Abstract
Inflammatory stimulation of the liver leads to the induction of nitric oxide (NO) biosynthesis. Because NO binds to the catalytic heme moiety of cytochromes P450 (CYPs), we investigated whether NO interferes with specific CYP-dependent metabolic pathways. In a first experimental approach V79 Chinese hamster cells genetically engineered for stable expression of rat and human CYP1A1 and -1A2 were used. Incubation with the NO donors sodium nitroprusside and S-nitrosylacetylpenicillamine led to a concentration-dependent inhibition of all four CYP enzymes. CYP1A1 was more sensitive to the inhibitory effect of NO than CYP1A2. In the second part of the study, endogenous NO synthesis was induced in rat hepatocytes by incubation with a mixture of cytokines and endotoxin. Concurrently, as NO production in hepatocytes increased within 24 hr, a decrease in CYP1A1-dependent benzo[a]pyrene turnover was observed to almost undetectable levels. The competitive inhibitor of NO synthesis, NG-monomethyl-L-arginine, was able to significantly restore CYP1A1 activity in the presence of cytokines and endotoxin. Inhibition of hepatocellular CYP activity by NO was predominantly due to a direct effect on the enzymes. However, NO-dependent inhibition of CYP expression at a transcriptional level was also demonstrated. Our results indicate that inhibition of NO biosynthesis in patients suffering from systemic inflammatory response syndromes may help to restore biotransformation capacity of the liver.
Full text
PDF

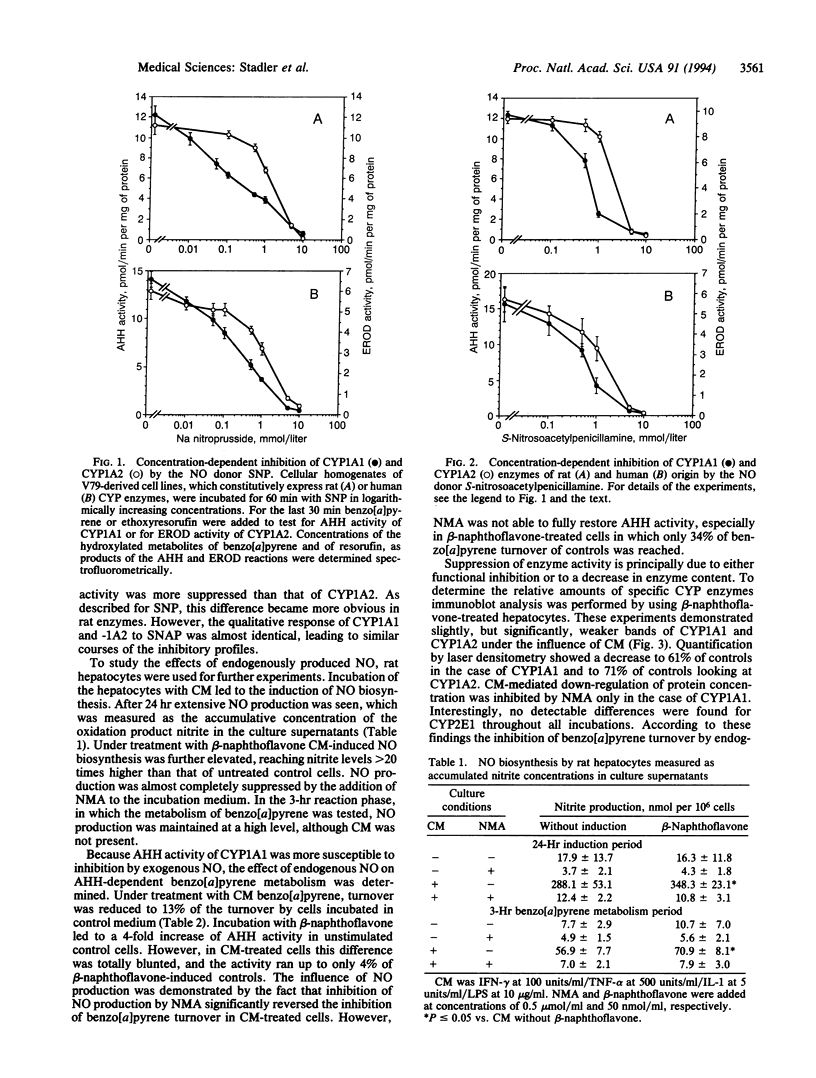
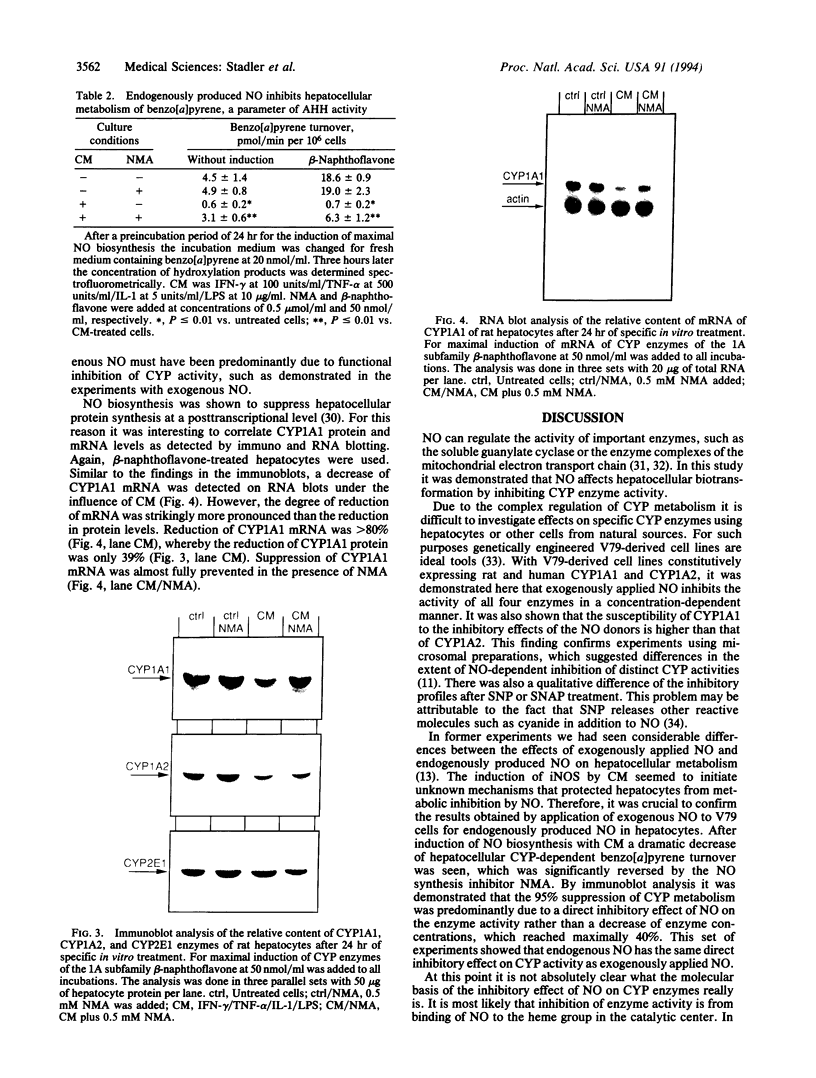

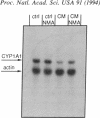
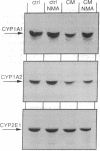
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker C. W., Fagan J. B., Pasco D. S. Interleukin-1 beta suppresses the induction of P4501A1 and P4501A2 mRNAs in isolated hepatocytes. J Biol Chem. 1992 Apr 25;267(12):8050–8055. [PubMed] [Google Scholar]
- Billiar T. R., Curran R. D., Stuehr D. J., West M. A., Bentz B. G., Simmons R. L. An L-arginine-dependent mechanism mediates Kupffer cell inhibition of hepatocyte protein synthesis in vitro. J Exp Med. 1989 Apr 1;169(4):1467–1472. doi: 10.1084/jem.169.4.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Curran R. D., Billiar T. R., Stuehr D. J., Hofmann K., Simmons R. L. Hepatocytes produce nitrogen oxides from L-arginine in response to inflammatory products of Kupffer cells. J Exp Med. 1989 Nov 1;170(5):1769–1774. doi: 10.1084/jem.170.5.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran R. D., Billiar T. R., Stuehr D. J., Ochoa J. B., Harbrecht B. G., Flint S. G., Simmons R. L. Multiple cytokines are required to induce hepatocyte nitric oxide production and inhibit total protein synthesis. Ann Surg. 1990 Oct;212(4):462–471. doi: 10.1097/00000658-199010000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran R. D., Ferrari F. K., Kispert P. H., Stadler J., Stuehr D. J., Simmons R. L., Billiar T. R. Nitric oxide and nitric oxide-generating compounds inhibit hepatocyte protein synthesis. FASEB J. 1991 Apr;5(7):2085–2092. doi: 10.1096/fasebj.5.7.1707021. [DOI] [PubMed] [Google Scholar]
- Doehmer J., Dogra S., Friedberg T., Monier S., Adesnik M., Glatt H., Oesch F. Stable expression of rat cytochrome P-450IIB1 cDNA in Chinese hamster cells (V79) and metabolic activation of aflatoxin B1. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5769–5773. doi: 10.1073/pnas.85.16.5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehmer J., Wölfel C., Dogra S., Doehmer C., Seidel A., Platt K. L., Oesch F., Glatt H. R. Applications of stable V79-derived cell lines expressing rat cytochromes P4501A1, 1A2, and 2B1. Xenobiotica. 1992 Sep-Oct;22(9-10):1093–1099. doi: 10.3109/00498259209051863. [DOI] [PubMed] [Google Scholar]
- Dogra S., Doehmer J., Glatt H., Mölders H., Siegert P., Friedberg T., Seidel A., Oesch F. Stable expression of rat cytochrome P-450IA1 cDNA in V79 Chinese hamster cells and their use in mutagenicity testing. Mol Pharmacol. 1990 May;37(5):608–613. [PubMed] [Google Scholar]
- Fukuda Y., Ishida N., Noguchi T., Kappas A., Sassa S. Interleukin-6 down regulates the expression of transcripts encoding cytochrome P450 IA1, IA2 and IIIA3 in human hepatoma cells. Biochem Biophys Res Commun. 1992 Apr 30;184(2):960–965. doi: 10.1016/0006-291x(92)90684-d. [DOI] [PubMed] [Google Scholar]
- Geller D. A., Lowenstein C. J., Shapiro R. A., Nussler A. K., Di Silvio M., Wang S. C., Nakayama D. K., Simmons R. L., Snyder S. H., Billiar T. R. Molecular cloning and expression of inducible nitric oxide synthase from human hepatocytes. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3491–3495. doi: 10.1073/pnas.90.8.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller D. A., Nussler A. K., Di Silvio M., Lowenstein C. J., Shapiro R. A., Wang S. C., Simmons R. L., Billiar T. R. Cytokines, endotoxin, and glucocorticoids regulate the expression of inducible nitric oxide synthase in hepatocytes. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):522–526. doi: 10.1073/pnas.90.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Haem-dependent activation of guanylate cyclase and cyclic GMP formation by endogenous nitric oxide: a unique transduction mechanism for transcellular signaling. Pharmacol Toxicol. 1990 Jul;67(1):1–7. doi: 10.1111/j.1600-0773.1990.tb00772.x. [DOI] [PubMed] [Google Scholar]
- Kanner J., Harel S., Granit R. Nitric oxide, an inhibitor of lipid oxidation by lipoxygenase, cyclooxygenase and hemoglobin. Lipids. 1992 Jan;27(1):46–49. doi: 10.1007/BF02537058. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Morgan E. T. Suppression of constitutive cytochrome P-450 gene expression in livers of rats undergoing an acute phase response to endotoxin. Mol Pharmacol. 1989 Nov;36(5):699–707. [PubMed] [Google Scholar]
- Nebert D. W., Gelboin H. V. Substrate-inducible microsomal aryl hydroxylase in mammalian cell culture. I. Assay and properties of induced enzyme. J Biol Chem. 1968 Dec 10;243(23):6242–6249. [PubMed] [Google Scholar]
- Nussler A. K., Di Silvio M., Billiar T. R., Hoffman R. A., Geller D. A., Selby R., Madariaga J., Simmons R. L. Stimulation of the nitric oxide synthase pathway in human hepatocytes by cytokines and endotoxin. J Exp Med. 1992 Jul 1;176(1):261–264. doi: 10.1084/jem.176.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nüssler A., Drapier J. C., Rénia L., Pied S., Miltgen F., Gentilini M., Mazier D. L-arginine-dependent destruction of intrahepatic malaria parasites in response to tumor necrosis factor and/or interleukin 6 stimulation. Eur J Immunol. 1991 Jan;21(1):227–230. doi: 10.1002/eji.1830210134. [DOI] [PubMed] [Google Scholar]
- O'Keeffe D. H., Ebel R. E., Peterson J. A. Studies of the oxygen binding site of cytochrome P-450. Nitric oxide as a spin-label probe. J Biol Chem. 1978 May 25;253(10):3509–3516. [PubMed] [Google Scholar]
- Rao D. N., Elguindi S., O'Brien P. J. Reductive metabolism of nitroprusside in rat hepatocytes and human erythrocytes. Arch Biochem Biophys. 1991 Apr;286(1):30–37. doi: 10.1016/0003-9861(91)90005-4. [DOI] [PubMed] [Google Scholar]
- Schmalix W. A., Mäser H., Kiefer F., Reen R., Wiebel F. J., Gonzalez F., Seidel A., Glatt H., Greim H., Doehmer J. Stable expression of human cytochrome P450 1A1 cDNA in V79 Chinese hamster cells and metabolic activation of benzo[a]pyrene. Eur J Pharmacol. 1993 Oct 1;248(3):251–261. doi: 10.1016/0926-6917(93)90052-r. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Stadler J., Bergonia H. A., Di Silvio M., Sweetland M. A., Billiar T. R., Simmons R. L., Lancaster J. R., Jr Nonheme iron-nitrosyl complex formation in rat hepatocytes: detection by electron paramagnetic resonance spectroscopy. Arch Biochem Biophys. 1993 Apr;302(1):4–11. doi: 10.1006/abbi.1993.1173. [DOI] [PubMed] [Google Scholar]
- Stadler J., Billiar T. R., Curran R. D., Stuehr D. J., Ochoa J. B., Simmons R. L. Effect of exogenous and endogenous nitric oxide on mitochondrial respiration of rat hepatocytes. Am J Physiol. 1991 May;260(5 Pt 1):C910–C916. doi: 10.1152/ajpcell.1991.260.5.C910. [DOI] [PubMed] [Google Scholar]
- Stadler J., Harbrecht B. G., Di Silvio M., Curran R. D., Jordan M. L., Simmons R. L., Billiar T. R. Endogenous nitric oxide inhibits the synthesis of cyclooxygenase products and interleukin-6 by rat Kupffer cells. J Leukoc Biol. 1993 Feb;53(2):165–172. doi: 10.1002/jlb.53.2.165. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Nathan C. F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989 May 1;169(5):1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink D. A., Osawa Y., Darbyshire J. F., Jones C. R., Eshenaur S. C., Nims R. W. Inhibition of cytochromes P450 by nitric oxide and a nitric oxide-releasing agent. Arch Biochem Biophys. 1993 Jan;300(1):115–123. doi: 10.1006/abbi.1993.1016. [DOI] [PubMed] [Google Scholar]
- Wortelboer H. M., de Kruif C. A., van Iersel A. A., Falke H. E., Noordhoek J., Blaauboer B. J. The isoenzyme pattern of cytochrome P450 in rat hepatocytes in primary culture, comparing different enzyme activities in microsomal incubations and in intact monolayers. Biochem Pharmacol. 1990 Dec 1;40(11):2525–2534. doi: 10.1016/0006-2952(90)90095-3. [DOI] [PubMed] [Google Scholar]
- Wölfel C., Heinrich-Hirsch B., Schulz-Schalge T., Seidel A., Frank H., Ramp U., Wächter F., Wiebel F. J., Gonzalez F., Greim H. Genetically engineered V79 Chinese hamster cells for stable expression of human cytochrome P450IA2. Eur J Pharmacol. 1992 Sep 1;228(2-3):95–102. doi: 10.1016/0926-6917(92)90017-7. [DOI] [PubMed] [Google Scholar]
- Wölfel C., Platt K. L., Dogra S., Glatt H., Wächter F., Doehmer J. Stable expression of rat cytochrome P450IA2 cDNA and hydroxylation of 17 beta-estradiol and 2-aminofluorene in V79 Chinese hamster cells. Mol Carcinog. 1991;4(6):489–498. doi: 10.1002/mc.2940040613. [DOI] [PubMed] [Google Scholar]