Abstract
The brain regions involved in vocal communication are well described for some species, including songbirds, but less is known about the neural mechanisms underlying motivational aspects of communication. Mesolimbic dopaminergic projections from the ventral tegmental area (VTA) are central to mediating motivated behaviors. In songbirds, VTA provides dopaminergic innervation to brain regions associated with motivation and social behavior that are also involved in sexually-motivated song production. Neurotensin (NT) is a neuropeptide that strongly modulates dopamine activity, co-localizes with dopamine in VTA, and is found in regions where dopaminergic cells project from VTA. Yet, little is known about how NT contributes to vocal communication or other motivated behaviors. We examined the relationships between sexually-motivated song produced by male European starlings (Sturnus vulgaris) and NT immunolabeling in brain regions involved in social behavior and motivation. Additionally, we observed relationships between NT labeling, non-vocal courtship behaviors (another measure of sexual motivation), and agonistic behavior to begin to understand NT’s role in socially-motivated behaviors. NT labeling in VTA, lateral septum, and bed nucleus of the stria terminalis correlated with sexually-motivated singing and non-vocal courtship behaviors. NT labeling in VTA, lateral septum, medial preoptic nucleus, and periaqueductal gray was associated with agonistic behavior. This study is the first to suggest NT’s involvement in song, and one of the few to implicate NT in social behaviors more generally. Additionally, our results are consistent with the idea that distinct patterns of neuropeptide activity in brain areas involved in social behavior and motivation underlie differentially motivated behaviors.
Keywords: Neurotensin, Neuropeptides, Songbird, Motivation, Vocal communication, Social behavior
1. Introduction
Vocal communication is necessary for appropriate social functioning in many vertebrates. Vocal signals are context-specific behaviors that convey information about motivational states and intents, e.g. sexual or agonistic [1]. The brain regions involved in the learning, production, and processing of vocalizations are well described for some species, including songbirds [2], but less is known about the neural mechanisms that underlie motivational aspects of communication.
Across vertebrates, mesolimbic dopaminergic projections from the ventral tegmental area (VTA) are involved in motivated, goal-directed behaviors [3,4], including sexually-motivated singing behavior used by male songbirds to attract females [5-7]. Dopamine, its metabolites, or enzymes involved in dopamine synthesis in VTA are tightly linked to sexually-motivated song [8-11], suggesting that dopaminergic projections from VTA may be crucial for controlling this type of communication. Neurotensin (NT) is a neuropeptide that strongly modulates activity in the mesolimbic dopamine system (reviewed in [12]). NT and NT1 receptors (the only known avian NT receptor [13]) are highly co-localized with dopamine neurons in VTA [14-16]. Regions with dopaminergic projections are enriched with NT-containing neurons and NT1 receptors [17-20], including areas involved in sexually-motivated song in songbirds (reviewed below). Despite the close relationship between NT and dopamine in multiple brain regions central to motivation and vocal production, NT’s role in vocal communication or other social behaviors is unclear.
VTA projects directly to brain areas involved in vocal production [21,22], and is reciprocally connected to additional NT- and dopamine-rich areas implicated in sexually-motivated song [23-28]. These areas include the lateral septum (LS), the bed nucleus of the stria terminalis (BNST), the medial preoptic area (POM), and the periaqueductal gray (PAG) [10,29-37]. These regions, in addition to others, are considered nodes in a “social behavior network,” a group of reciprocally connected brain regions in which distinct patterns of activity underlie specific social behaviors [38,39]. Some authors suggest that input from the mesolimbic dopamine system to these social behavior regions constitutes a larger “social decision-making” network that facilitates appropriate social behaviors in a given context, and that LS and BNST specifically may serve as relay nodes between these circuits for social behavior and motivation [40]. It has been proposed that neuropeptides act within these social behavior/social decision-making regions to generate wide variation in social behaviors [38-40]. The dense NT presence in these areas along with the strong interactions between NT and dopamine suggest that NT may be an important modulator of activity within the social brain network.
Little is known about how NT contributes to social behaviors generally, but evidence suggests it inversely relates to at least one form of agonistic behavior. In mice, higher NT gene expression in the preoptic area (analogous to POM in birds) as measured via real-time polymerase chain reaction is associated with lower levels of maternal defense of offspring, an agonistic behavior [41]. Intracerebroventricular injections of NT decrease maternal defense of offspring in mice, and several social brain regions, including LS, BNST, POM, and dorsomedial PAG, exhibit increased c-Fos expression in response to injections of NT [42], suggesting these regions as sites in which NT may act to inhibit maternal defense. Since NT is seen so robustly in multiple social brain regions that are implicated in the motivation to sing, highly interacts with DA, and is involved in aspects of social behavior, NT is a strong candidate neuropeptide to contribute to the regulation of sexually-motivated vocal communication.
To gain insight into a possible role for NT in sexually-motivated song production, we determined the extent to which NT immunolabeling in VTA, LS, BNST, POM, and PAG related to sexually-motivated singing behavior in male European starlings, Sturnus vulgaris. These areas were selected as starting points because prior work has implicated dopamine in VTA, POM, and PAG in sexually-motivated song [11,32]. Furthermore, activity in LS and BNST is related to sexually-motivated song production [31,32], and these are also sites in which neuropeptides, including NT, act to influence social behaviors [42-47]. Additionally, we examined other non-vocal social behaviors to explore the possibility that distinct patterns of NT activity in the social behavior network relate to separate social behaviors. We examined relationships between NT labeling and non-vocal courtship behaviors indicative of sexual motivation, agonistic behavior, and non-socially motivated behaviors as controls. This is the first study to our knowledge to examine the relationship between NT and birdsong, and one of the few studies to explore the relationship between NT and social behavior.
2. Materials & Methods
2.1 Animals
20 male and 4 female starlings were captured in the winter of 2009-10 on a farm in Madison, WI with baited fly-in traps. Birds were then housed indoors in single sex groups of 5 in stainless steel cages (91 cm × 47 cm × 47 cm) at the University of Wisconsin-Madison. Food and water were available ad libitum. All procedures and protocols were in accordance with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and a protocol approved by the University of Wisconsin Institutional Care and Use Committee.
2.2 Housing Conditions
Birds were housed in captivity for at least a year after capture. Before the study began, birds were placed on photoperiods of 18 h light (L):6 h dark (D) for 6 weeks, and then 8L:16D for 6 weeks. Experiencing this sequence of photoperiods causes photosensitivity in starlings, such that males subsequently exposed to day lengths longer than 11 hours demonstrate increased gonad volume and plasma T concentrations indicative of spring breeding condition [48]. Males were placed in groups of 5 into outdoor aviaries (2.13 m × 2.4 m × 1.98) that contained nest boxes, perches, nesting material, a water bath, and food and water ad libitum. Birds were exposed to the 13L:11D natural light conditions of this time of year (April). Birds habituated to the aviaries for 12 days before behavioral observations began.
2.3 Behavioral Observations
Each aviary was observed in a rotating order at one time each day over a period of 4 days. A single experimenter recorded observations of all birds in a given aviary for 20 minutes. Starlings tend to not perform multiple behaviors simultaneously or rapidly change locations while behaving, enabling observations of several birds to be performed by a single observer, as is common in studies of starling behavior such as [10,49,50] To observe male courtship singing and behavior, a female starling was released into the aviary, and fresh nesting materials (grass clippings and green leaves) were placed in the aviary. One of 4 different females was released into the aviaries each day, i.e. the same female was used in each aviary for a single observation day, although a different female was used each day.
Singing behaviors observed were time singing (secs) and number of complete songs (defined as songs lasting more than 10 secs). Non-vocal courtship behaviors consisted of the sum of the number of times a male entered a nest box, looked in a nest box, gathered nest material, and wing waved; these behaviors are clear indicators of sexual motivation, as they are performed only by male starlings during the spring breeding season as part of mate attraction [50,51]. We also measured the number of times a bird displaced a conspecific, an agonistic behavior, specified as a male approaching within 5 cm of another male who then departed after the approach. Non-specific behaviors included bouts of feeding and preening, with a bout defined as occurring at least 2 secs after a previous behavior, and calling, which is a non-specific non-song vocalization. Tissue was collected for NT analysis only from birds observed to sing at least once during the 4 observation days or on any of the 12 preceding days of habituation, resulting in n = 12.
2.4 Tissue Preparation for Immunohistochemistry
Immediately after the final observation period for an aviary, the female was removed and each male was rapidly decapitated. Brains were subsequently removed, submerged in 5% acrolein (Sigma Aldrich Cat. #110221) for 24 h, placed in 30% sucrose, and stored at 4 °C. For 3 days, the sucrose was replaced every 24 h. Brains were then flash frozen in dry ice and stored at 80 °C. For slicing, brains were sectioned coronally at 40 μm microns on a cryostat at -17 °C in triplicate serial sections and stored in antifreeze cryoprotectant (PBS, polyvinylpyrrolidone, sucrose, and ethylene glycol mixture) at -20 °C.
2.5 Validation of antibody specificity
A Western immunoblot was run to determine antibody specificity. Micropunched tissue from starling brain (from a bird not part of this behavioral study) was homogenized with a mixture of RIPA buffer, protease inhibitor cocktail (P8340, Sigma, St. Louis, MO, USA), phosphatase inhibitor cocktail (P0044, Sigma, St. Louis, MO, USA), and PMSF. Samples were then centrifuged at 12,000 rpm for 10 min at 4 °C. Following collection of supernatant, BCA Protein Assay (Pierce Chemical Co., Rockford, IL, USA) determined protein concentration. 15 μl of protein was gel electrophoresed using 4-20% precast Tris-HCl gel (Bio-Rad, Hercules, CA) and transferred to a PVDF membrane. The membrane was washed in 0.1 M Tris-buffered saline (TBS) with 0.05% Tween 20 (TBST) for 10 min, blocked for 1 hr in 0.1 M TBST in 5% nonfat dry milk with agitation at room temperature, washed in TBST, and incubated overnight at 4° C with agitation in primary anti-neurotensin protein antibody prepared in rabbit (Immunostar, Hudson, WI, CAT #20072) at 1:5000 with 5% nonfat dry milk and TBST. The following day, the membrane was washed in TBST, incubated with agitation in secondary antibody for 1 hr at room temperature, washed in TBST for 10 min, and washed in TBS for 10 min. Bands reacted with a chemiluminescent detection reagent kit (Amersham ECL Prime, GE Healthcare UK, Buckinghamshire, UK) and were visualized with a C-DiGit blot scanner (LI-COR, Lincoln, NE, USA) and the program ImageStudio 3.1. A band is at the approximate molecular weight of the NT precursor neuromedin N at 19 kDA [52] indicated by the arrow (Fig. 1). Two additional bands are also seen at approximately 49 kDA and 51 kDA. These may represent NT bound to the two protein subunits of the NT1 receptor, whose molecular weights are 49 kDA and 51 kDA [53]. This antibody displays an anatomical distribution in tissue that is similar to previous studies, further suggesting it is specific for NT [35,37].
Figure 1.
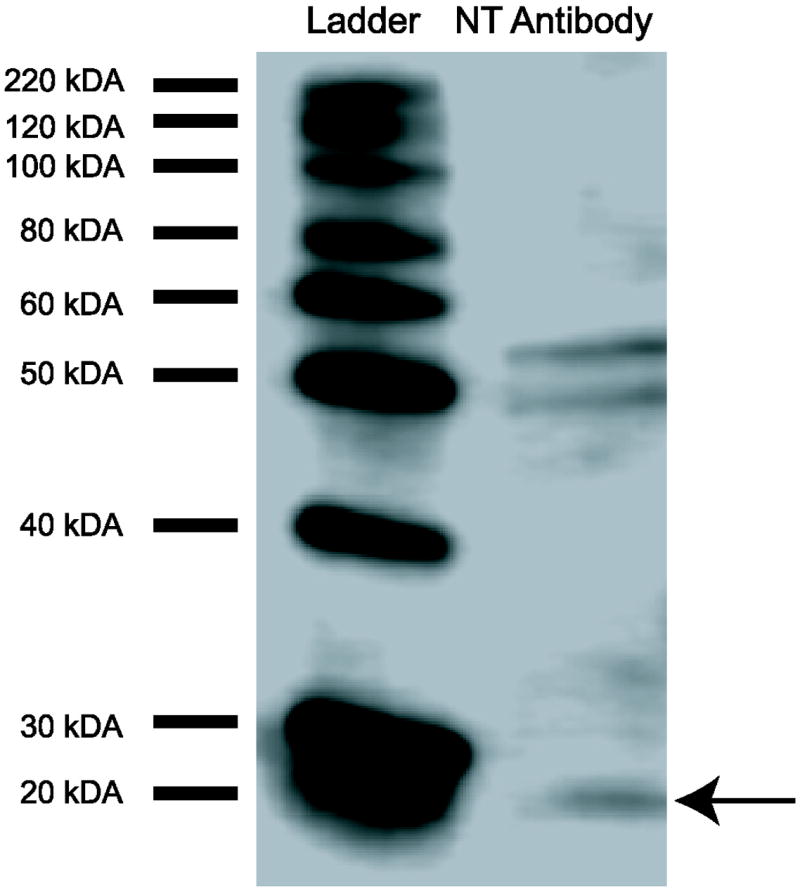
Western blot results for NT antibody (Immunostar). A band is at the approximate molecular weight of the NT precursor neuromedin N at 19 kDA [52], indicated by the arrow. Two additional bands are also seen at approximately 49 kDA and 51 kDA, which may represent NT bound to the 2 subunits of the NT1 receptor [53].
2.6 Immunohistochemistry
Tissue from the 12 males that exhibited singing behavior was rinsed in phosphate-buffered (PBS) saline for 20 min, washed in 0.5% sodium borohydride (NaBH4) for 20 min, washed in PBS for 20 min, washed in 0.5% hydrogen peroxide (H2O2) for 15 min, washed in PBS for 10 min, washed in PBS with 0.2% triton (PBS-T) for 10 min, blocked in 20% normal goat serum (PBS-T) for 1 hour, and then incubated overnight in primary anti-neurotensin protein antibody prepared in rabbit (Immunostar, Hudson, WI, CAT #20072) at 1:15000 with 20% normal goat serum (made in PBS-T). The next day, the tissue was washed in PBS-T for 20 min, incubated in secondary antibody (biotinylated goat anti-rabbit at 1:1000, Vector Laboratories, Burlingame, CA) for 90 min, washed in PBS-T for 20 min, incubated in avidin-biotin (AB) solution (Vectastain Elite ABC, Vector Laboratories) for 1 hr, washed in PBS-T for 20 min, incubated in 3,3’-diaminobenzidine (DAB) (Sigma-Aldrich, St. Louis, MO) for 10 min to visualize the AB complex, and lastly washed in PBS for 20 min. All tissue was mounted on gelatin-coated slides, dehydrated in 70% ethanol (EtOH) for 5 min, in 95% EtOH for 20 min, in 100% EtOH for 20 min, in Xylene for 20 min, and then cover slipped.
2.7 Quantification of NT immunolabeling
Images of NT labeled brain regions were taken using a camera (AxioCam HRC, Carl Zeiss) attached to a Zeiss microscope. Pictures of brain slices were analyzed using MetaMorph (Version 7.8.4). Measures of both the density of NT label (average intensity; calculated as the absolute value of the sum of gray value divided by the number of pixels, such that greater values represent darker label) and the area covered by labeled fibers (pixel area) were collected in VTA, PAG, POM, LS, and BNST (Fig. 2). LS was divided into 3 distinct subdivisions as described in [54]: the lateral septum, rostral division (LSr); ventral zone of the lateral septum, caudal division (LSc.v); and the ventrolateral zone of the lateral septum, caudal division (LSc.vl). Total quantification values consist of an average of left and right images for 3 consecutive brain slices in these areas, after the subtraction of a background image that contained no labeling on the same slice for each area. In cases where tissue damage prevented acquisition of an image, an additional slice was included. All measures were made within boxes at the center of each region (VTA: 0.37 mm × 0.41 mm; PAG: 0.32 mm × 0.21 mm; POM: 0.33 mm × 0.42 mm; LS: 0.16 mm × 0.16 mm; BNST: 0.33 mm × 0.24 mm). For each region a different threshold was used to measure pixel area due to differences in background staining, but the same threshold was used for a single region. In each case the threshold included labeled fibers and not unlabeled background. Dendritic and punctate staining was observed in all areas, except LS also contained some cell body staining (Fig. 3).
Figure 2.
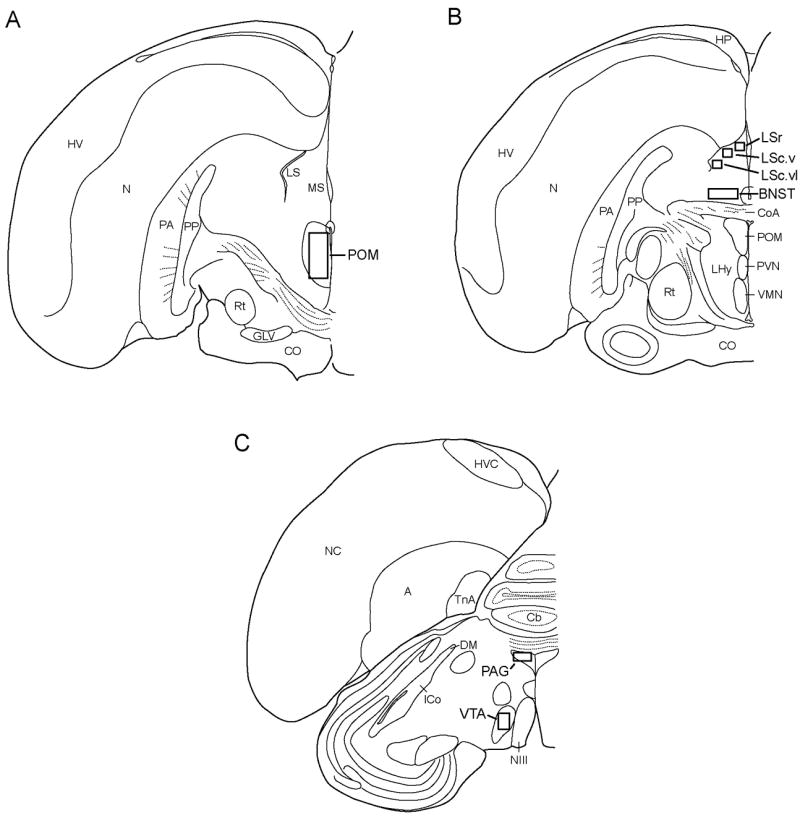
Illustrations of coronal sections of one hemisphere of starling brain with areas quantified for NT labeling indicated by boxes. Abbreviations: A, arcopallium; BNST, bed nucleus of the stria terminalis; Cb, cerebellum; CoA, anterior commissure; CO, optic chiasm; PAG, periaqueductal gray; GLV, nucleus geniculatus lateralis, pars ventralis; HP, hippocampus; ICo, nucleus intercollicularis; LS, lateral septum; LSc.v, lateral septum, caudal part, ventral zone; LSc.vl, lateral septum, caudal part, ventrolateral zone; LSr, lateral septum, rostral part; MS, medial septum; NIII, third cranial nerve; N, nidopallium; POM, medial preoptic nucleus; Rt, nucleus rotundus; TnA, nucleus taeniae of the amygdala; VMN, ventromedial nucleus of the hypothalamus; VTA, ventral tegmental area.
Figure 3.
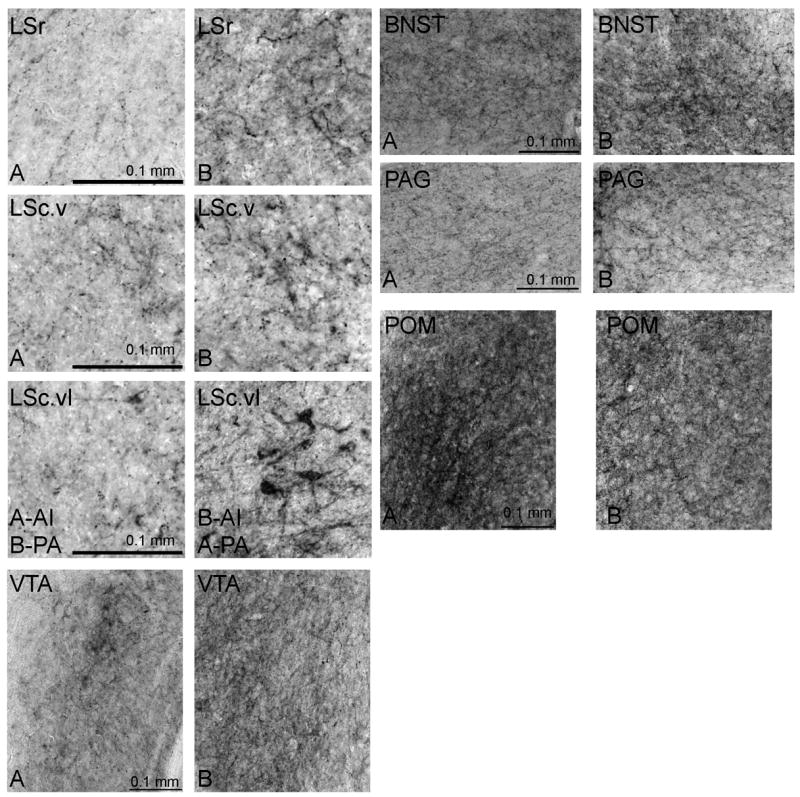
Photomicrographs of representative NT labeling in LS, BNST, POM, VTA, and PAG in relation to low (A) and high (B) rates of behavior. Relationships are consistent for measures of average intensity (AI) and pixel area (PA) except for LSc.vl, as indicated in the figure. Scale bar is indicated in bottom left of panel A for each region.
2.8 Statistical Analysis
Data were analyzed with the statistical software program Statistica (Version 6.0, StatSoft, Tulsa, OK). The Kolmogorov-Smirnov test was used to assess normality of distribution for all variables. Only one variable, time singing, violated normal assumptions and was log-transformed log10(x+1) to attain normality For measures of both average intensity and pixel area, linear regression analyses were performed with the brain regions POM, VTA, PAG, BNST, LSr, and LSc.vl entered as independent variables. LSc.v was correlated with both LSr and LSc.vl, so separate analyses were run with the other brain regions (POM, VTA, PAG, BNST) as independent variables and LSc.v substituted in place of LSr and LSc.vl. Behaviors were entered as the dependent variables for each analysis. Assumptions were tested using residual plots and outliers were removed from analyses if they fell more than 2 standard deviations outside the mean. We ran forward and backward stepwise regressions and report the strongest models according to the adjusted R2 and standard error of the estimates. Significance was accepted at p < 0.05 for the entire model and for the beta coefficients of the predictor variables.
3. Results
3.1 Singing behavior
Multiple regression analyses with NT average intensity in POM, VTA, PAG, BNST, LSr, and LSc.vl entered as independent variables and singing behaviors entered as dependent variables were significant. Time singing (log transformed; adjusted R2 = 0.74, p = 0.0056) was statistically explained by NT average intensity in BNST (beta = 0.64, p = 0.0055) and LSr (beta = 0.39, p = 0.048) (Fig. 3; Table 1). The number of complete songs (adjusted R2 = 0.70, p = 0.0053) was similarly explained by NT average intensity in LSr (beta = 0.51, p = 0.032) and BNST (beta =0.46, p = 0.026) (Fig. 3; Fig. 4; Table 1). One data point was removed from this analysis of time singing because it was more than 2 standard deviations outside the mean.
Table 1.
Regression models demonstrating brain areas in which NT density immunolabeling statistically explain behavioral variation.
| Behavior | Model Statistics | Variables | Beta | p-Values |
|---|---|---|---|---|
| Time singing | Adjusted R2 = 0.74, F3,7 = 10.48, p=0.0056 | BNST | 0.64 | 0.0055 |
| LSr | 0.39 | 0.048 | ||
| VTA | -0.37 | 0.054 | ||
| Complete songs | Adjusted R2 = 0.70, F3,8 = 9.40, p=0.0053 | LSr | 0.51 | 0.032 |
| BNST | 0.46 | 0.026 | ||
| PAG | 0.22 | 0.28 | ||
| Non-vocal courtship behaviors | Adjusted R2 = 0.86, F2,9 = 34.17, p<0.001 | LSr | 0.78 | <0.001 |
| BNST | 0.39 | 0.0092 | ||
| Adjusted R2 = 0.53, F3,8 = 5.06, p=0.030 | LSc.v | 0.71 | 0.012 | |
| Agonistic behavior | Adjusted R2 = 0.73, F4,6 = 7.70, p=0.015 | LSc.vl | 0.51 | 0.034 |
| POM | -0.78 | 0.0054 | ||
| PAG | 0.49 | 0.037 | ||
| BNST | 0.19 | 0.33 | ||
| Adjusted R2 = 0.78, F4,6 = 9.69, p=0.0087 | LSc.v | 0.61 | 0.011 | |
| VTA | 0.49 | 0.025 | ||
| POM | -0.61 | 0.014 | ||
| Feeding | No significant model | (none) | ||
| Preening | No significant model | (none) | ||
| Calling | No significant model | (none) |
Figure 4.
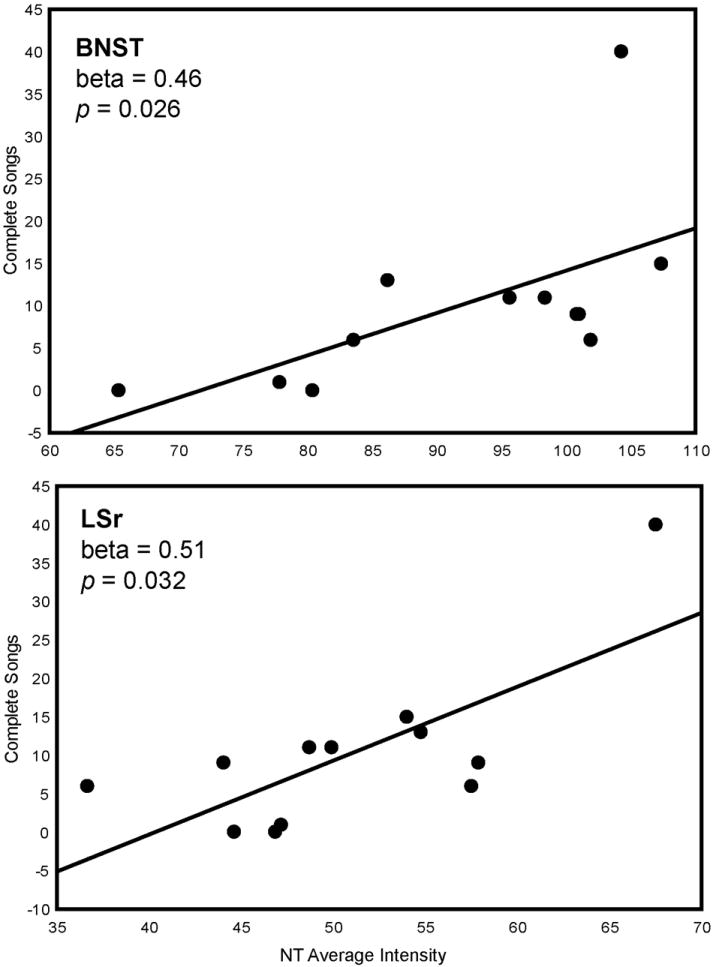
Two-dimensional visualization of a multiple regression model with 2 significant variables, in this case indicating the relationship between NT average intensity (X-axis) in BNST and LSr and complete songs (Y-axis). Statistics were computed in a multiple regression model and do not represent values of linear correlations. Each point represents data from a single individual.
Multiple regression analyses with NT pixel area in POM, VTA, PAG, BNST, and LS entered as independent variables and singing behaviors entered as dependent variables were significant. Time singing (log transformed; adjusted R2 = 0.49, p = 0.040) was statistically explained by NT pixel area in VTA (beta = 0.58, p = 0.040) (Fig. 3; Fig. 5; Table 2). Complete songs (adjusted R2 = 0.83, p = 0.0038) were explained by NT pixel area in BNST (beta = 0.71, p = 0.0039), LSc.vl (beta = -0.45, p =0.041), and VTA (beta = 0.37, p = 0.047) (Fig. 3; Fig. 6; Table 2). One data point was removed from the analysis of complete songs because it was more than 2 standard deviations outside the mean.
Figure 5.
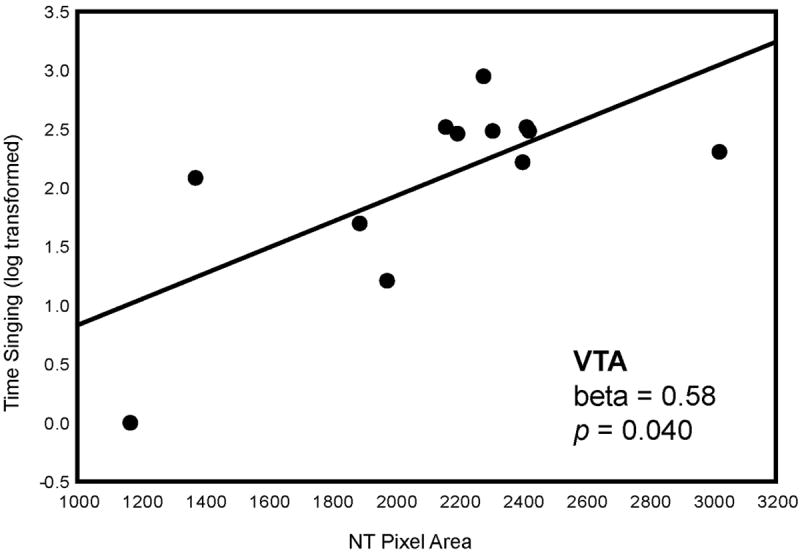
Two-dimensional visualization of a multiple regression model with 2 significant variables, in this case indicating the relationship between NT pixel area (X-axis) in VTA and time singing (Y-axis). Statistics were computed in a multiple regression model and do not represent values of linear correlations. Each point represents data from a single individual.
Table 2.
Regression models demonstrating brain areas in which NT pixel area immunolabeling statistically explain behavioral variation.
| Behavior | Model Statistics | Variables | Beta | p-Values |
|---|---|---|---|---|
| Time singing | Adjusted R2 = 0.49, F3,8 = 4.47, p=0.040 | VTA | 0.58 | 0.040 |
| BNST | 0.40 | 0.14 | ||
| LSr | -0.27 | 0.27 | ||
| Complete songs | Adjusted R2 = 0.83, F4,6 = 13.41, p=0.0038 | BNST | 0.71 | 0.0039 |
| LSc.vl | -0.45 | 0.041 | ||
| VTA | 0.37 | 0.047 | ||
| LSr | -0.20 | 0.26 | ||
| Non-vocal courtship behaviors | Adjusted R2 = 0.82, F5,6 = 10.76, p=0.0059 | BNST | 0.52 | 0.019 |
| LSr | 0.60 | 0.018 | ||
| VTA | 0.35 | 0.056 | ||
| POM | -0.26 | 0.16 | ||
| LSc.vl | -0.21 | 0.31 | ||
| Adjusted R2 = 0.79, F4,7 = 11.42, p=0.0035 | BNST | 0.77 | 0.0038 | |
| PAG | 0.51 | 0.024 | ||
| LSc.v | 0.56 | 0.018 | ||
| POM | -0.40 | 0.053 | ||
| Agonistic behavior | Adjusted R2 = 0.56, F2,9 = 7.97, p=0.010 | VTA | 0.65 | 0.016 |
| BNST | 0.28 | 0.23 | ||
| Feeding | No significant model | (none) | ||
| Preening | Adjusted R2 = 0.35, F2,9 = 5.76, p=0.024 | BNST | 0.89 | 0.0079 |
| PAG | 0.49 | 0.094 | ||
| Calling | No significant model | (none) |
Figure 6.
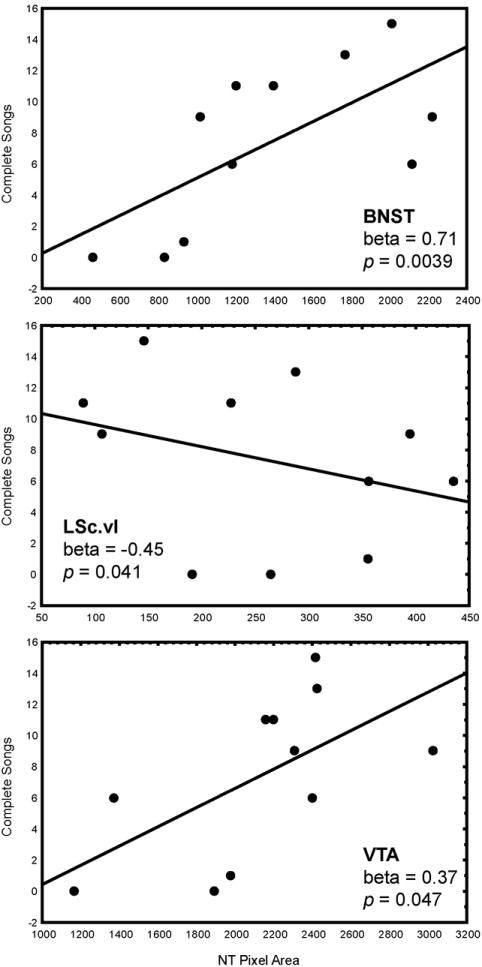
Two-dimensional visualization of a multiple regression model with 2 significant variables, in this case indicating the relationship between NT pixel area (X-axis) in BNST, LSc.vl, VTA, and complete songs (Y-axis). Statistics were computed in a multiple regression model and do not represent values of linear correlations. Each point represents data from a single individual.
3.2 Non-vocal courtship behaviors
A multiple regression analysis with NT average intensity in POM, VTA, PAG, BNST, LSr, and LSc.vl entered as independent variables, and non-vocal courtship behaviors (the sum of times looking in a nest box, entering a nest box, gathering nest material, and wing waving) entered as the dependent variable, was significant (adjusted R2 = 0.86, p < 0.001). NT average intensity in LSr (beta = 0.78, p < 0.001) and BNST (beta = 0.39, p = 0.0092) statistically explained variation in these sexually-motivated behaviors (Fig. 3; Fig. 7; Table 1). A separate analysis with LSc.v substituted for LSr and LSc.vl was significant (adjusted R2 = 0.53, p = 0.030) and revealed that NT average intensity in LSc.v (beta = 0.71, p = 0.012) similarly explained variation in these behaviors (Fig. 3; Fig. 5; Table 1).
Figure 7.
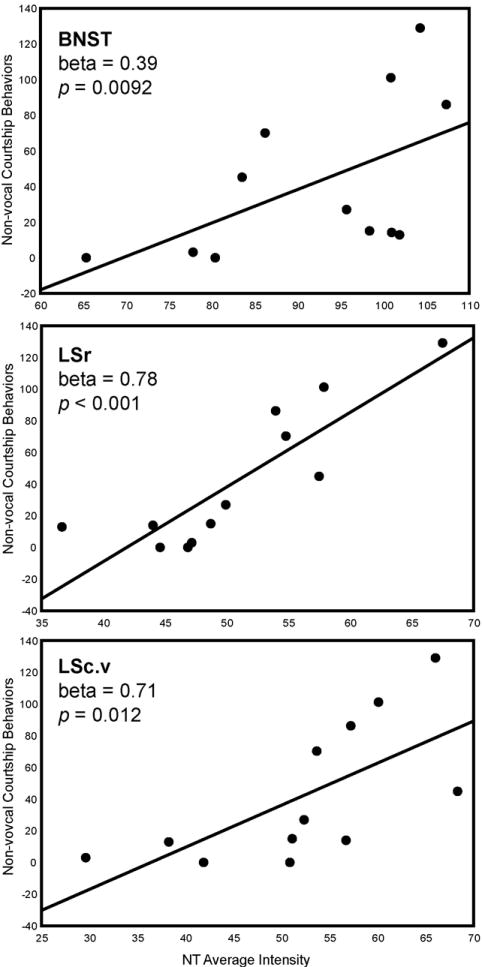
Two-dimensional visualization of a multiple regression model with 3 significant variables, in this case indicating the relationship between NT average intensity (X-axis) in BNST, LSr, and LSc.v and non-vocal courtship behaviors (Y-axis). Statistics were computed in multiple regression models and do not represent values of linear correlations. Each point represents data from a single individual.
A multiple regression analysis with NT pixel area in POM, VTA, PAG, BNST, LSr, and LSc.vl entered as independent variables, and non-vocal courtship behaviors entered as the dependent variable, was significant (adjusted R2 = 0.82, p = 0.0059). NT pixel area in BNST (beta = 0.52, p = 0.019) and LSr (beta = 0.60, p = 0.018) (with a similar trend observed for VTA (beta = 0.35, p = 0.056)) statistically explained variation in these sexually-motivated behaviors (Fig. 3; Table 2). A separate analysis with LSc.v substituted for LSr and LSc.vl was significant (adjusted R2 = 0.79, p = 0.0035) and revealed that NT pixel area in PAG (beta = 0.51, p = 0.024) and LSc.v (beta = 0.56, p = 0.018) similarly explained variation in these behaviors (Fig. 3; Table 2).
3.3 Agonistic behavior
A multiple regression analysis with NT average intensity in POM, VTA, PAG, BNST, LSr, and LSc.vl entered as independent variables significantly explained displacements (adjusted R2 = 0.73, p = 0.015). NT average intensity in LSc.vl (beta = 0.51, p = 0.034), POM (beta = -0.78, p = 0.0054), and PAG (beta = 0.49, p = 0.037) statistically explained variation in this behavior (Fig. 3; Table 1). One outlier was removed from this analysis. A separate analysis with LSc.v substituted for LSr and LSc.vl was significant (adjusted R2 = 0.78, p = 0.0087) and demonstrated that NT average intensity in LSc.v (beta = 0.61, p = 0.011), VTA (beta = 0.49, p = 0.025), and POM (beta = -0.61, p = 0.014) statistically explained variation in agonistic behavior (Fig. 3; Table 1). One outlier was removed because it was more than 2 standard deviations outside the mean.
A multiple regression analysis with NT pixel area in POM, VTA, PAG, BNST, LSr and LSc.vl entered as independent variables and displacements entered as dependent variables was significant (adjusted R2 = 0.56, p = 0.010). NT labeling pixel area in VTA (beta = 0.65, p = 0.016) statistically explained variation in agonistic behavior (Fig. 3; Fig. 8; Table 2).
Figure 8.
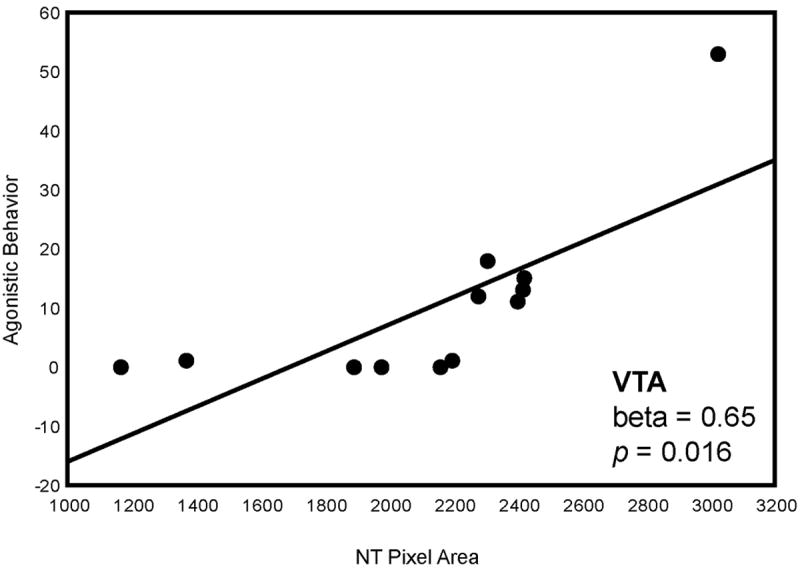
Two-dimensional visualization of a multiple regression model with 2 significant variables, in this case indicating the relationship between NT pixel area (X-axis) in VTA and agonistic behavior (Y-axis). Statistics were computed in a multiple regression model and do not represent values of linear correlations. Each point represents data from a single individual.
3.4 Non-specific behaviors
Multiple regression analyses with NT average intensity in the brain regions described above entered as independent variables did not statistically explain variation in feeding, preening, or calling (p > 0.05) (Table 1). A multiple regression analysis with NT pixel area in POM, VTA, PAG, BNST, LSr and LSc.vl entered as independent variables yielded a significant model for preening (adjusted R2 = 0.35, p = 0.024) with BNST (beta = 0.89, p = 0.0079) as a significant predictor (Fig. 3; Table 2).
4. Discussion
Our results demonstrate close linear associations between individual variability in sexually-motivated communication and NT immunolabeling measures in VTA, LS, and BNST. NT labeling in VTA, LS, and BNST also related positively to non-vocal courtship behaviors, suggesting a general role for NT in these regions in sexually-motivated behavior. Furthermore, NT labeling measures in LS, POM, and PAG were associated with agonistic behavior. These results suggest that NT may act in these regions to modify sexually-motivated communication and other social behaviors. It is also possible that the act of producing these behaviors induces changes in NT in these regions. However, as NT manipulations in past studies show NT to modify at least one form of social behavior (maternal defense of offspring [42]), we interpret these findings as consistent with a regulatory role for NT in sexually-motivated vocal communication. Overall, the present findings offer further support for the involvement of VTA, LS, and BNST in sexually-motivated song and, for the first time, indicate that NT may act within these regions to modulate this form of communication. Our results also support the idea that NT may be a neuromodulator in multiple social brain regions for the differential control of social behaviors.
4.1 NT labeling measures in VTA, LS, and BNST statistically explained sexually-motivated singing behavior
The area covered by NT labeling in VTA was associated positively with sexually-motivated singing behavior. This suggests that singing may involve non-localized NT innervation of large portions of VTA. Previous research shows strong positive correlations between c-Fos labeling in this region and sexually-motivated song production [33], and multiple studies correlate neuronal firing in VTA with the production of sexually-motivated male song [7,55]. Our data support the proposition that activity in VTA regulates vocal communication and that VTA is involved in mediating motivated social behaviors.
NT labeling density in LSr and BNST positively predicted sexually-motivated singing behavior, as did NT pixel area labeling in BNST. The area covered by NT labeling in LSc.vl related negatively to the number of complete songs. These patterns are somewhat consistent with past work showing positive correlations between neural activity (reflected in labeling for immediate early genes) in BNST and sexually-motivated song, but negative correlations between labeling in LSc.vl and sexually-motivated song [31,32,56]. Past studies, however, have not implicated LSr in sexually-motivated song [31,32]. It is not clear why NT would relate positive to song in one part of LS and negatively in another, but this may reflect functionally distinct subdivisions of LS, as discussed below in Section 4.6. Furthermore, septal lesions in male zebra finches reduce sexually-motivated song directed towards females [57]. The present findings thus provide further support for the involvement of VTA, LS, and BNST in sexually-motivated song and, for the first time, indicate that NT may act within these regions to modulate this form of communication. It is also possible that this type of singing behavior induces synthesis and/or release of NT in these regions.
LS and BNST are proposed to functionally link the social behavior network with mesolimbic dopamine reward/motivation systems [40]. These two areas are dense with neurons containing NT and neurons containing dopamine [42]. This anatomical relationship between NT and dopamine in LS and BNST is consistent with the possibility that NT may interact with dopamine-mediated information in these systems. Dopaminergic projections from VTA innervate both LS and BNST [58], and electrical stimulation of either LS or BNST activates dopamine neurons in VTA [59,60], suggesting the involvement of these regions in the modulation of goal-directed behaviors. Dopamine modulates NT activity in BNST [61], and the circuitry between VTA and BNST in particular has received attention in motivation literature due to its role in drug-seeking [62]. The positive correlations seen between NT in LS and BNST, sexually-motivated song, and non-vocal courtship behaviors (other measures of sexual motivation) further suggest that NT in these regions may be an important regulator of highly motivated, dopamine-mediated, goal-directed behaviors.
4.2 NT in LS, VTA, PAG, and POM was related to agonistic behavior
NT labeling in LS, VTA, PAG, and POM was associated with the number of times a male displaced another individual from a perch. Each of these regions has been implicated in agonistic behavior in past research. LS lesions reduce agonistic behaviors in birds and mammals [63,64]. In VTA and PAG of song sparrows, c-Fos-like immunoreactivity increases in response to simulated territorial intrusions and is correlated with territorial song [65]. The negative relationship observed between NT immunolabeling density in POM and agonistic behavior suggests that that POM may be a site in which NT acts to inhibit agonistic behavior. Intracerebroventricular injections of NT have been shown to reduce maternal defense of offspring in mice and increase neural activity in POM as measured by c-Fos expression [42]. Again, however, variation in NT labeling could be due to changes in behavior.
4.3 Distinct patterning of activity in social brain regions and the neuropeptidergic modulation of social behaviors
The overall patterning of activity by nuclei in the social behavior network and the action of neuropeptides are central to the modulation of social behaviors [38,39]. We found that the area covered by NT label in VTA was associated with multiple motivated behaviors, including courtship song, non-vocal courtship behaviors, and agonistic behavior, suggesting that activity in VTA may play a general role in motivating multiple behaviors. NT labeling in LS and BNST related to sexually-motivated behaviors (both vocal and non-vocal). This consistent relationship between NT labeling density in these regions and sexually-motivated behaviors may indicate that NT acts in LS and BNST to signify sexual motivation, and this information is then relayed to song control nuclei to stimulate courtship song and to motor nuclei to activate non-vocal courtship behaviors. The finding that a different relationship was seen between NT labeling density (in LS, VTA, PAG, POM) and agonistic behavior is consistent with the notion that distinct patterns of activity in social brain regions are associated with separate social behaviors and that NT may be a neuropeptidergic modulator in the social behavior network. Interestingly, slightly different relationships were observed for NT labeling density and sexually-motivated vocal behaviors (LSr, BNST) and sexually-motivated non-vocal behaviors (LSr, LSc.v, BNST), which may suggest that unique patterns of activity also underlie different behaviors within the same motivational state.
The present study and a past study on NT and maternal defense of offspring in mice [42] have implicated NT in LS and BNST as having a potential modulatory role in social behavior. A growing body of literature implicates LS and BNST, and interactions between the two, in a range of social behaviors including: recognition, approach, avoidance, affiliation, and bonding [43-47,66-69]. Future work could explore the extent to which NT in these regions influences additional social behaviors, and the directionality of these relationships
4.4 NT was not strongly implicated in non-specific behaviors
No significant relationship were identified between NT labeling intensity and the non-specific behaviors feeding, preening or calling. For pixel area no significant relationships were found between NT labeling and feeding or calling; however, NT pixel area labeling in BNST positively related to preening. This finding is surprising because past studies demonstrate that NT has inhibitory effects on grooming, as studied in rats (e.g. [70]). Additional studies are needed to understand this relationship; however, the overall lack of relationship identified between NT and non-specific behaviors suggests that NT in social brain regions is selectively involved in highly motivated, goal-directed behaviors. Feeding and preening are related to homeostatic or regulatory functions, not social motivation. Although calling is a vocalization produced in social situations [51], its purpose is not known and it is not used exclusively in a specific situation, suggesting it may not be a behavior that is as highly goal directed as sexually-motivated song. These findings therefore are consistent with a role for NT activity in specifically highly socially-motivated or goal-directed behaviors.
4.5 Regional specificity of LS
We found a positive correlation between NT labeling density in LSr and sexually-motivated communication, a negative correlation between NT pixel area and sexually-motivated song and additional positive relationships between NT labeling density in LSr and LSc.v and non-vocal courtship behaviors as well as NT labeling density in LSc.v and LSc.vl in agonistic behavior. LS is known to have neurochemically distinct anatomical sub-regions [54]. Past studies suggest these regions may also be functionally distinct. Agonistic behavior in white-throated sparrows is correlated positively with vasotocin and vasoactive intestinal peptide levels in LSc.vl, but not with the entire region when subdivisions are not accounted for [71]. Neural activity, as measured by ZENK labeling, in LSc.v and LSc.vl relates negatively to sexually-motivated song [31]. Activity in LS.cv as measured by c-Fos immunoreactivity relates negatively to the number of songs produced, but activity in the caudal dorsal portion of LS as measured by c-Fos and ZENK immunoreactivity relates negatively to average song bout length [56]. The relationship between LS sub-regions and behavior is thus not clear, but it is possible that the associations we observed between NT staining in sub-regions of LS may differ due to specifics in motivational state (sexual or agonistic) and/or class of behavior (vocal or non-vocal).
4.6 Conclusion
Overall, our results are an initial step in elucidating the role of NT in brain regions implicated in motivation and social behavior on sexually-motivated vocal communication. Additionally, they provide insight into NT’s contributions to social behaviors in general. This study provides support for the idea that distinct patterns of NT activity in multiple brain regions may underlie, or be induced by, variation in specific social behaviors. Future work is now needed to more closely examine the nature of NT and dopamine interactions in VTA, LS, and BNST, to determine the causality of the relationship between NT in these areas and sexually-motivated song, and to observe NT’s potential involvement in other social behaviors.
Highlights.
Neurotensin in VTA, LS and BNST related to sexually-motivated song.
Neurotensin in VTA, LS, PAG, and POM related to agonistic behavior.
Unique patterns of neurotensin labeling explained sexual and agonistic behaviors.
Results suggest a role for neurotensin in birdsong and other social behaviors.
Study supports idea that neuropeptides in social nuclei modulate behavior.
Acknowledgments
This work was funded by The National Institute of Mental Health (R01 MH080225 to LVR). The authors thank Dr. M. Susan DeVries and Allison L. Childs for experimental assistance and manuscript comments and Chris Elliott for starling care.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bradbury JW, Vehrencamp SL. Principles of animal communication. Sunderland: Mass.: Sinauer Associates; 2011. [Google Scholar]
- 2.Zeigler HP, Marler P. Neuroscience of birdsong. Cambridge, UK: Cambridge University Press; 2008. [Google Scholar]
- 3.Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 4.Björklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Kubikova L’, Košt’ál L’. Dopaminergic system in birdsong learning and maintenance. J Chem Neuroanat. 2010;39:112–23. doi: 10.1016/j.jchemneu.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riters LV. The role of motivation and reward neural systems in vocal communication in songbirds. Front Neuroendocrinol. 2012;33:194–209. doi: 10.1016/j.yfrne.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yanagihara S, Hessler NA. Modulation of singing-related activity in the songbird ventral tegmental area by social context. Eur J Neurosci. 2006;24:3619–27. doi: 10.1111/j.1460-9568.2006.05228.x. [DOI] [PubMed] [Google Scholar]
- 8.Bharati IS, Goodson JL. Fos responses of dopamine neurons to sociosexual stimuli in male zebra finches. Neuroscience. 2006;143:661–70. doi: 10.1016/j.neuroscience.2006.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodson JL, Kabelik D, Kelly AM, Rinaldi J, Klatt JD. Midbrain dopamine neurons reflect affiliation phenotypes in finches and are tightly coupled to courtship. Proc Natl Acad Sci. 2009;106:8737–42. doi: 10.1073/pnas.0811821106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heimovics SA, Riters LV. Evidence that dopamine within motivation and song control brain regions regulates birdsong context-dependently. Physiol Behav. 2008;95:258–66. doi: 10.1016/j.physbeh.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heimovics SA, Salvante KG, Sockman KW, Riters LV. Individual differences in the motivation to communicate relate to levels of midbrain and striatal catecholamine markers in male European starlings. Horm Behav. 2011;60:529–39. doi: 10.1016/j.yhbeh.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Binder EB, Kinkead B, Owens MJ, Nemeroff CB. Neurotensin and Dopamine Interactions. Pharmacol Rev. 2001;53:453–86. [PubMed] [Google Scholar]
- 13.Numao M, Sudo H, Yamamoto I, Nakao N, Kaiya H, Miyazato M, et al. Molecular characterization of structure and tissue distribution of chicken neurotensin receptor. Gen Comp Endocrinol. 2011;171:33–8. doi: 10.1016/j.ygcen.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 14.Hökfelt T, Everitt BJ, Theodorsson-Norheim E, Goldstein M. Occurrence of neurotensinlike immunoreactivity in subpopulations of hypothalamic, mesencephalic, and medullary catecholamine neurons. J Comp Neurol. 1984;222:543–59. doi: 10.1002/cne.902220407. [DOI] [PubMed] [Google Scholar]
- 15.Palacios JM, Kuhar MJ. Neurotensin receptors are located on dopamine-containing neurones in rat midbrain. Nature. 1981;294:587–9. doi: 10.1038/294587a0. [DOI] [PubMed] [Google Scholar]
- 16.Seroogy K, Ceccatelli S, Schalling M, Hökfelt T, Frey P, Walsh J, et al. A subpopulation of dopaminergic neurons in rat ventral mesencephalon contains both neurotensin and cholecystokinin. Brain Res. 1988;455:88–98. doi: 10.1016/0006-8993(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 17.Köhler C, Eriksson LG. An immunohistochemical study of somatostatin and neurotensin positive neurons in the septal nuclei of the rat brain. Anat Embryol (Berl) 1984;170:1–10. doi: 10.1007/BF00319452. [DOI] [PubMed] [Google Scholar]
- 18.Moyse E, Rostène W, Vial M, Leonard K, Mazella J, Kitabgi P, et al. Distribution of neurotensin binding sites in rat brain: A light microscopic radioautographic study using monoiodo [125I]Tyr3-neurotensin. Neuroscience. 1987;22:525–36. doi: 10.1016/0306-4522(87)90350-2. [DOI] [PubMed] [Google Scholar]
- 19.Roberts GW, Woodhams PL, Polak JM, Crow TJ. Distribution of neuropeptides in the limbic system of the rat: The amygdaloid complex. Neuroscience. 1982;7:99–131. doi: 10.1016/0306-4522(82)90156-7. [DOI] [PubMed] [Google Scholar]
- 20.Uhl GR, Snyder SH. Neurotensin: a neuronal pathway projecting from amygdala through stria terminalis. Brain Res. 1979;161:522–6. doi: 10.1016/0006-8993(79)90681-4. [DOI] [PubMed] [Google Scholar]
- 21.Appeltants D, Ball GF, Balthazart J. The origin of catecholaminergic inputs to the song control nucleus RA in canaries. Neuroreport. 2002;13:649–53. doi: 10.1097/00001756-200204160-00023. [DOI] [PubMed] [Google Scholar]
- 22.Appeltants D, Absil P, Balthazart J, Ball GF. Identification of the origin of catecholaminergic inputs to HVc in canaries by retrograde tract tracing combined with tyrosine hydroxylase immunocytochemistry. J Chem Neuroanat. 2000;18:117–33. doi: 10.1016/s0891-0618(99)00054-x. [DOI] [PubMed] [Google Scholar]
- 23.Absil P, Riters LV, Balthazart J. Preoptic Aromatase Cells Project to the Mesencephalic Central Gray in the Male Japanese Quail (Coturnix japonica) Horm Behav. 2001;40:369–83. doi: 10.1006/hbeh.2001.1702. [DOI] [PubMed] [Google Scholar]
- 24.Atoji Y, Saito S, Wild JM. Fiber connections of the compact division of the posterior pallial amygdala and lateral part of the bed nucleus of the stria terminalis in the pigeon (Columba livia) J Comp Neurol. 2006;499:161–82. doi: 10.1002/cne.21042. [DOI] [PubMed] [Google Scholar]
- 25.Balthazart J, Absil P. Identification of catecholaminergic inputs to and outputs from aromatase-containing brain areas of the Japanese quail by tract tracing combined with tyrosine hydroxylase immunocytochemistry. J Comp Neurol. 1997;382:401–28. [PubMed] [Google Scholar]
- 26.Montagnese CM, Zachar G, Bálint E, Csillag A. Afferent connections of septal nuclei of the domestic chick (Gallus domesticus): A retrograde pathway tracing study. J Comp Neurol. 2008;511:109–50. doi: 10.1002/cne.21837. [DOI] [PubMed] [Google Scholar]
- 27.Montagnese CM, Székely AD, Ádám Á, Csillag A. Efferent connections of septal nuclei of the domestic chick (Gallus domesticus): An anterograde pathway tracing study with a bearing on functional circuits. J Comp Neurol. 2004;469:437–56. doi: 10.1002/cne.11018. [DOI] [PubMed] [Google Scholar]
- 28.Riters LV, Alger SJ. Neuroanatomical evidence for indirect connections between the medial preoptic nucleus and the song control system: possible neural substrates for sexually motivated song. Cell Tissue Res. 2004;316:35–44. doi: 10.1007/s00441-003-0838-6. [DOI] [PubMed] [Google Scholar]
- 29.Alger SJ, Riters LV. Lesions to the medial preoptic nucleus differentially affect singing and nest box-directed behaviors within and outside of the breeding season in European starlings (Sturnus vulgaris) Behav Neurosci. 2006;120:1326–36. doi: 10.1037/0735-7044.120.6.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atoji Y, Shibata N, Yamamoto Y, Suzuki Y. Distribution of neurotensin-containing neurons in the central nervous system of the pigeon and the chicken. J Comp Neurol. 1996;375:187–211. doi: 10.1002/(SICI)1096-9861(19961111)375:2<187::AID-CNE2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 31.Heimovics SA, Riters LV. ZENK labeling within social behavior brain regions reveals breeding context-dependent patterns of neural activity associated with song in male European starlings (Sturnus vulgaris) Behav Brain Res. 2007;176:333–43. doi: 10.1016/j.bbr.2006.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heimovics SA, Riters LV. Breeding-context-dependent relationships between song and cFOS labeling within social behavior brain regions in male European starlings (Sturnus vulgaris) Horm Behav. 2006;50:726–35. doi: 10.1016/j.yhbeh.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heimovics SA, Riters LV. Immediate early gene activity in song control nuclei and brain areas regulating motivation relates positively to singing behavior during, but not outside of, a breeding context. J Neurobiol. 2005;65:207–24. doi: 10.1002/neu.20181. [DOI] [PubMed] [Google Scholar]
- 34.Heimovics SA, Cornil CA, Ball GF, Riters LV. D1-like dopamine receptor density in nuclei involved in social behavior correlates with song in a context-dependent fashion in male European starlings. Neuroscience. 2009;159:962–73. doi: 10.1016/j.neuroscience.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jennes L, Stumpf WE, Kalivas PW. Neurotensin: Topographical distribution in rat brain by immunohistochemistry. J Comp Neurol. 1982;210:211–24. doi: 10.1002/cne.902100302. [DOI] [PubMed] [Google Scholar]
- 36.Riters LV, Pawlisch BA, Kelm-Nelson CA, Stevenson SA. Inverted-U shaped effects of D1 dopamine receptor stimulation in the medial preoptic nucleus on sexually motivated song in male European starlings. Eur J Neurosci. 2014;39:650–62. doi: 10.1111/ejn.12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uhl GR. Distribution of neurotensin and its receptor in the central nervous system. Ann N Y Acad Sci. 1982;400:132–49. doi: 10.1111/j.1749-6632.1982.tb31565.x. [DOI] [PubMed] [Google Scholar]
- 38.Goodson JL. The vertebrate social behavior network: Evolutionary themes and variations. Horm Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Newman SW. The Medial Extended Amygdala in Male Reproductive Behavior A Node in the Mammalian Social Behavior Network. Ann N Y Acad Sci. 1999;877:242–57. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- 40.O’Connell LA, Hofmann HA. The Vertebrate mesolimbic reward system and social behavior network: A comparative synthesis. J Comp Neurol. 2011;519:3599–639. doi: 10.1002/cne.22735. [DOI] [PubMed] [Google Scholar]
- 41.Gammie SC, Auger AP, Jessen HM, Vanzo RJ, Awad TA, Stevenson SA. Altered gene expression in mice selected for high maternal aggression. Genes Brain Behav. 2007;6:432–43. doi: 10.1111/j.1601-183X.2006.00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gammie SC, D’Anna KL, Gerstein H, Stevenson SA. Neurotensin inversely modulates maternal aggression. Neuroscience. 2009;158:1215–23. doi: 10.1016/j.neuroscience.2008.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bielsky IF, Hu S-B, Ren X, Terwilliger EF, Young LJ. The V1a Vasopressin Receptor Is Necessary and Sufficient for Normal Social Recognition: A Gene Replacement Study. Neuron. 2005;47:503–13. doi: 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 44.Landgraf R, Frank E, Aldag JM, Neumann ID, Sharer CA, Ren X, et al. Viral vector-mediated gene transfer of the vole V1a vasopressin receptor in the rat septum: improved social discrimination and active social behaviour. Eur J Neurosci. 2003;18:403–11. doi: 10.1046/j.1460-9568.2003.02750.x. [DOI] [PubMed] [Google Scholar]
- 45.Landgraf R, Gerstberger R, Montkowski A, Probst JC, Wotjak CT, Holsboer F, et al. V1 vasopressin receptor antisense oligodeoxynucleotide into septum reduces vasopressin binding, social discrimination abilities, and anxiety-related behavior in rats. J Neurosci. 1995;15:4250–8. doi: 10.1523/JNEUROSCI.15-06-04250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Y, Curtis JT, Wang Z. Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster) Behav Neurosci. 2001;115:910–9. doi: 10.1037//0735-7044.115.4.910. [DOI] [PubMed] [Google Scholar]
- 47.Lukas M, Toth I, Veenema AH, Neumann ID. Oxytocin mediates rodent social memory within the lateral septum and the medial amygdala depending on the relevance of the social stimulus: Male juvenile versus female adult conspecifics. Psychoneuroendocrinology. 2013;38:916–26. doi: 10.1016/j.psyneuen.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 48.Dawson A. Plasma gonadal steroid levels in wild starlings (Sturnus vulgaris) during the annual cycle and in relation to the stages of breeding. Gen Comp Endocrinol. 1983;49:286–94. doi: 10.1016/0016-6480(83)90146-6. [DOI] [PubMed] [Google Scholar]
- 49.Cordes MA, Stevenson SA, Riters LV. Status-appropriate singing behavior, testosterone and androgen receptor immunolabeling in male European starlings (Sturnus vulgaris) Horm Behav. 2014;65:329–39. doi: 10.1016/j.yhbeh.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eens M, Pinxten R, Verheyen RF. On the function of singing and wing-waving in the European Starling Sturnus vulgaris. Bird Study. 1990;37:48–52. [Google Scholar]
- 51.Feare C. The starling. Oxford: Oxford University Press; 1984. [Google Scholar]
- 52.Dobner PR, Barber DL, Villa-Komaroff L, McKiernan C. Cloning and sequence analysis of cDNA for the canine neurotensin/neuromedin N precursor. Proc Natl Acad Sci. 1987;84:3516–20. doi: 10.1073/pnas.84.10.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mazella J, Kitabgi P, Vincent J-P. Molecular properties of neurotensin receptors in rat brain. Identification of subunits by covalent labeling. J Biol Chem. 1985;260:508–14. [PubMed] [Google Scholar]
- 54.Goodson JL, Evans AK, Lindberg L. Chemoarchitectonic subdivisions of the songbird septum and a comparative overview of septum chemical anatomy in jawed vertebrates. J Comp Neurol. 2004;473:293–314. doi: 10.1002/cne.20061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hara E, Kubikova L, Hessler NA, Jarvis ED. Role of the midbrain dopaminergic system in modulation of vocal brain activation by social context. Eur J Neurosci. 2007;25:3406–16. doi: 10.1111/j.1460-9568.2007.05600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alger SJ, Maasch SN, Riters LV. Lesions to the medial preoptic nucleus affect immediate early gene immunolabeling in brain regions involved in song control and social behavior in male European starlings. Eur J Neurosci. 2009;29:970–82. doi: 10.1111/j.1460-9568.2009.06637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goodson JL, Eibach R, Sakata J, Adkins-Regan E. The effect of septal lesions on male song and aggression in the colonial zebra finch (Taeniopygia guttata) and the territorial field sparrow (Spizella pusilla) Behav Brain Res. 1999;98:167–80. [PubMed] [Google Scholar]
- 58.Swanson LW. The projections of the ventral tegmental area and adjacent regions: A combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–53. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- 59.Georges F, Aston-Jones G. Potent Regulation of Midbrain Dopamine Neurons by the Bed Nucleus of the Stria Terminalis. J Neurosci. 2001;21:RC160–RC160. doi: 10.1523/JNEUROSCI.21-16-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maeda H, Mogenson GJ. Electrophysiological responses of neurons of the ventral tegmental area to electrical stimulation of amygdala and lateral septum. Neuroscience. 1981;6:367–76. doi: 10.1016/0306-4522(81)90130-5. [DOI] [PubMed] [Google Scholar]
- 61.Day HEW, Vittoz NM, Oates MM, Badiani A, Watson SJ, Jr, Robinson TE, et al. A 6-Hydroxydopamine lesion of the mesostriatal dopamine system decreases the expression of corticotropin releasing hormone and neurotensin mRNAs in the amygdala and bed nucleus of the stria terminalis. Brain Res. 2002;945:151–9. doi: 10.1016/s0006-8993(02)02747-6. [DOI] [PubMed] [Google Scholar]
- 62.Jalabert M, Aston-Jones G, Herzog E, Manzoni O, Georges F. Role of the bed nucleus of the stria terminalis in the control of ventral tegmental area dopamine neurons. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1336–46. doi: 10.1016/j.pnpbp.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goodson JL. Territorial Aggression and Dawn Song are Modulated by Septal Vasotocin and Vasoactive Intestinal Polypeptide in Male Field Sparrows (Spizella pusilla) Horm Behav. 1998;34:67–77. doi: 10.1006/hbeh.1998.1467. [DOI] [PubMed] [Google Scholar]
- 64.Patil SN, Brid SV. Relative Role of Neural Substrates in the Aggressive Behavior of Rats. J Basic Clin Physiol Pharmacol. 2011;21:357–68. doi: 10.1515/jbcpp.2010.21.4.357. [DOI] [PubMed] [Google Scholar]
- 65.Maney DL, Ball GF. Fos-like immunoreactivity in catecholaminergic brain nuclei after territorial behavior in free-living song sparrows. J Neurobiol. 2003;56:163–70. doi: 10.1002/neu.10227. [DOI] [PubMed] [Google Scholar]
- 66.Goodson JL, Evans AK, Wang Y. Neuropeptide binding reflects convergent and divergent evolution in species-typical group sizes. Horm Behav. 2006;50:223–36. doi: 10.1016/j.yhbeh.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kirkpatrick B, Carter CS, Newman SW, Insel TR. Axon-sparing lesions of the medial nucleus of the amygdala decrease affiliative behaviors in the prairie vole (Microtus ochrogaster): Behavioral and anatomical specificity. Behav Neurosci. 1994;108:501. doi: 10.1037//0735-7044.108.3.501. [DOI] [PubMed] [Google Scholar]
- 68.Lehman MN, Winans SS, Powers JB. Medial Nucleus of the Amygdala Mediates Chemosensory Control of Male Hamster Sexual Behavior. Science. 1980;210:557–60. doi: 10.1126/science.7423209. [DOI] [PubMed] [Google Scholar]
- 69.Luiten PGM, Koolhaas JM, de Boer S, Koopmans SJ. The cortico-medial amygdala in the central nervous system organization of agonistic behavior. Brain Res. 1985;332:283–97. doi: 10.1016/0006-8993(85)90597-9. [DOI] [PubMed] [Google Scholar]
- 70.Sandoval SL, Kulkosky PJ. Effects of peripheral neurotensin on behavior of the rat. Pharmacol Biochem Behav. 1992;41:385–90. doi: 10.1016/0091-3057(92)90115-v. [DOI] [PubMed] [Google Scholar]
- 71.Maney DL, Erwin KL, Goode CT. Neuroendocrine correlates of behavioral polymorphism in white-throated sparrows. Horm Behav. 2005;48:196–206. doi: 10.1016/j.yhbeh.2005.03.004. [DOI] [PubMed] [Google Scholar]


