electrode distances while at short inter-electrode distances coherences were decreased in the theta and beta range, particularly in the frontal region. The enhanced coherences at long inter-electrode distances suggest that during sleep in children with infantile spasms widely spread cortical region do not have functional differentiation whereas in the frontal lobe there is reduced functional connectivity and integration of local cortical regions. Children with continued seizures and developmental delay showed persistent abnormalities in coherence.
Conclusion
This study demonstrates that hypsarrhythmic EEGs have marked abnormalities in coherence spectral power and such abnormalities may be related to cognitive impairment.
Keywords: hypsarrhythmia, coherence, phase lag, connectivity, development
Introduction
West syndrome is a devastating, age-related epileptic encephalopathy characterized by infantile spasms, developmental delay and a pathognomonic EEG pattern of hypsarrhythmia, an abnormal EEG pattern that is typically characterized by a high amplitude, arrhythmic, and asynchronous electrical activity consisting of slow and sharp waves and multi-focal spikes1. The markedly aberrant oscillations in hypsarrhythmia may play a critical role in the cognitive impairment seen in West syndrome since it is known that brain oscillations are essential for binding cooperating neuronal assemblies in the representation, processing, storage and retrieval of information2–7. While the hypsarrhythmic pattern suggests that connectivity between different brain structures may be reduced, functional connectivity has not been evaluated in hypsarrhythmia.
In this study, oscillatory activity from children with infantile spasms was compared with age-matched controls, with a particular emphasis on coherence. On a frequency by frequency basis, EEG spectral coherence represents the consistency of the phase difference between two EEG signals when compared over time. Coherence is a measure of synchronization between two EEG signals based mainly on phase consistency. EEG coherence is often interpreted as a measure of “coupling” and as a measure of the functional association between two brain regions8,9. High coherence values are taken as a measure of strong connectivity between the brain regions that produce the compared EEG signals10.
Based on the classical description of hypsarrhythmia of a high amplitude, arrhythmic, and asynchronous electrical pattern consisting of slow and sharp waves and multi-focal spikes we hypothesized that compared to controls: i) infants with hysarrhythmia would have reduced coherences at both long and short inter-electrode distances; and ii) infants with hypsarrhythmia would have high absolute power in the delta bandwidths and a higher relative delta and lower alpha power.
We report here that our hypothesis that coherences would be lower in children with West syndrome was rejected. Children with West syndrome differed markedly from age-matched normal children in showing increased coherences, particularly at long inter-electrode distances. The second hypothesis that infants with hypsarrhtymia have higher absolute higher in the delta bandwidth was proven. These marked abnormalities in connectivity and power may relate to the cognitive deficits occurring in infantile spasms.
Methods
The study population consisted of 28 infants, 12 with infantile spasms (IS) and 16 age-matched controls (CONT) who had routine or long-term EEG/video recordings. All children in the IS group had a history of infantile spasms (flexor, extensor or mixed) and had abnormal EEGs at the time of the study, showing either hypsarrhythmia or modified hypsarrhythmia (Suppl. Fig. 1). CONT children were those who referred for EEGs because of a history suggestive of seizures and who had normal awake and asleep recordings. The mean age of the IS groups and CONT did not differ; IS: 13.00±1.79 months (range 6–24 months); CONT: 15.06±1.77 (range 6–28 months)(p = 0.428). Many of the children had had infantile spasms for months prior to the EEG recordings used in this study, thus accounting for the older ages of the children than typically seen at the onset of infantile spasms. Four of the 12 children with IS had cerebral dysgenesis (two with bilateral frontal lobe pachygyria, one with polymicrogyria and one with schizencephaly). All four of the children with cerebral dysgenesis were having daily infantile spasms at the time of the EEEG. In the other eight children neuroimaging was normal and an etiology for the IS was not determined. In these eight children 6 of the 8 were having spasms at the time of the EEG.
Seven children had follow-up EEGs from 13–24 months after the original EEG (mean age 27.57±2.96 months). In six children the hypsarrhythmia was no longer present and the EEGs demonstrated diffuse slowing with multifocal or generalized epileptiform discharges. The six children with abnormal EEGs continued to have seizures and showed few developmental gains since the onset of the spasms. In one child the EEG was normal and the child was developmentally normal. Follow-up EEGs were analyzed and compared to the CONT group. The study was approved by the Institutional Review Board of Dartmouth College.
EEGs were performed in the Clinical Neurophysiology Laboratory at Dartmouth-Hitchcock Medical Center or Fletcher Allen Health Care. Digital EEGs were recorded during the fully awake, drowsy and sleep states using the 10–20 System of Electrode placement. Ten minute segments of awake and sleep (Stage II) were selected for analysis. Sections were selected in which artifact was minimal although because of the young age some muscle and movement artifact occasionally occurred. Portions of records with suppression bursts or ictal events were excluded. Wakefulness was assessed by evaluation of eye blinks and technologist observations. If there was a concern about possible drowsiness the epoch was not evaluated. The sleep epochs were obtained during stage two of sleep when the child’s eyes were closed at a time sleep spindles and vertex sharp waves were present in the controls. Since sleep architecture was severely disturbed in the children with IS, sleep was assessed behaviorally when the child’s eyes were closed and there was no muscle or movement artifact. Not all of the children progressed beyond stage two of sleep and therefore slow wave sleep was not analyzed. None of the children had seizures during the EEG.
Analysis was performed using Neuroguide software (Applied Neuroscience, Inc., St. Petersburg, Florida) using a linked-ears montage. Details regarding the methods are provided in the Supplementary Material. A prior hypotheses were formulated with the hypothesis proven or discarded with unpaired t tests, doubled tail (p<0.025) using NeurostaEEG Statistical software (Applied Neuroscience, Inc., St. Petersburg, Florida).
Results
Absolute power during sleep in the IS group demonstrated significantly higher power in all bandwidths in the IS group than in the CONT (Suppl. Fig. 2). Relative power evaluation showed relatively more delta and less theta, beta and gamma in the IS compared to the CONT (Fig. 1). FFT mean power ratios showed that in comparison to the CONT, IS children had marked increases in delta/theta, delta/beta, delta/high beta, theta/high beta, alpha/beta, alpha/high beta and beta/high beta and marked decreases in the delta/alpha, theta/alpha and theta/beta ratios. Findings during the awake were similar (results not shown).
Fig. 1.
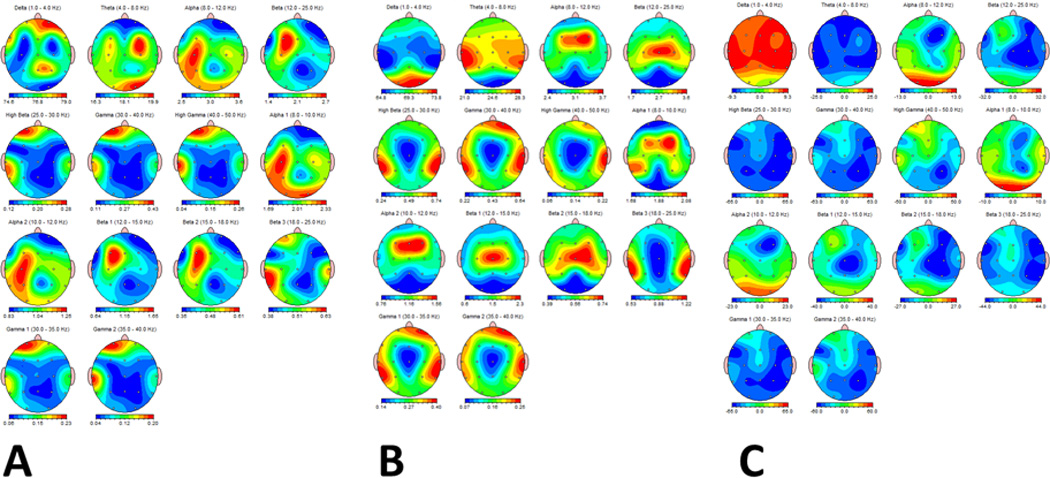
Relative power (ratio of bandwidth compared to total power) during the awake state in IS (A) and CONT (B) groups. C. Percent difference of relative power between IS and CONT. Relatively more delta and less theta, beta and gamma was present in the IS compared to the CONT. Frequencies are arranged from left to right, top to bottom: Delta (0–4 Hz), Theta (4–8 Hz), Alpha (8–12 Hz), Alpha1 (8–10 Hz), Alpha2 (10–12 Hz), Beta (12–25 Hz), High Beta (25–30 Hz). Beta1 (12–15 Hz), Beta 2 (15–18 Hz), Beta 3 (18–25 Hz), Gamma (30–40 Hz), High Gamma (40–50 Hz), Gamma1 (30–35 Hz) and Gamma2 (35–40 Hz). In A and B relative power is presented in color from low (blue) to high (red). In C percentage differences are measured from negative (blue) to positive (red).
During sleep coherence in the IS short distance inter-electrode coherence was decreased in frontal and anterior temporal regions while longer distant inter-electrode coherence was increased (Fig. 2). Comparison of FFT coherence between CONT and IS groups during the awake state showed similar finding to those seen when the infants were asleep (results not shown).
Fig. 2.
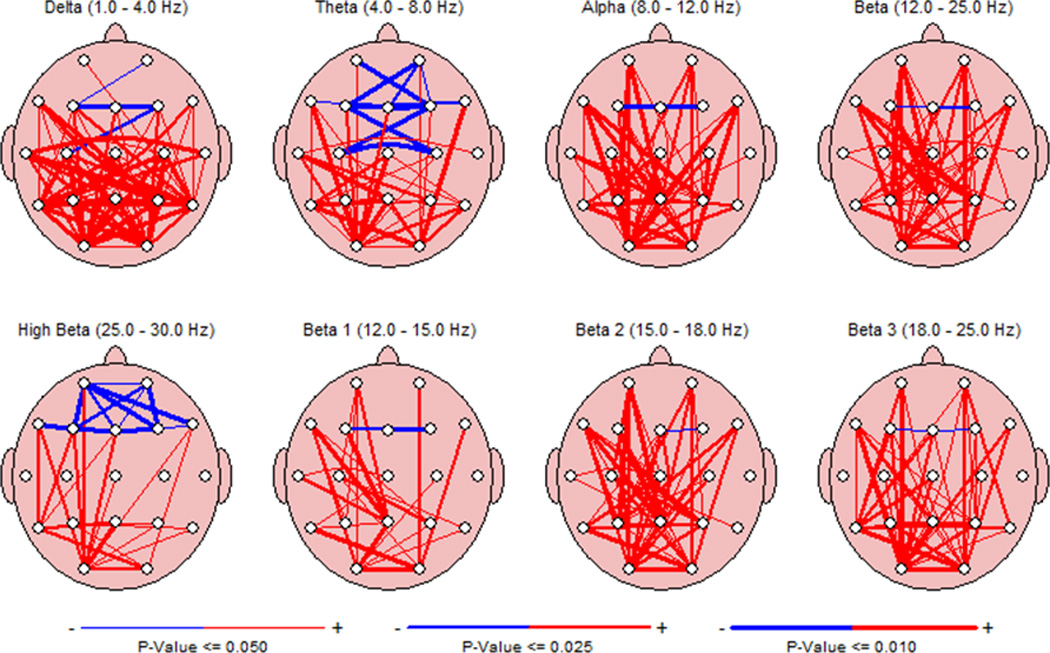
Comparison of FFT coherence between CONT and IS groups during sleep. Red lines indicate increased coherence in the IS compared to the CONT and blue lines indicate decrease coherences with the thickness of the lines reflecting degree of significance (thin = p value of <=0.05; medium p<=0.025; thick p<=0.01). During sleep short distance inter-electrode coherence is decreased in frontal and anterior temporal regions while longer distant inter-electrode coherence is increased.
In the six infants who had follow-up abnormal EEGs and continued developmental delay the coherences remained markedly increased compared to the CONT (Suppl. Fig. 3). In the one child with a normal EEG the coherences did not differ significantly from the CONT. There was not a relationship between etiology of the IS and EEG findings. For example, the child with schizencephaly did not have any demonstrable clear differences in coherence or power from the other children with IS.
Discussion
The primary finding in this study is that EEGs from children with infantile spasms and hypsarrhythmia EEGs have marked abnormalities both in spectral power and coherence. Not surprisingly, hypsarrhythmic EEGs, with high voltage, slow, disorganized and asynchronous background showed high spectral powers in the low frequencies. Both the profound slowing and reduction in faster frequencies could contribute to impairment in informational processing.
The most remarkable finding in this study was the enhanced, rather than decreased coherence, compared to normal children of similar ages. During sleep increases in delta, theta, alpha and alpha coherences were seen, particularly at long inter-electrode distances while in the theta bandwidth there were decreased coherences at short inter-electrode differences in the frontal region. The findings during the waking state were in a similar direction but were less dramatic.
Coherence between two directly-connected structures at certain frequencies reflects the strength of the communication between them and, therefore, provides information about the functional integrity of the network. Not surprising, our findings show that neuronal networks are markedly altered in children with infantile spasms, particular during sleep. However what was surprising was the finding that there were marked increases in coherence, indicating that the brain was operating in a widely synchronous, time-locked state. Increased coherences can be caused by enhanced connectivity between cerebral structures or related to cortico-subcortical circuitry contributing to the synchronization of some components of cortical activity.
Intuitively one would expect that enhanced coherences would be beneficial for normal brain development, high coherences have been associated with low intelligence and autistic spectrum disorder. Similar to our children with IS, Duffy et al.11 found that in children with autism had enhanced short-distance coherences and increased long-distant coherences. However, it should be noted that comparing coherences in children with autism and West syndrome is difficult. Most children with autism have normal to mildly abnormal EEGs whereas the hypsarrhythmic pattern in West syndrome is markedly abnormal. Nevertheless, the enhanced coherences, especially at long inter-electrode in the children with infantile spasms suggest that during the “default” state of sleep the brains of children with infantile spasms do not have functional differentiation, i.e. signaling is not region specific.
The consequences of such aberrant connectivity are unknown. We found that in six children with continued EEG abnormalities, seizures and developmental delay that coherence remained high. Coherences were normal in one child who was developing normally. While few conclusions can be drawn from such a limited number of patients, these findings raise the possibility that aberrant coherences may be physiological marker of cognitive impairment in infants with a history of infantile spasms. Other factors, such as etiology, are known to influence outcome in West syndrome. Future studies should determine whether coherences in West syndrome are related to etiology and whether the prognosis in children with persistent abnormalities in coherence differs from those children in which coherences normalize.
Supplementary Material
Highlights.
Whether hypsarrhythmia contributes to epileptic encephalopathies is controversial
Coherence measures synchronization between EEG signals based on phase consistency
Long-distance coherences are increased in hypsarrhythmia
Short-distant frontal lobe coherences are decreased in hypsarrhythmia
Aberrant coherences may contribute to developmental delay in infantile spasms
Acknowledgements
Supported by grants from the National Institutes of Health (NINDS): NS073083, NS057563, NS074450 and the Emmory R. Shapses Research Fund and the Michael J. Pietroniro Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gibbs FA, Gibbs EL. Atlas of Electroencephalography, Volume 2, Epilepsy. 2 . Cambridge, Mass: Addison-Wesley; 1952. pp. 1–422. [Google Scholar]
- 2.Senior TJ, Huxter JR, Allen K, et al. Gamma oscillatory firing reveals distinct populations of pyramidal cells in the CA1 region of the hippocampus. J Neurosci. 2008;28:2274–2286. doi: 10.1523/JNEUROSCI.4669-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vertes RP, Kocsis B. Brainstem-diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience. 1997;81:893–926. doi: 10.1016/s0306-4522(97)00239-x. [DOI] [PubMed] [Google Scholar]
- 4.Chrobak JJ, Buzsaki G. High-frequency oscillations in the output networks of the hippocampal-entorhinal axis of the freely behaving rat. J Neurosci. 1996;16:3056–3066. doi: 10.1523/JNEUROSCI.16-09-03056.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lisman J. The theta/gamma discrete phase code occuring during the hippocampal phase precession may be a more general brain coding scheme. Hippocampus. 2005;15:913–922. doi: 10.1002/hipo.20121. [DOI] [PubMed] [Google Scholar]
- 6.van Vugt MK, Schulze-Bonhage A, Litt B, et al. Hippocampal gamma oscillations increase with memory load. J Neurosci. 2010;30:2694–2699. doi: 10.1523/JNEUROSCI.0567-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lega BC, Jacobs J, Kahana M. Human hippocampal theta oscillations and the formation of episodic memories. Hippocampus. 2012;22:748–761. doi: 10.1002/hipo.20937. [DOI] [PubMed] [Google Scholar]
- 8.Thatcher RW, Krause PJ, Hrybyk M. Cortico-cortical associations and EEG coherence: a two-compartmental model. Electroencephalogr Clin Neurophysiol. 1986;64:123–143. doi: 10.1016/0013-4694(86)90107-0. [DOI] [PubMed] [Google Scholar]
- 9.Thatcher RW, Walker RA, Giudice S. Human cerebral hemispheres develop at different rates and ages. Science. 1987;236:1110–1113. doi: 10.1126/science.3576224. [DOI] [PubMed] [Google Scholar]
- 10.Srinivasan R, Winter WR, Ding J, et al. EEG and MEG coherence: measures of functional connectivity at distinct spatial scales of neocortical dynamics. J Neurosci Methods. 2007;166:41–52. doi: 10.1016/j.jneumeth.2007.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duffy FH, Als H. A stable pattern of EEG spectral coherence distinguishes children with autism from neuro-typical controls - a large case control study. BMC Med. 2012;10:64. doi: 10.1186/1741-7015-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


