Abstract
We examined the feasibility of recruiting US adults ≥45 years old with Fabry disease (FD) for telephone assessments of cognitive functioning. A case–control design matched each FD participant on age, sex, race, and education to four participants from a population-based study. Fifty-four participants with FD age 46–72 years were matched to 216 controls. Standardized cognitive assessments, quality of life (QOL), and medical histories were obtained by phone, supplemented by objective indices of comorbidities. Normalized scores on six cognitive tasks were calculated. On the individual tasks, scores on list recall and semantic fluency were significantly lower among FD participants (p-values < 0.05), while scores on the other four tasks did not differ. After averaging each participant’s normalized scores to form a cognitive composite, we examined group differences in composite scores, before and after adjusting for multiple covariates using generalized estimating equations. The composite scores of FD cases were marginally lower than controls before covariate adjustments (p = 0.08). QOL and mental health variables substantially attenuated this finding (p = 0.75), highlighting the influence of these factors on cognition in FD. Additional adjustment for cardiovascular comorbidities, kidney function, and stroke had negligible impact, despite higher prevalence in the FD sample. Telephone-based cognitive assessment methods are feasible among adults with FD, affording access to a geographically dispersed sample. Although decrements in discrete cognitive domains were observed, the overall cognitive function of older adults with FD was equivalent to that of well-matched controls before and after accounting for multiple confounding variables.
Introduction
Fabry disease (FD) is a rare X-linked lysosomal storage disorder with potential impact on cognitive function. Deficient alpha-galactosidase A results in accumulation of globotriaosylceramide and related glycosphingolipids in cardiomyocytes and in vascular endothelial, neural, and renal cells, leading to tissue remodeling, fibrosis, ischemia, and potential end-organ damage affecting the heart, kidneys, and brain (Desnick et al. 2001), although phenotypic variations are striking (Wilcox et al. 2008).
Reports of cognitive difficulties are not uncommon among persons with FD (Segal et al. 2010; Low et al. 2007), but assessment of cognitive function has been limited. Samples available for clinical cognitive assessments have been small, spanning a broad age spectrum. While some researchers have found a pattern of below average scores in discrete cognitive abilities (Elstein et al. 2012), others have found no pattern of deficits (Segal et al. 2010; Low et al. 2007).
There are multiple pathways whereby cognitive dysfunction might occur. Transient ischemic attacks and strokes are important causes of cognitive impairment and debilitation in FD (Desnick et al. 2001). In the absence of clinical events, cognitive decrements could occur due to nonspecific cerebrovascular changes (Elstein et al. 2012; Moore et al. 2007). Hearing loss also can affect cognitive processing and correlates with neuropathic and vascular damage in FD (Ries et al. 2007). White matter changes also are important findings; however, to date, the relation of brain abnormalities to described minor changes in cognitive function in FD is not compelling (Moore et al. 2007; Muller et al. 2005; Schermuly et al. 2011; Fellgiebel et al. 2012). Chronic distress associated with FD may have more influence on cognitive function than do pathologic alterations in the central nervous system (Segal et al. 2010; Schermuly et al. 2011).
We conducted a pilot study to examine the feasibility of telephone-based cognitive assessments as a means of expanding access to persons with FD. Such methods previously have been found to be reliable and precise (Unverzagt et al. 2007). We focused on persons middle-aged and older to represent patients experiencing a prolonged lifespan attributable to improved renal transplants and the availability of enzyme replacement therapies (ERT). We capitalized on telephone assessment technologies being used in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) population-based cohort study (Howard et al. 2005) and used a subset of that cohort as age-, sex-, race-, and education-matched controls.
We expected that the cooperation rate of FD patients would be higher than that of the REGARDS cohort due to differences in the samples and recruitment methods and that the cognitive assessment would yield usable data even among patients using assistive hearing devices. We hypothesized that FD participants would perform worse than REGARDS control participants on a cognitive composite and component tests of learning, memory, and executive function. We predicted that controlling for effects of quality of life (QOL), perceived stress, depressive symptoms, income, cardiovascular disease, kidney function, and stroke would attenuate group differences in cognitive function. These variables have been associated with cognitive function in REGARDS (Kurella Tamura et al. 2011; Unverzagt et al. 2011; Addison-Brown et al. 2014).
Methods
Participants and Procedures
Participants with Fabry Disease
Participants with FD were enrolled from September 2009 through May 2011. They were recruited primarily through Fabry Registry physicians’ offices and secondarily through two patient websites. Community-dwelling, English-speaking men and women with FD ≥ 45 years old who were able to communicate by telephone were eligible, whether treated or untreated with ERT. Neither participants nor physicians were provided with incentives for enrollment.
Interested patients called a toll-free number at the Edward R. Roybal Center for Translational Research on Aging and Mobility at the University of Alabama at Birmingham (UAB) to schedule a telephone assessment of demographics, cognitive function, perceived stress, depressive symptoms, and QOL. Verbal informed consent was obtained, and assessments were conducted by the Survey Research Unit (SRU) in the School of Public Health at UAB using standardized scripts used in the REGARDS study. Written informed consent and medication logs were executed by mail.
In October 2012, we recontacted FD participants by phone for self-reported cardiovascular risk factor histories, and we gathered by mail an addendum consent form granting permission to obtain objective medical information from (1) the Fabry Registry, using Registry IDs released by Registry physicians, or (2) from non-Registry physicians primarily responsible for patients’ Fabry disease care.
Control Participants
Control participants were drawn from the database of the REGARDS study, a population-based study designed to examine geographic and racial differences in stroke and cognitive decline (Howard et al. 2005; Wadley et al. 2011). REGARDS participants (N = 30,239) were recruited from January 2003 to October 2007 through mailings followed by telephone contacts. Eligibility criteria included age ≥45, self-reported race of black or white, English-speaking, residing in the community, no life-limiting illness, and able to communicate by telephone. Following verbal informed consent, an interview assessing demographics, perceived stress, depressive symptoms, QOL, and cardiovascular risk factors was conducted by telephone, followed by a home visit including written informed consent, blood pressure measurement, blood and urine samples, and an electrocardiogram (ECG) that was centrally read. The SRU began conducting telephone-based cognitive assessments of learning, memory, and executive function between 2006 and 2009.
The same personnel, custom computer programs, standardized cognitive assessment and scoring techniques, and abbreviated mental health scales were used in both FD patient and REGARDS control samples. Each telephone assessment lasted less than 30 min on average.
Each enrolled FD participant was matched on age (±2.5 years), education level, sex, and race to 4 REGARDS participants randomly selected from the pool of all potential matches in the REGARDS database. The 4:1 matching scheme was selected to provide stable estimates and adequate power to detect group differences in overall cognitive function after adjustment for covariates. For the present comparison of cognitive function of persons with FD to matched control participants, only initial assessments of REGARDS participants’ cognitive function were used.
Measures
A detailed account of study measures, administration, and scoring is available in the Appendix. The measures are listed here only briefly.
Age, sex, race, education, and income were self-reported. Cognitive function was assessed with six tasks drawn from the National Institute of Neurological Disorders and Stroke–Canadian Stroke Network (NINDS-CSN) 5-minute battery (Hachinski et al. 2006) and the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) battery (Morris et al. 1989). Learning, memory, and executive function were assessed with CERAD word list learning (WLL) and word list delayed recall (WLDR), NINDS-CSN 5-item recall and 6-item orientation (Hachinski et al. 2006; Morris et al. 1989; Nasreddine et al. 2005), letter fluency (letter F), and semantic fluency (Hachinski et al. 2006; Morris et al. 1989). Health-related QOL was measured using a modified interviewer script version of the Short Form-12 (SF-12; Quality Metrics) survey (Ware et al. 1996). Perceived stress was measured with the 4-item Perceived Stress Scale (PSS-4; Cohen et al. 1983). The Center for Epidemiologic Studies Depression Scale – 4-item version (CES-D-4; Melchior et al. 1993) – was used to evaluate depressive symptoms. A combination of self-report and objective assessments was used to ascertain cardiovascular comorbidities of hypertension, diabetes, dyslipidemia, and heart disease, as well as kidney function and history of stroke.
Statistical Approach
The cooperation rate of the FD sample was calculated (Morton et al. 2006), and the feasibility of the telephone-based assessment approach was assessed via interviewer ratings of participants’ hearing, comprehension, and compliance with the assessment. Usable data from at least 85% of FD participants was established a priori as the threshold for acceptable feasibility.
Scores on each cognitive measure were converted to z-scores based on the distributions observed in the combined sample of REGARDS and FD participants. The average z-score across available measures was calculated to form an overall cognitive composite score for each participant. Composite scores of FD participants were compared to those of REGARDS participants after adjustment for potential confounding variables using linear regression.
Baseline characteristics of the two samples were compared using generalized estimating equation (GEE) models in matched analyses. A compound symmetry covariate structure was fitted to determine whether the cognitive composite z-score of FD participants differed from that of REGARDS participants before and after adding covariate groups (QOL and mental health, income, cardiovascular comorbidities, kidney function, stroke history).
Results
Participant Characteristics, Cooperation Rates, and Feasibility
Fifty-six participants were screened for the FD sample; one was ineligible due to severe hearing difficulties and one due to comprehension difficulties unrelated to hearing. The final FD sample included 54 participants (45 who learned about the study from Fabry Registry physicians’ offices and nine from patient websites). Four clinicians participating in the Fabry Registry of North America (authors Sims, Warnock, Hopkin, and Laney) contributed the majority of study enrollees. After matching each patient with FD to four REGARDS participants, the full study sample consisted of 270 participants (54 with FD, 216 controls).
Table 1 presents the baseline characteristics of each population. By design, the matching variables of race, sex, and education did not differ between groups; mean age differed only marginally. FD participants were 46–72 years old; matched REGARDS participants were 45–74 years old. FD participants had higher prevalence of elevated depressive symptoms, higher perceived stress, and lower QOL than controls. Thirty-two FD participants were using ERT (agalsidase beta at 1 mg/kg) at the time of the cognitive assessment.
Table 1.
Baseline factors and cognitive scores for participants with Fabry disease and controls
| n | Fabry disease (n = 54) | n | REGARDS controls (n = 216) | p-value | |
|---|---|---|---|---|---|
| Age M (SD) | 54 | 55.7 (6.7) | 216 | 56.0 (6.6) | 0.074 |
| White race n (%) | 54 | 53 (98) | 216 | 212 (98) | 0.99 |
| Men n (%) | 54 | 17 (31) | 216 | 68 (31) | 0.99 |
| Education n (%) | 54 | 216 | 0.99 | ||
| <HS (%) | 2 (4) | 8 (4) | |||
| HS grad (%) | 8 (15) | 32 (15) | |||
| Some college (%) | 16 (30) | 64 (30) | |||
| College grad (%) | 28 (52) | 112 (52) | |||
| Quality of life M (SD) | 53 | 214 | |||
| SF-12 MCS scorea,b | 47.7 (11.1) | 53.1 (9.0) | 0.002 | ||
| SF-12 PCS scorea,b | 38.0 (11.4) | 48.0 (9.9) | 0.001 | ||
| Perceived stress, PSS-4 scorea,b M (SD) | 53 | 6.4 (2.3) | 214 | 3.2 (2.7) | <0.0001 |
| Elevated depressive symptoms CES-D-4b n (%) | 54 | 15 (28) | 214 | 21 (10) | 0.007 |
| Income n (%) | 54 | 216 | 0.33 | ||
| <$20 K (%) | 3 (6) | 26 (12) | |||
| $20 K–$34 K (%) | 10 (19) | 54 (25) | |||
| $35 K–$74 K (%) | 20 (37) | 67 (31) | |||
| >=$75 K (%) | 15 (28) | 54 (25) | |||
| Refused (%) | 6 (11) | 15 (7) | |||
| Systolic blood pressurec M (SD) (mmHg) | 17 | 120 (17) | 216 | 120 (15) | 0.96 |
| Diastolic blood pressurec M (SD) (mmHg) | 16 | 74 (8.9) | 216 | 76 (11.0) | 0.96 |
| Hypertension n (%) | 54 | 38 (70) | 216 | 81 (38) | <0.0001 |
| Diabetesd n (%) | 54 | 5 (9) | 213 | 24 (11) | 0.56 |
| Heart disease d n (%) | 54 | 31 (57) | 206 | 36 (17) | <0.0001 |
| Atrial fibrillationc,d n (%) | 38 | 17 (45) | 206 | 10 (5) | <0.0001 |
| LVHc,d n (%) | 26 | 19 (73) | 81 | 2 (2) | <0.0001 |
| Dyslipidemia d n (%) | 54 | 38 (70) | 211 | 102 (47) | 0.0044 |
| Total cholesterolc,d M (SD) (mg/dL) | 26 | 180 (42) | 210 | 201 (36) | 0.021 |
| LDLc,d M (SD) (mg/dL) | 26 | 96 (30) | 210 | 119 (31) | 0.0006 |
| HDLc,d M (SD) (mg/dL) | 26 | 63 (22) | 210 | 53 (17) | 0.013 |
| Triglyceridesc,d M (SD) (mg/dL) | 26 | 102 (56) | 211 | 152 (69) | 0.0019 |
| Kidney function, eGFR ml/min/1.73 m2c,d M (SD) | 27 | 63 (26) | 211 | 85 (20) | 0.0001 |
| History of stroke e n (%) | 54 | 6 (11) | 216 | 10 (5) | 0.071 |
REGARDS stands for the Reasons for Geographic and Racial Differences in Stroke study
Four controls were randomly selected from all possible REGARDS participants who could be matched to each Fabry disease study participant on age (±2.5 years), race, sex, and education
Variables in bold print were used in subsequent generalized estimating equation models
Missing data:
a1 FD ppt refused to answer these questions
b2 REGARDS ppts refused to answer these questions
cMissing data for FD ppts on these variables was due to lack of available information in the Fabry Registry or physicians’ medical records
dMissing data for REGARDS ppts on these variables was due to missing or inadequate blood samples or ECG data
e14 FD ppts responded “don’t know” regarding self-reported stroke and had no stroke history documentation; these ppts were classified as “no stroke”
Cooperation rate (number enrolled divided by number contacted and eligible) for FD participants from the Fabry Registry was 74% (45 enrollees from a pool of 61 Registry patients who were invited and deemed eligible). As expected, this cooperation rate was higher than the 49% cooperation rate of the REGARDS epidemiological population study (Wadley et al. 2011). We were not able to include in the cooperation rate calculation the nine enrollees with FD recruited from patient websites, due to an unknown number of potentially eligible persons who may have viewed the study information but did not contact us.
With respect to feasibility of telephone-based assessments within the FD participants, the mean time for interview completion was 30.0 min (for men, the mean time was 29.2 min [range 12–48], and for women the mean time was 30.9 min [range 18–48]). The REGARDS participant telephone interviews were shorter by 6 min on average because in that study, the verbal consent process and medication inventory were done at baseline visits prior to the cognitive assessments. Interviewer ratings revealed one participant with moderate hearing loss and one displaying modest motivation/effort. Most of the data provided by these two participants was considered usable, but selected cognitive tests affected by the identified issues were set to missing. Interviewer error or equipment malfunction resulted in missing data on a subset of tests for two additional participants. Thus, all FD participants (100%) provided sufficient usable cognitive data for calculating the overall cognitive composite, exceeding the feasibility threshold of 85%. Applying a more rigorous standard of usable and complete data on all cognitive tests, feasibility remained acceptable at 92.5% (50 of 54 participants).
Cognitive Function Analyses
Table 2 presents the two groups’ raw scores and z-scores for each of the cognitive tests and their average z-score composites. Figure 1 displays the pattern of z-scores and 95% confidence intervals of FD participants on each test and overall, based on the means and SDs of the full sample. Mean z-scores of participants with FD were significantly lower than controls on WLL delayed recall and semantic fluency tasks and did not differ from the other tasks or the overall composite.
Table 2.
Raw cognitive scores and normalized z-scores
| Fabry disease (n = 54) | REGARDS controls (n = 216) | |||
|---|---|---|---|---|
| Raw score | Z-score (SD) | Raw score (SD) | Z-score (SD) | |
| Word list learning | 19.4 (4.7) | −0.17 (0.9) | 20.2 (5.0) | 0.0 (1.0) |
| Word list delayed recall | 6.6 (2.1) | −0.43 (1.0) | 7.4 (2.0) | 0.0 (1.0) |
| NINDS-CSN recall | 4.4 (1.1) | −0.05 (1.2) | 4.4 (0.9) | 0.0 (1.0) |
| NINDS-CSN orientation | 5.7 (0.6) | −0.08 (1.3) | 5.8 (0.5) | 0.0 (1.0) |
| Semantic fluency | 17.6 (5.7) | −0.29 (0.8) | 19.7 (7.4) | 0.0 (1.0) |
| Letter fluency | 12.8 (5.4) | 0.24 (1.3) | 11.8 (4.3) | 0.0 (1.0) |
| Average Z composite score | – | −0.16 (0.7) | – | 0.0 (0.6) |
REGARDS stands for the Reasons for Geographic and Racial Differences in Stroke study
Numbers missing for FD participants: word list learning, 3; word list recall, 3; NINDS-CSN recall, 1; NINDS-CSN orientation, 0; semantic fluency, 4; letter fluency, 4; and average Z composite, 0. No REGARDS participants were missing cognitive data
Fig. 1.
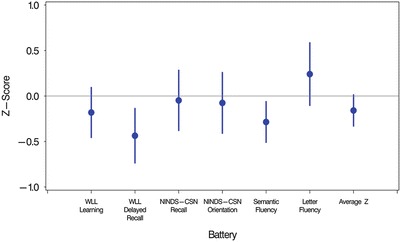
Z-scores of participants with Fabry disease relative to matched controls by cognitive task and overall composite. Z-scores (mean = 0, SD = 1.0) on the y-axis were computed from raw score means and SDs of the full sample available for each cognitive task (Ns range from 266 to 270) and the average composite z-score (N = 270). Error bars represent 95% confidence intervals
Table 3 presents GEE results examining group differences between average z-score composites, with p-values for tests of whether each difference score is greater than zero. Model 1 presents the univariate analyses. Models 2 through 6 present the multivariable analyses, with each subsequent model including all variables from the prior models. Model 2 added QOL and mental health factors (PCS, MCS, PSS-4, CES-D-4). Model 3 added income. Model 4 added cardiovascular comorbidities that differed between FD and REGARDS participants in univariate analyses. Model 5 added kidney function (eGFR). Model 6 added stroke history. The two cohorts differed marginally on the overall cognitive composite (p = 0.08) prior to consideration of covariates. This effect was attenuated after accounting for QOL and mental health variables (p = 0.75) and remained nonsignificant after the entry of each subsequent group of covariates.
Table 3.
Average composite z-score results from GEE models
| Magnitude of difference (SE) | p-value | ||
|---|---|---|---|
| Model 1 (n = 270) | Univariate | −0.16 (0.090) | 0.08 |
| Model 2 (n = 266) | + MCS, PCS, PSS-4, CES-D-4 | 0.034 (0.11) | 0.75 |
| Model 3 (n = 266) | + Income | −0.043 (0.11) | 0.69 |
| Model 4 (n = 254) | + Hypertension, dyslipidemia, heart disease | −0.051 (0.12) | 0.67 |
| Model 5 (n = 229) | + eGFR | −0.14 (0.19) | 0.46 |
| Model 6 (n = 229) | + Stroke | −0.14 (0.18) | 0.45 |
GEE stands for generalized estimating equation. SE stands for standard error. A negative magnitude of difference indicates that participants with Fabry disease have a lower z-score than REGARDS control participants
MCS stands for mental component score. PCS stands for physical component score. PSS-4 stands for the 4-item Perceived Stress Scale. CES-D-4 stands for the 4-item the Center for Epidemiologic Studies Depression Scale. eGFR stands for estimated glomerular filtration rate
Due to possible sex differences in cognitive function, particularly among FD patients, we performed post hoc analyses using unadjusted GEE models to test the interaction between group (FD vs. REGARDS) and sex for each cognitive measure. These interactions were nonsignificant for all measures (p-values from 0.19 to 0.79), indicating that score variations due to sex were no different for the FD sample than for the control sample.
Discussion
We examined cognitive function in middle-aged and older adults with Fabry disease using matched controls and telephone-based assessments. Our results suggest that telephone-based cognitive assessment methods are feasible among many patients with Fabry disease. All participants with FD provided usable data on the primary cognitive composite outcome, and over 90% provided complete data on the cognitive battery. Importantly, we failed to establish a significant difference in overall cognitive function among FD participants relative to controls matched on sex, education, age, and race. A trend for lower cognitive functioning in FD participants was substantially attenuated by adjustment for the expected differences between groups in QOL and depression measures, even prior to accounting for key medical comorbidities. Our matched sample design and adjustment for multiple comorbidities and confounders suggest that this null finding with respect to global cognition is fairly robust.
Among eligible potential participants who learned about the study from their Fabry Registry physicians’ offices, 74% enrolled in the study. It is possible that enrollment could be enhanced in future studies with careful consideration of incentives, including provision of individual feedback to participants and providers. Even so, to our knowledge, our FD sample is the largest to participate in a study of cognitive function and is also the oldest, with a mean age of 56 years and a range of 46–72 years.
Our finding of no difference in composite cognitive performance in participants with FD compared to well-matched control participants is similar to that of Fellgiebel et al. (2012). These investigators enrolled 25 FD patients and 20 age-matched controls in a neuroimaging study that included measures of learning and memory. Although patients with FD had significantly smaller hippocampal volumes than controls, memory performance was not associated with hippocampal volume, and patients did not differ from controls on cognitive measures. Similarly, Low et al. (2007) conducted a neuroimaging study in 22 FD patients aged 20–62 years. They demonstrated increased lesion prevalence with age but no differences in cognitive performance compared to normative data on two global screening instruments. While it is possible that cognitive decrements may be too subtle to detect in small patient samples, our results in a somewhat larger sample with a large demographically matched control group are consistent with prior research reporting no global deficits and are strengthened after accounting for key variables related to cognitive function.
Although our global composite did not differ between participants with FD and controls, it is of interest to note performance patterns on the component cognitive tasks. On the individual tests, FD participants’ performance was lower than controls on 5 of the 6 tasks and significantly lower on delayed recall and semantic fluency tasks representing memory and executive function. Mild but diffuse cognitive impairments on subdomains of a computerized cognitive battery have previously been reported (Elstein et al. 2012), as have marginal differences between FD patients and controls on subdomain scores of the Neuropsychiatry Unit Cognitive Screen (Low et al. 2007). Semantic fluency and memory deficits are characteristic of Alzheimer’s disease and Parkinson’s disease (Henry et al. 2004; Henry and Crawford 2004), and white matter lesions contribute independently to memory impairment in preclinical dementia (Grambaite et al. 2011). Thus, linkages between semantic fluency and memory, white matter lesions, and dementia risk in FD warrant future investigation.
Disease-related factors help explain variability in cognitive performance among patients with FD. We found greater prevalence of elevated depressive symptoms, higher ratings of perceived stress, and lower ratings of QOL in patients with FD compared to controls. Twenty-eight percent of our FD sample screened positive for depressive symptoms on the 4-item CES-D, consistent with survey findings using the full CES-D (Cole et al. 2007). Likewise, lower QOL in both mental and physical domains is consistent with previous reports (Low et al. 2007). Prior research found that mild deficits in executive function among 25 patients with FD compared to 20 controls were no longer significant after controlling for depression (Schermuly et al. 2011). In our study, controlling for depressive symptoms, perceived stress, and QOL variables substantially attenuated the trend for lower composite cognitive scores among FD participants. Further control for comorbid cardiovascular, renal, and cerebrovascular disease did not change these results, despite significantly higher prevalence in the FD sample of all comorbidities except diabetes. It is notable, however, that our sample attained high levels of education and income (82% with at least some college, 28% with household incomes over $75K) in spite of disease-related restrictions. Further, it may be considered a testament to their coping capacities that they generally scored in ranges that were only mildly abnormal on scales of depressive symptoms and quality of life.
Our findings, though largely consistent with those of smaller clinical studies with control groups, should be interpreted in light of certain limitations. Our sample of persons with FD was able to participate meaningfully in telephone-based cognitive assessments but may not represent the full spectrum of persons with FD, particularly those with prominent hearing loss and survivors of severe stroke. In addition, men with FD were underrepresented in our study, with women comprising 70% of our sample. Disease severity in women with FD can range from asymptomatic carriers to classic symptomatic cases with involvement of multiple organ systems (Wang et al. 2007). Our study design allowed us to test whether cognitive score differences attributable to sex were different in our FD sample than in the control sample. The relationship of sex to cognitive scores did not differ across the two groups on any of the cognitive tests, thus minimizing the possibility of confounding due to sex differences in FD severity.
Strengths of this research are the relatively large sample, collection of important covariates, and a control group matched on four key demographic variables. The older mean age of our study sample extends previous research by capturing a population that is aging with Fabry disease. We demonstrate the feasibility of a low-cost and accessible telephone cognitive assessment methodology that is amenable to future studies of FD and other rare disorders. Such studies might apply this data collection method to children, young adults, and non-English-speaking persons both within and outside of the United States. Future investigations also are needed in which ERT effects on cognitive function in FD are explicitly examined.
Acknowledgements
This research was supported by an investigator-initiated grant funded by Genzyme: a Sanofi Company. The REGARDS study is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health, and Department of Health and Human Service. The authors thank the investigators, staff, and participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org. An unrestricted investigator-initiated research grant from Amgen, Inc. supported this project, as did the Edward R. Roybal Center for Translational Research on Aging and Mobility, P30 AG022838, from the National Institute on Aging. Representatives of Genzyme: a Sanofi Company and NINDS were involved in the review and approval of the manuscript but were not directly involved in the collection, management, analysis, or interpretation of the data. The content of the article has not been influenced by the sponsors.
Synopsis
In a relatively large sample of older adults with Fabry disease matched to controls on age, sex, race, and education, we demonstrated the feasibility of telephone assessments of cognitive function, accounted for important psychosocial and disease comorbidities, and found no substantial differences between groups in overall cognitive function based on six tasks.
Compliance with Ethics Guidelines
Conflict of Interest
Virginia Wadley has received an investigator-initiated award from Genzyme: a Sanofi Company. David Warnock has received investigator-initiated awards from Genzyme: a Sanofi Company and Amgen, Inc.; he is a consultant for Genzyme: a Sanofi Company and Amgen, Inc.
Robert Hopkin has received research funding from Genzyme: a Sanofi Company.
Dawn Laney has received investigator-initiated awards from Genzyme: a Sanofi Company, Amicus Therapeutics, and Shire Corporation.
Virginia Clarke has received a coinvestigator award from Genzyme: a Sanofi Company. Katherine Sims has received research funding from Genzyme: a Sanofi Company, Synageva, and Biogen Idec.
Leslie McClure, Caroline Lassen-Greene, Manjula Kurella Tamura, and George Howard declare that they have no conflict of interest.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committees on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Author Contributions
Virginia Wadley designed the study, obtained funding, collected data, contributed to statistical analyses and interpretation, and drafted the manuscript.
Leslie McClure contributed to study design, randomly selected matched controls from the REGARDS study, created and merged the datasets, analyzed and interpreted the data, and critically revised the manuscript.
David Warnock contributed to the study conception and design, recruited participants, contributed to data interpretation, and critically revised the manuscript.
Caroline Lassen-Greene collected data, created databases, contributed to data interpretation, and critically revised the manuscript.
Robert Hopkin recruited participants, contributed to data interpretation, and critically revised the manuscript.
Dawn Laney recruited participants, contributed to data interpretation, and critically revised the manuscript.
Virginia Clarke recruited participants, contributed to data interpretation, and critically revised the manuscript.
Manjula Kurella Tamura contributed to data interpretation and critically revised the manuscript.
George Howard designed the REGARDS study, contributed to the design of the present study, contributed to statistical analyses and interpretation, and critically revised the manuscript.
Katherine Sims contributed to the study design, recruited participants, contributed to data interpretation, and critically revised the manuscript.
Appendix: Detailed Methods
Demographics
Matching variables of age, sex, race (black or white), and education level (less than high school, high school, some college, college graduate) were self-reported. Income also was self-reported (<$20 K, $20 K–$34 K, $35 K–$75 K, ≥$75 K, Refused).
Cognitive Function
Cognitive function was assessed with six tasks drawn from the National Institute of Neurological Disorders and Stroke–Canadian Stroke Network (NINDS-CSN) 5-minute battery (Hachinski et al. 2006) and the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) battery (Morris et al. 1989). The NINDS-CSN 5-minute battery was recommended for use in studies calling for very brief assessments, epidemiological studies, and/or telephone administration (Hachinski et al. 2006).
Learning and Memory
Learning and memory were assessed with word list learning (WLL) and word list delayed recall (WLDR) from the CERAD battery and NINDS-CSN 5-item recall and 6-item orientation (Hachinski et al. 2006; Morris et al. 1989; Nasreddine et al. 2005). WLL consists of three learning trials of a list of ten words which are presented in fixed orders that vary across the three trials, followed by a free recall trial (WLDR) after a 5-minute delay (Welsh et al. 1995). WLL and WLDR were administered according to standard protocols with minor modifications for telephone administration (Addison-Brown et al. 2014). Instructions and word lists were administered via a recording so that all participants were exposed to the same narrator. For WLL, correct responses on the three trials are summed. Scores range from 0 to 30. For WLDR, participants recall as many of the ten words as possible. Scores range from 0 to 10. Scores for NINDS-CSN 5-item recall and 6-item orientation from the Montreal Cognitive Assessment (Nasreddine et al. 2005) range from 0–5 and 0–6, respectively.
Executive Function
Executive function was assessed with letter fluency (letter F) from the NINDS-CSN battery and semantic fluency (animals) from the CERAD battery (Hachinski et al. 2006; Morris et al. 1989). Letter fluency and semantic fluency tests prompt participants to name as many words as they can beginning with the letter “F” in one minute and, subsequently, to name as many animals as they can in one minute. Scores on each consist of the total number of valid responses produced by each participant in 60 s. With explicit verbal permission, the assessments were recorded in digital waveform audio files and then played back later for scoring following standard scoring protocols.
Quality of Life
Health-related QOL was measured using a modified interviewer script version of the Short Form-12 (SF-12) survey (Ware et al. 1996; Quality Metrics). The survey yields Physical and Mental Component Summary scores (PCS-12 and MCS-12), each with a mean of 50 and SD of 10, facilitating comparisons to general population norms.
Perceived Stress
Perceived stress was measured with the 4-item Perceived Stress Scale (PSS-4; Cohen et al. 1983). This questionnaire elicits perceptions of stress during the past month. Each response is assigned a value of 0–4. Scores range from 0 (no stress) to 16 (high stress).
Depressive Symptoms
The Center for Epidemiologic Studies Depression Scale – 4-item version (CES-D-4; Melchior et al. 1993) – was used to evaluate depressive symptoms. Each of the 4 items assesses emotional symptoms of depression; no somatic symptoms are included in the scale. Each response is assigned a value of 0–3. Total scores range from 0 to 12; a score ≥4 suggests a clinically significant level of psychological distress (Melchior et al. 1993).
Cardiovascular Comorbidities
Cardiovascular comorbidities of hypertension, diabetes, dyslipidemia, and heart disease were assessed, as well as kidney function and history of stroke. A combination of self-report and objective assessments was used. In the FD participants, objective measures were drawn from Fabry Disease Registry information or medical records; for REGARDS participants, they were measured during the baseline home visit. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic pressure ≥90 mmHg, documented or self-reported physician diagnosis, or use of antihypertensive medications. Diabetes was defined as fasting glucose >126 mg/dL, non-fasting glucose >200 mg/dL, documented or self-reported physician diagnosis, or use of diabetes medications. Dyslipidemia was defined as total cholesterol ≥240 mg/dL, low-density lipoprotein cholesterol ≥160 mg/dL, or high-density lipoprotein cholesterol <40 mg/dL; documented or self-reported physician diagnosis; or use of lipid-lowering medications. Heart disease was defined as presence of atrial fibrillation, left ventricular hypertrophy (LVH), coronary artery disease (CAD), or heart failure. Atrial fibrillation and LVH were defined by ECG or documented diagnosis. CAD was defined by ECG or documented or self-reported physician diagnosis of myocardial infarction or coronary revascularization. Heart failure was defined by self-reported or documented physician diagnosis of orthopnea or paroxysmal nocturnal dyspnea.
Kidney function was a continuous variable derived from blood assays. In REGARDS, serum creatinine was measured with isotope dilution mass spectrometry, and estimated glomerular filtration rate (eGFR) was calibrated using the Modification of Diet in Renal Disease equation. In FD participants, local laboratory values for eGFR were used, representing variable creatinine calibration methods.
For FD participants, stroke was defined as documented or self-reported physician diagnosis. In REGARDS, baseline history of stroke was defined by self-reported physician diagnosis, and reported incident strokes occurring during follow-up but prior to the initial cognitive battery assessment were confirmed by retrieval of medical records, which were adjudicated by REGARDS study physicians using the World Health Organization and clinical stroke criteria. For cases in which death occurred with no medical records surrounding the event, death certificates were examined, and proxy interviews were conducted for detection and adjudication of stroke events.
Footnotes
Competing interests: None declared
Contributor Information
Virginia G. Wadley, Email: vwadley@uab.edu
Collaborators: Johannes Zschocke, Matthias Baumgartner, K Michael Gibson, Marc Patterson, and Shamima Rahman
References
- Addison-Brown K, Yaggi K, Letter A, et al. Association between cognition and health-related quality of life in Obstructive Sleep Apnea while controlling for demographic, co-morbid medical, and psychological factors. J Sleep Res. 2014;23(1):69–76. doi: 10.1111/jsr.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Social Behav. 1983;24:385–396. doi: 10.2307/2136404. [DOI] [PubMed] [Google Scholar]
- Cole AL, Lee PJ, Hughes DA, Deegan PB, Waldek S, Lachmann RH. Depression in adults with Fabry disease: a common and under-diagnosed problem. J Inherited Metab Dis. 2007;30(6):943–951. doi: 10.1007/s10545-007-0708-6. [DOI] [PubMed] [Google Scholar]
- Desnick R, Ioannou Y, Eng C. Alpha-galactosidase A deficiency: Fabry disease. In: Scriver C, Beaudet A, Sly W, Valle D, editors. The metabolic bases of inherited disease. 8. New York: McGraw-Hill; 2001. pp. 3733–3774. [Google Scholar]
- Elstein D, Doniger GM, Altarescu G. Cognitive testing in Fabry disease: pilot using a brief computerized assessment tool. Israel Med Assoc J. 2012;14(10):624–628. [PubMed] [Google Scholar]
- Fellgiebel A, Wolf DO, Kolodny E, Müller MJ. Hippocampal atrophy as a surrogate of neuronal involvement in Fabry disease. J Inherited Metab Dis. 2012;35(2):363–367. doi: 10.1007/s10545-011-9390-9. [DOI] [PubMed] [Google Scholar]
- Grambaite R, Reinvang I, Selnes P, et al. Pre-dementia memory impairment is associated with white matter tract affection. J Int Neuropsychol Soc. 2011;17(1):143–153. doi: 10.1017/S1355617710001360. [DOI] [PubMed] [Google Scholar]
- Hachinski V, Iadecola C, Petersen RC, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37(9):2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- Henry JD, Crawford JR. Verbal fluency deficits in Parkinson’s disease: a meta-analysis. J Int Neuropsychol Soc. 2004;10(4):608–622. doi: 10.1017/S1355617704104141. [DOI] [PubMed] [Google Scholar]
- Henry JD, Crawford JR, Phillips LH. Verbal fluency performance in dementia of the Alzheimer’s type: a meta-analysis. Neuropsychologia. 2004;42(9):1212–1222. doi: 10.1016/j.neuropsychologia.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepi. 2005;25(3):135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- Kurella Tamura M, Muntner P, Wadley VG, et al. Albuminuria, kidney function and the incidence of cognitive impairment among adults in the United States. Am J Kidney Dis. 2011;58(5):756–763. doi: 10.1053/j.ajkd.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M, Nicholls K, Tubridy N, et al. Neurology of Fabry disease. Int Med J. 2007;37(7):436–447. doi: 10.1111/j.1445-5994.2007.01366.x. [DOI] [PubMed] [Google Scholar]
- Melchior LA, Huba GJ, Brown VB, Reback CJ. A short depression index for women. Educ Psychol Measurement. 1993;53:1117–1125. doi: 10.1177/0013164493053004024. [DOI] [Google Scholar]
- Moore DF, Kaneski CR, Askari H, Schiffmann R. The cerebral vasculopathy of Fabry disease. J Neurol Sci. 2007;257:258–263. doi: 10.1016/j.jns.2007.01.053. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39(9):1159–1165. doi: 10.1212/WNL.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Morton LM, Cahill J, Hartge P. Reporting participation in epidemiologic studies: a survey of practice. Am J Epidemiol. 2006;63:197–203. doi: 10.1093/aje/kwj036. [DOI] [PubMed] [Google Scholar]
- Muller MJ, Muller KM, Dascalescu A, et al. Psychiatric and neuropsychological signs and symptoms in patients with Fabry disease: literature review. Fortschr Neurol Psychiatr. 2005;73:687–693. doi: 10.1055/s-2004-830300. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Ries M, Kim HJ, Zalewski CK, et al. Neuropathic and cerebrovascular correlates of hearing loss in Fabry disease. Brain. 2007;130:143–150. doi: 10.1093/brain/awl310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schermuly I, Muller MJ, Muller KM, et al. Neuropsychiatric symptoms and brain structural alterations in Fabry disease. Euro J Neurol. 2011;18:347–353. doi: 10.1111/j.1468-1331.2010.03155.x. [DOI] [PubMed] [Google Scholar]
- Segal P, Kohn Y, Pollak Y, Altarescu G, Galili-Weisstub E, Raas-Rothschild A. Psychiatric and cognitive profile in Anderson-Fabry patients: a preliminary study. J Inherited Metab Dis. 2010;33(4):429–436. doi: 10.1007/s10545-010-9133-3. [DOI] [PubMed] [Google Scholar]
- Unverzagt FW, Monahan PO, Moser LR, et al. The Indiana University Telephone-based Assessment of Neuropsychological Status: a new method for large scale neuropsychological assessment. J Int Neuropsychol Soc. 2007;13(5):799–806. doi: 10.1017/S1355617707071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unverzagt FW, McClure LA, Wadley VG, et al. Vascular risk factors are predictive of cognitive impairment in a stroke-free Cohort. Neurology. 2011;77(19):1729–1736. doi: 10.1212/WNL.0b013e318236ef23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadley VG, Unverzagt FW, McGuire LC, et al. Incident cognitive impairment is elevated in the stroke belt: the REGARDS study. Ann Neurol. 2011;70(2):229–236. doi: 10.1002/ana.22432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RY, Lelis A, Mirocha J, Wilcox WR (2007) Heterozygous Fabry women are not just carriers, but have a significant burden of disease and impaired quality of life. Genet Med 9(1):34–45 [DOI] [PubMed]
- Ware JE, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Welsh KA, Fillenbaum G, Wilkinson W, et al. Neuropsychological test performance in African-American and white patients with Alzheimer’s disease. Neurology. 1995;45:2207–2211. doi: 10.1212/WNL.45.12.2207. [DOI] [PubMed] [Google Scholar]
- Wilcox WR, Oliveira J-P, Hopkin RJ, et al. Females with Fabry disease frequently have major organ involvement: lessons from the Fabry Registry. Molec Genet Metab. 2008;93(2):112–128. doi: 10.1016/j.ymgme.2007.09.013. [DOI] [PubMed] [Google Scholar]


