Abstract
A study in a mouse model of immune-mediated glomerular disease and in people with rapidly progressive glomerulonephritis shows activation of epidermal growth factor receptor (EFGR) signaling in podocytes by a molecule expressed in the kidney (pages 1242–1250). Blocking this axis may open new doors to treat inflammatory kidney conditions.
Evolution has fashioned the glomerulus of the mammalian kidney into an integrated unit to provide essential blood filtration. Each of the million or so glomeruli in the human kidney consists of a cluster of capillary loops composed of a fenestrated endothelium overlaid by interdigitating foot processes of specialized epithelia called podocytes, which, together with the endothelial cells, is the source of an interposing glomerular basement membrane (GBM). Mesenchymal mesangial cells and their associated extracellular matrix support the interstices of these capillary tufts. Bowman’s capsule, which is composed of parietal epithelial cells, separates the tuft from surrounding renal parenchyma by forming a urinary space connecting to a proximal tubular lumen.
Unfortunately, the glomerulus is the target for a variety of inflammatory and noninflammatory forms of injury. The most severe inflammatory injury is rapidly progressive glomerulonephritis (RPGN), which is characterized by a precipitous loss of glomerular filtration and by the accumulation of CD4+ T cells and macrophages in the tuft, proliferation of endogenous glomerular cells and the development of cellular crescents, which result from glomerular capillary damage and subsequent leakage of plasma proteins into Bowman’s space. Crescents consist of fibrous material and proliferating cells arising from both the parietal epithelial cells and from podocytes, as well as infiltrating macrophages and myofibroblasts1.
RPGN is a clinical syndrome that can result from a number of diseases, one of which is anti-GBM disease, an autoimmune condition in which individuals develop cytotoxic antibodies targeted to the GBM. In the current issue of Nature Medicine, Bollée et al.2 in a mouse model of anti-GBM disease show that the activation of EGFR in podocytes by one of its ligands, heparin-binding EGF (HB-EGF), results in the development and progression of RPGN. Pharmacological blockade or genetic deletion of the HB-EGF–EGFR axis in podocytes improved the course of RPGN in mice and prevented infiltration of inflammatory cells. These findings may open new therapeutic avenues to tackle crescentic and immune-mediated glomerular disease.
The EGFR is a member of the family of ErbB receptors, which is widely expressed, including in mammalian kidney3. Upon activation, there is phosphorylation of residues that serve as docking sites for a variety of signaling molecules that activate intracellular pathways controlling cell proliferation, differentiation and apoptosis4. Members of the EGF peptide growth factor–related family4, 5, such as EGF, amphiregulin, transforming growth factor-α (TGF-α), HB-EGF, betacellulin and epiregulin are produced as transmembrane proteins that are cleaved by metalloproteinases, especially ADAMs, to release a soluble active moiety that can activate EGFR. A number of these ligands have been implicated in experimental models of renal disease6. Notably, TGF-α has previously been implicated in progressive renal injury, including that seen in response to chronic exposure to angiotensin II or severe ablation of functioning renal tissue7–9.
One of these ligands, HB-EGF, is constitutively expressed in the kidney, especially the epithelial cells of the distal nephron, and its expression increases in response to acute is chemic or toxic injury10. A study in a rat model of experimental anti-GBM disease showed there was also increased expression of HB-EGF in podocytes and mesangial cells and that a blocking antibody to HB-EGF reversed the acute decreases in glomerular filtration in response to administration of anti-GBM antisera11, but, despite showing amelioration of acute renal hemodynamic abnormalities, this group did not examine the long-term consequences of interrupting the HB-EGF–EGFR signaling axis.
Bollée et al.2 have now performed an extensive set of studies to follow up these initial observations. Using a mouse model of anti-GBM disease in which the mice develop severe crescenteric glomerulonephritis and loss of renal function over 8 d, the authors demonstrated that mice with genetic deletion of HB-EGF show markedly decreased functional and structural injury. Their studies confirmed increased expression of HB-EGF in podocytes and parietal epithelial cells in wild-type mice with RPGN and showed substantially increased glomerular immunoreactive HB-EGF in human biopsies of individuals with RPGN2. HB-EGF activation of EGFR in cultured podocytes led to dedifferentiation and conversion to a proliferative and migratory phenotype, consistent with what has been reported to occur in vivo in crescent formation1. Selective, temporally controlled ablation of podocyte EGFR lessened the severity of glomerular injury to a similar extent as HB-EGF deletion.
As further proof that HB-EGF is the primary culprit, they tested other EGFR ligands, TGF-α and epiregulin, and failed to see any amelioration2. And although T cells and macrophages express HB-EGF, selective deletion of HB-EGF in hematopoietic cells did not alter the course of the disease, suggesting that it is the increased expression of HB-EGF in the glomerulus that is pathogenic.
The current treatment for RPGN involves broad immunosuppressive therapy, accompanied by plasmapheresis if there is evidence for antibody involvement. One of the most exciting translational aspects of the study by Bollée et al.2 is the identification of EGFR signaling as a potential therapeutic target in this condition. In their studies, treatment with EGFR tyrosine kinase inhibitors had beneficial effects similar to genetic deletion of either HB-EGF or EGFR. Of special note, one of these inhibitors, erlotinib, was effective even when treatment was begun 4 d after the initiation of the injury, which could have important clinical implications, as individuals with RPGN often do not see a physician until the disease manifests clinically.
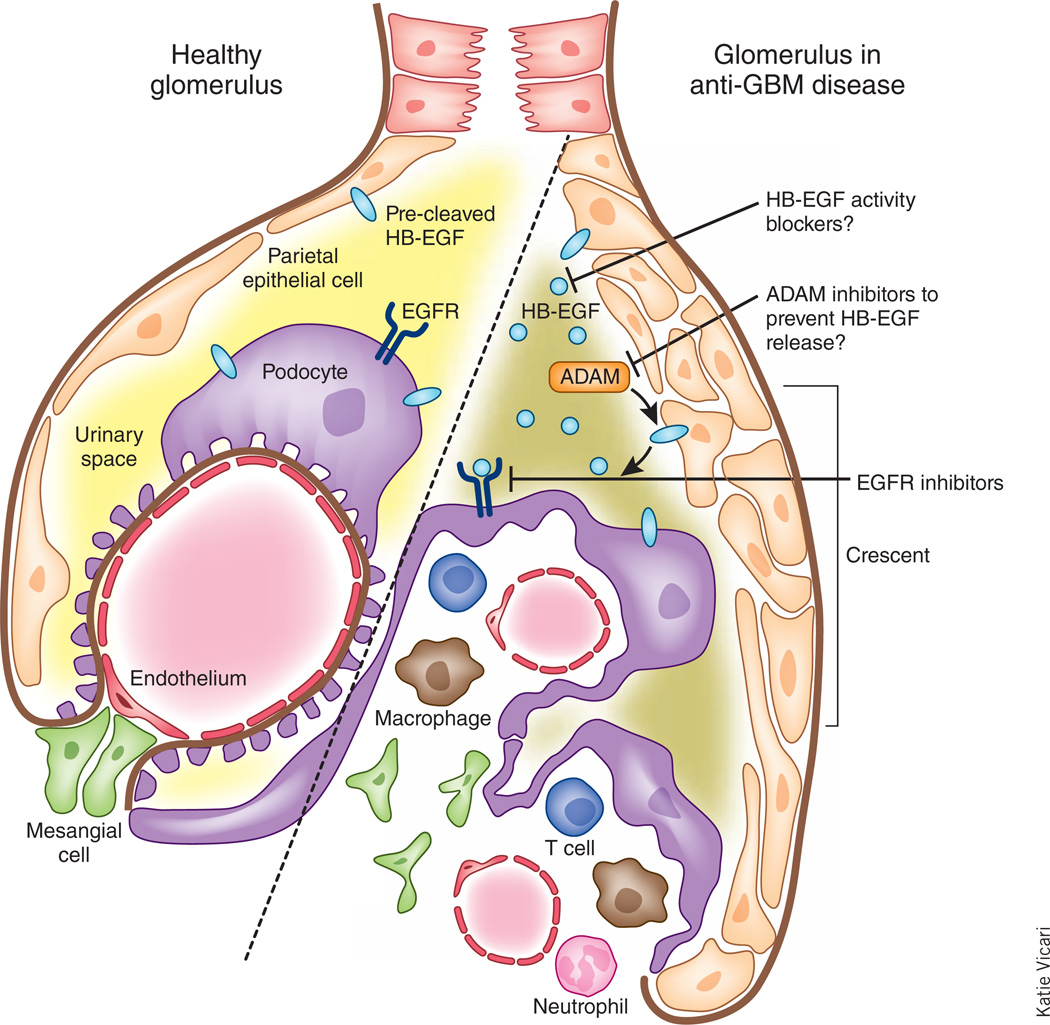
The authors convincingly demonstrated a crucial pathogenic role for HB-EGF activation of glomerular EGFR signaling in a mouse model of anti-GBM–induced RPGN (Fig. 1). Future studies will need to address whether this signaling pathway is involved in the pathogenesis of other types of progressive glomerulonephritis, such as IgA nephropathy or in systemic diseases that can affect the glomerulus, such as lupus nephritis or diabetic nephropathy.
Figure 1.
Activation of the EGFR pathway by HB-EGF in the glomerulus in RPGN. HB-EGF is expressed in the kidney in an anti-GBM disease model in mice. Bollée et al.2 now show that this molecule activates EGFR on podocytes and parietal epithelial cells to drive crescent formation during glomerulonephritis, renal failure and the clinical features of RPGN. Blockade of the HB-EGF–EGFR axis with EGFR inhibitors or HB-EGF activity or release blockers may be a potential way of therapeutic intervention.
In addition, these current studies raise the possibility that EGFR kinase inhibitors and/ or blocking antibodies could be efficacious in the treatment of human RPGN. It may also be possible to block the activation of EGFR by interfering with HB-EGF, either by preventing its release with ADAM inhibitors, or, as HB-EGF is the diphtheria toxin receptor, blocking its actions with the nontoxic diphtheria toxin analog CRM197. Further studies will also be required to determine whether urinary HB-EGF might serve as a biomarker of glomerular disease activity.
Footnotes
COMPETING FINANCIAL INTERESTS
The author declares no competing financial interests.
References
- 1.Thorner PS, Ho M, Eremina V, Sado Y, Quaggin S. J. Am. Soc. Nephrol. 2008;19:495–502. doi: 10.1681/ASN.2006101115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bollée G, et al. Nat. Med. 2011;17:1242–1250. doi: 10.1038/nm.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Citri A, Yarden Y. Nat. Rev. Mol. Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 4.Avraham R, Yarden Y. Nat. Rev. Mol. Cell Biol. 2011;12:104–117. doi: 10.1038/nrm3048. [DOI] [PubMed] [Google Scholar]
- 5.Harris RC, Chung E, Coffey RJ. Exp. Cell Res. 2003;284:2–13. doi: 10.1016/s0014-4827(02)00105-2. [DOI] [PubMed] [Google Scholar]
- 6.Zeng F, Singh AB, Harris RC. Exp. Cell Res. 2009;315:602–610. doi: 10.1016/j.yexcr.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lautrette A, et al. Nat. Med. 2005;11:867–874. doi: 10.1038/nm1275. [DOI] [PubMed] [Google Scholar]
- 8.Pillebout E, et al. J. Clin. Invest. 2003;112:843–852. doi: 10.1172/JCI17647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laouari D, et al. J. Am. Soc. Nephrol. 2011;22:327–335. doi: 10.1681/ASN.2010040356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakai M, et al. J. Clin. Invest. 1997;99:2128–2138. doi: 10.1172/JCI119386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng L, et al. J. Clin. Invest. 2000;105:341–350. doi: 10.1172/JCI2869. [DOI] [PMC free article] [PubMed] [Google Scholar]