Abstract
We investigated peripheral blood T-lymphocyte subpopulations and intracellular expression of IFN-γ, IL-4, IL-10, and IL-13, by whole blood flow cytometry, in 22 type I Gaucher disease (GD) patients. Results were compared with those of 19 sex- and age-matched controls. Patients with GD exhibited decreased frequencies and absolute numbers of CD3+/CD4+ helper T lymphocytes (40.8 ± 9.8% vs. 49.4 ± 5.7%, p = 0.002, and 0.77 ± 0.33 vs. 1.04 ± 0.28 × 109/μL, p = 0.011), as well as increased frequencies of CD3+CD8+ suppressor T lymphocytes (23.8 ± 8.0% vs. 18.4 ± 3.8%, p = 0.010), resulting in a significantly decreased CD4/CD8 cell ratio (p < 0.001). Moreover, they had significantly increased percentages of IFNγ-producing both CD4+ and CD8+ T cells (p = 0.0003 and p = 0.023, respectively), implying a TH-1 polarization pattern. Finally, patients with GD had decreased percentages and absolute numbers of CD4+CD25dim T lymphocytes (p = 0.033 and p = 0.007, respectively), of CD4+CD25high T lymphocytes (p = 0.039 and p = 0.016, respectively), and of CD4+CD25highFOXP3+ regulatory T cells (p = 0.036 and p = 0.019, respectively). Our results demonstrate that patients with GD have a significant numerical impairment of T-helper lymphocytes and a constitutive TH1 direction pattern of activation of both CD4+ and CD8+ cells, associated with a significant decrease of T-regs. Ineffective T-cell control may explain the chronic inflammatory reaction and the increased incidence of lymphoid malignancies, which have been repeatedly reported among patients with GD.
Introduction
GD is the most common lysosomal storage disorder, resulting from an abnormal structure and/or impaired function of the enzyme glucocerebrosidase, which leads to inadequate cleavage of glucosylceramide to glucose and ceramide. Consequently, undigested glucosylceramide is accumulated in the macrophageal lysosomes of the reticuloendothelial system, inducing them the characteristic morphology of Gaucher cells and producing the main clinical features of the disease.
There are three clinical variables of GD: the most common non-neuronopathic type 1, the early neuronopathic type 2, and type 3 or the late neuronopathic variant. Besides neurological degeneration, encountered in type 2 and 3 variants, other common clinical features of GD include hepatosplenomegaly of various severity, bone disease, and an unspecific chronic proinflammatory status (Shoenfeld et al. 1982), whose pathogenesis is as yet obscure.
Clinical and experimental studies have shown that GD patients exhibit various immunological abnormalities, such as increased susceptibility to infections (Aker et al. 1993; Maródi et al. 1995), numerical dendritic cell defects (Micheva et al. 2006), and an increased incidence of B-lymphoproliferative disorders and solid tumors (Arends et al. 2013). Laboratory findings include elevated levels of acute-phase proteins (Rogowski et al. 2005) and serum immunoglobulin levels (de Fost et al. 2008), frequent occurrence of monoclonal gammopathy (Arends et al. 2013), detection of various autoantibodies in patients’ serum (Shoenfeld et al. 1995), and increased plasma levels of proinflammatory cytokines, including IL-1β, IL-6, IL-8, IL-10, TNF-α, and TGF-β (Allen et al. 1997; Barak et al. 1999; Michelakakis et al. 1996; Deegan et al. 2005; Pérez Calvo et al. 2000). These findings imply that chronic inflammatory status possibly reflects a chronic stimulation of the immune system.
T lymphocytes are the key cells, involved in all immune processes, and they play a pivotal role in immune homeostasis. Nevertheless, little is known about their status and role in GD. Among few studies, Balreira et al. (2005) demonstrated that patients, either under enzyme replacement therapy (ERT) or not, have decreased proportion of CD4+ cells and increased proportion of CD8+ cells. Lacerda et al. (1999), however, found that both CD4+ and CD8+ absolute numbers were decreased in patients with bone manifestations. Moreover, Burstein et al. (1987) reported decreased number of NKT cells, whereas Braudeau et al. (2013) demonstrated that untreated patients exhibit low NK, Tγδ2, and naive CD4+ cells and high CD4+ memory T-cell counts.
We therefore aimed to investigate aspects of T-cell immunity in patients with GD, including enumeration of PB lymphocyte subpopulations and T-regs and their activation pattern and possible contribution to disease phenotype.
Patients, Materials, and Methods
Subjects
Twenty-two patients with type 1 GD (12 females, 10 males, median age 40 years, range 16–67) were studied. Results were compared with those of 19 healthy controls (10 females, 9 males, median age 41 years, range 26–70). Blood was obtained during a scheduled appointment for patients’ evaluation. All estimations were performed in the absence of any acute or chronic infection or any other known, active inflammatory condition. All procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients and controls.
Diagnosis of GD had been confirmed by the detection of low beta-glucosidase activity in peripheral blood (PB) lymphocytes and mutations in the GBA gene. The dominant mutation was N370S, which was identified in 20 patients, of whom 7 had both alleles mutated (homozygous state). At the time of this workup, five patients had detectable bone lesions (mainly osteopenia), 6 had been splenectomized, and another one had manifested nodular-sclerosing Hodgkin’s lymphoma, but was in sustained complete remission for 5 years, following high-dose chemotherapy and autologous stem-cell transplantation (Symeonidis et al. 2011). Seventeen patients were receiving ERT, 3 on substrate reduction treatment (SRT), and 2 were not receiving any treatment.
Sample Collection: Labeling of Lymphocyte Surface Markers
For each experiment, 7 mL of PB was collected, of which 4 mL in a heparinized tube and 3 mL in an EDTA tube. The following monoclonal antibodies were used: CD3-FITC (clone UCHT1), CD4-PC5 (clone 13B8.2), CD8-ECD (clone SFCI21Thy2D3), and HLA-DR-PE (clone L243) or CD25-PE (BD Biosciences, San Jose, USA). The following lymphocyte subsets were identified: CD3+, CD3+CD4+, CD3+CD8+, CD4+CD8+, CD4+CD25+, CD4+HLA-DR+, CD8+CD25+, and CD8+HLA-DR+. At least 5 × 104 cells were analyzed per sample by a Coulter FC5000 flow cytometer. Data was processed using the Kaluza software. Determination of absolute counts of the various lymphocyte subsets was estimated from the whole WBC counts, conducted the same day of the experiments, using a Sysmex ΧΤ-1800 automatic cell counter.
T-Regs Analysis
For T-reg labeling, the eBioscience (San Diego, USA) kit was used, which contained CD4-FITC (clone RPA-T4), CD25-PE (clone BC96) for cell surface, and Foxp3-PE-Cy5 (clone PCH101) for nuclear and cytoplasmic staining.
The analysis was performed by a Coulter Epics-XL-MCL flow cytometer. The lymphocytic population was initially defined in a forward (FSC) and a side scatter (SSC) gate. In a dot plot CD4 versus CD25, the CD4+CD25+ subset was determined, based on the exclusion of the CD4-CD25- and CD4+CD25- subsets. Subsequently, two gates of equal size were created, dividing the CD4+CD25+ in two subsets: CD4+CD25dim και CD4+CD25high. The CD4+CD25high cells were defined as T-regs. The intracellular expression of FOXP3 per T-reg cell was determined by a histogram plot. The intracellular expression of FOXP3 in the CD4+CD25- cells was used as an internal control. At least 1 × 105 cells were analyzed, and data were processed using the Flowing Software analysis software.
Intracellular Labeling of Cytokines
For estimation of the intracellular expression of IFN-γ, IL-13, IL-4, and IL-10 in CD4+ cells and IFN-γ in CD8+ cells, a slightly modified previously described technique was followed (Karakantza et al. 2004), based on the stimulation of lymphocytes with PMA/ionomycin, in the presence of BFA, for 5 h at 37°C. The following antibodies were used: CD3-ECD (clone UCHT1), CD4-FITC (clone Leu3a-3b, BDIS, Beckman Coulter, Miami, USA), CD8-FITC (clone B9.11, Beckman Coulter), anti-IFNγ-PE (clone 45.15), anti-IL-4-PE (Beckman Coulter Immunotech), anti-IL-10-PE (Beckman Coulter Immunotech), and anti-IL-13-PE (clone JES10-5A2, BD Pharmingen). The monoclonal antibody CD69-PC5 (clone TP1.55.3, Beckman Coulter) was used as a marker of early activation. At least 5 × 104 cells were analyzed, and data were processed using the Kaluza software.
Subject Grouping and Statistical Analysis
Healthy control subjects were nominated group A. Patients as a whole consisted group B. Patients were then divided in three subgroups: those without osteopenia and/or splenectomy (n = 11, group C), patients with osteopenia and an intact spleen (n = 5, group D), and splenectomized patients irrespective of osteopenia (n = 6, group E). For the assessment of intracellular cytokine expression, since the number of patients was rather small (n = 15, all under ERT), patients were divided in 2 groups: those without osteopenia or splenectomy (n = 9, group III) and those with osteopenia and/or splenectomy (n = 6, of whom 4 splenectomized: group IV).
Statistical analysis was performed following logarithmic transformation. Results were analyzed using student’s t-test. Data are described using mean ± standard deviation, which were determined according to the raw form (normal) of data and not the logarithmic. For statistical analysis, GraphPad Prism software was used.
Results
Total Lymphocytes and Total T lymphocytes
No statistically significant difference in the WBC count between patients and controls, as well as in the total lymphocyte count (TLC) between patient group B or group C and controls, was observed. However, group D patients had significantly lower TLC (p = 0.048), whereas group E patients had significantly higher TLC (p = 0.006).
The total T-cell population (CD3+ cells) was not significantly different between patient group B or D and controls. CD3+ cells were significantly lower in patient group C and higher with marginal significance in group E (p = 0.023 and p = 0.052, respectively, Table 1).
Table 1.
Basic lymphocyte subpopulations and regulatory T-cells subsets in patients and controls
| (Sub)population | Controls | PT group B | PT group C | PT group D | PT group E | p (Gr B) | p (Gr C) | p (Gr D) | p (Gr E) |
|---|---|---|---|---|---|---|---|---|---|
| N = 19 (mean ± SD) | N = 22 (mean ± SD) | N = 11 (mean ± SD) | N = 5 (mean ± SD) | N = 6 (mean ± SD) | (Log-trans.) | (Log-trans.) | (Log-trans.) | (Log-trans.) | |
| WBC count (×109/L) | 7.15 ± 1.31 | 6.13 ± 2.42 | 5.95 ± 2.39 | 4.32 ±1.15 | 7.95 ± 1.95 | 0.036 | 0.029 | <0.001 | n.s. |
| Abs. lymphoc. count × (109/L) | 2.07 ± 0.49 | 2.05 ± 0.98 | 1.73 ± 0.80 | 1.59 ± 0.58 | 3.05 ± 0.84 | n.s. | 0.059 | 0.048 | 0.006 |
| CD3+ (%) | 71.8 ± 6.21 | 70.3 ± 11.3 | 70.3 ± 13.1 | 74.7 ± 6.00 | 66.6 ± 9.70 | n.s. | n.s. | n.s. | n.s. |
| CD3+ (abs. count ×109/L) | 1.51 ± 0.39 | 1.40 ± 0.58 | 1.15 ± 0.37 | 1.21 ± 0.50 | 2.00 ± 0.53 | n.s. | 0.023 | n.s. | 0.052 |
| CD3+CD4+ (%) | 49.4 ± 5.72 | 40.8 ± 9.78 | 41.8 ± 11.0 | 43.7 ± 6.1 | 36.6 ± 8.3 | 0.002 | 0.013 | n.s. | <0.001 |
| CD3+CD4+ (abs. count ×109/L) | 1.04 ± 0.28 | 0.77 ± 0.33 | 0.67 ± 0.21 | 0.72 ± 0.34 | 1.08 ± 0.29 | 0.011 | <0.001 | 0.022 | n.s. |
| CD3+CD8+ (%) | 18.4 ± 3.76 | 23.8 ± 7.99 | 21.2 ± 7.83 | 24.8 ± 6.32 | 27.6 ± 7.84 | 0.010 | n.s. | 0.018 | 0.001 |
| CD3+CD8+ (abs. count ×109/L) | 0.38 ± 0.11 | 0.48 ± 0.32 | 0.35 ± 0.12 | 0.40 ± 0.18 | 0.85 ± 0.40 | n.s. | n.s. | n.s. | <0.001 |
| CD4+/CD8+ ratio | 2.85 ± 0.80 | 1.91 ± 0.76 | 2.18 ± 0.86 | 1.88 ± 0.48 | 1.45 ± 0.49 | <0.001 | 0.018 | 0.006 | <0.001 |
| CD20+ (%) | 12.3 ± 3.18 | 13.1 ± 8.34 | 11.3 ± 4.12 | 10.9 ± 3.51 | 18.4 ± 13.5 | n.s. | n.s. | n.s. | n.s. |
| CD20+ (abs. count ×109/L) | 0.23 ± 0.11 | 0.26 ± 0.18 | 0.20 ± 0.10 | 0.14 ± 0.04 | 0.45 ± 0.23 | n.s. | n.s. | 0.015 | 0.035 |
| CD4+DR+ (%) | 2.29 ± 0.69 | 2.67 ± 2.46 | 1.70 ± 1.23 | 3.23 ± 2.50 | 4.00 ± 3.50 | n.s. | 0.032 | n.s. | n.s. |
| CD4+DR+ (abs. count ×106/L) | 48.1 ± 15.1 | 54.3 ± 50.8 | 28.5 ± 15.7 | 51.9 ± 50.8 | 103.7 ± 56.4 | n.s. | 0.003 | n.s. | 0.003 |
| CD4+CD25+ (% as CD4+ subset) | 4.36 ± 1.03 | 6.62 ± 6.17 | 3.97 ± 1.30 | 7.10 ± 4.98 | 11.1 ± 9.10 | n.s. | n.s. | n.s. | 0.002 |
| CD4+CD25+ (%) | 1.01 ± 0.39 | 0.60 ± 0.36 | 0.59 ± 0.36 | 0.71 ± 0.22 | 0.42 ± 0.28 | 0.027 | 0.020 | n.s. | 0.005 |
| CD4+CD25+ (abs. count ×106/L) | 20.4 ± 9.1 | 10.7 ± 6.9 | 9.6 ± 7.09 | 9.3 ± 2.97 | 11.3 ± 5.96 | 0.007 | 0.009 | n.s. | 0.016 |
| CD8+DR+ (%) | 1.92 ± 0.67 | 3.69 ± 2.92 | 2.41 ± 1.46 | 3.17 ± 2.31 | 6.38 ± 3.55 | 0.019 | n.s. | n.s. | <0.001 |
| CD8+DR+ (abs. count ×106/L) | 41.3 ± 17.2 | 87.7 ± 111 | 40.5 ± 24.7 | 56.5 ± 54.1 | 201 ± 155 | n.s. | n.s. | n.s. | <0.001 |
| CD8+DR+ (% as CD8+ subset) | 11.1 ± 4.13 | 15.3 ± 8.56 | 11.9 ± 6.40 | 14.9 ± 7.51 | 23.1 ± 8.26 | n.s. | n.s. | n.s. | 0.009 |
| CD4+CD25+dim (%) | 0.78 ± 0.34 | 0.50 ± 0.30 | 0.50 ± 0.36 | 0.60 ± 0.20 | 0.35 ± 0.23 | 0.033 | 0.085 | n.s. | 0.074 |
| CD4+CD25+dim (abs. count ×106/L) | 17.1 ± 7.7 | 7.7 ± 6.3 | 8.7 ± 6.8 | 7.9 ± 2.7 | 9.3 ± 4.9 | 0.007 | 0.033 | n.s. | n.s. |
| CD4+ CD25+dim (% as CD4+ subset) | 2.29 ± 0.74 | 1.51 ± 0.74 | 1.48 ± 0.79 | 1.84 ± 0.56 | 1.16 ± 0.60 | 0.014 | 0.027 | n.s. | 0.042 |
| CD4+CD25+dim FOXP3+ (%) | 0.43 ± 0.23 | 0.24 ± 0.17 | 0.26 ± 0.21 | 0.22 ± 0.04 | 0.21 ± 0.16 | 0.021 | 0.030 | 0.041 | n.s. |
| CD4+CD25+dimFOXP3+ (abs. count ×106/L) | 9.79 ± 5.47 | 4.75 ± 4.01 | 4.88 ± 4.58 | 3.07 ± 1.13 | 5.65 ± 3.58 | 0.008 | 0.064 | 0.045 | n.s. |
| CD4+CD25+high (%) | 0.15 ± 0.06 | 0.10 ± 0.05 | 0.11 ± 0.06 | 0.11 ± 0.04 | 0.07 ± 0.05 | 0.039 | n.s. | n.s. | 0.029 |
| CD4+CD25+high (abs. count) | 3.10 ± 1.46 | 2.09 ± 1.28 | 1.92 ± 1.36 | 1.40 ± 0.41 | 1.98 ± 1.22 | 0.016 | 0.038 | 0.042 | n.s. |
| CD4+CD25+highFOXP3+ (%) | 0.14 ± 0.05 | 0.08 ± 0.04 | 0.09 ± 0.05 | 0.08 ± 0.03 | 0.06 ± 0.04 | 0.036 | 0.030 | 0.054 | 0.028 |
| CD4+CD25+highFOXP3+ (abs. count) | 2.90 ± 1.28 | 1.57 ± 1.12 | 1.68 ± 1.31 | 1.07 ± 0.31 | 1.55 ± 0.89 | 0.019 | 0.028 | 0.031 | n.s. |
| FOXP3+ (on CD4+CD25+dim %) | 56.9 ± 17.5 | 50.2 ± 19.4 | 50.5 ± 19.2 | 39.1 ± 9.90 | 57.6 ± 7.46 | n.s. | n.s. | n.s. | n.s. |
| FOXP3+ (on CD4+CD25+high, %) | 90.2 ± 6.8 | 81.9 ± 11.8 | 83.9 ± 8.7 | 79.5 ± 16.4 | 78.9 ± 2.1 | 0.031 | 0.083 | n.s. | 0.021 |
Major T-Lymphocyte Subpopulations
Patients as a whole (group B) had significantly decreased both percentage and absolute CD4+ T-lymphocyte count (p = 0.002 and p = 0.011, respectively) compared to controls. The same was true for patient group C (p = 0.013 and p < 0.001, respectively). Patient group D exhibited lower absolute CD4+ cell count alone (p = 0.022), whereas splenectomized patients had significantly decreased percentage of CD4+ cells (p < 0.001). Moreover, patient groups B and D exhibited significantly increased percentages, but not absolute counts of CD8+ T lymphocytes, compared to controls (p = 0.010 and p = 0.018, respectively). Splenectomized patients had significantly increased both percentage and absolute CD8+ T lymphocytes (p = 0.001 and p < 0.001, respectively). The CD4/CD8 ratio was significantly lower in all patient groups compared to controls (Table 1).
B Lymphocytes
The absolute CD20+ lymphocyte count was significantly lower in the osteopathic patient group compared to controls (p = 0.015), whereas splenectomized patients had higher absolute CD20+ lymphocyte counts (p = 0.035). The CD20+CD5+ cells (autoreactive B lymphocytes) did not differ significantly between the controls and any patient group (data not shown).
Activated T lymphocytes
The presence of activated T lymphocytes was determined by the CD25 and HLA-DR surface antigen expression. Patients as a whole exhibited significantly higher frequencies of CD8+HLA-DR+ cells compared to controls (p = 0.019), but HLA-DR expression on CD8+ cells was not different, as was also absolute CD8+HLA-DR+ cell count. HLA-DR expression on both CD4+ and CD8+ cells was higher in splenectomized patients (p = 0.089 and p < 0.001, respectively). The same was observed for the absolute CD4+HLA-DR+ lymphocyte count (p = 0.003) and the percentage and the absolute CD8+HLA-DR+ cell count (p < 0.001 and p < 0.001, respectively). HLA-DR expression on CD4+ cells did not differ significantly between patients and controls. Surprisingly, non- osteopathic patients had significantly decreased CD4+HLA-DR+ cells, but HLA-DR expression on CD4+ cells was similar. This could be attributed to the lower percentage of CD4+ cells exhibited in these patients (Table 1).
CD25 Expression on CD4+ Cells and T-Regs
CD25 is an early activation T-cell marker and also a marker of T-regs. The CD4+CD25dim cell subset represents the compartment of activated CD4+ T lymphocytes. Patient groups B and C exhibited decreased both percentages (p = 0.033 and p = 0.085, respectively) and absolute CD4+CD25dim cell counts (p = 0.007 and p = 0.033, respectively) compared to controls. These results were also confirmed by the significantly reduced expression of CD25dim on CD4+ cells in patient groups B, C, and E compared to controls.
The CD4+CD25high subpopulation is considered representative of T-regs. This population was significantly reduced, as percentage in patient groups B (p = 0.039) and E (p = 0.029) and as absolute count in patient group B (p = 0.016), group C (p = 0.038), and group D (p = 0.042), compared to controls (Fig. 1). The same was also found for the CD4+CD25highFOXP3+ cell subset. Patient group E also exhibited significantly lower percentages of CD4+CD25high and CD4+CD25highFOXP3+ cells (p = 0.029 and p = 0.028, respectively), but not absolute counts of these populations. The proportion of FOXP3 expressing cells among the CD4+CD25+highcells was significantly reduced in patient groups B and E compared to controls (Table 1).
Fig. 1.
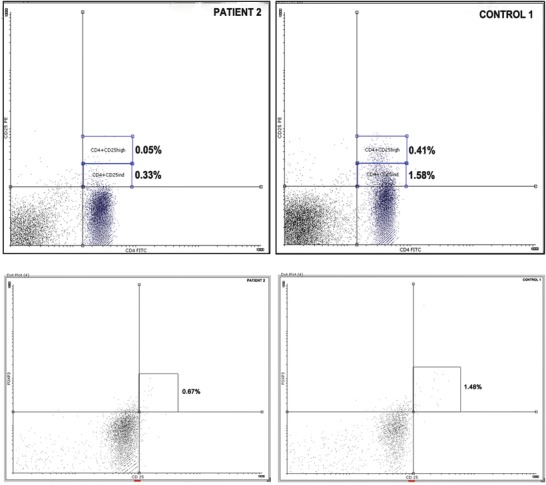
Comparative results for the CD4+CD25dim+, CD4+CD25high+, and CD25+FOXP3+ cell population of a typical patient and a healthy control subject. These cell populations were significantly reduced in the patient group compared to those of the healthy controls
The CD8+CD25+ subpopulation exhibited substantial variation among patients, and no significant difference between any patient group and controls was found (data not shown).
NK Cells
We examined the various NK- and of NKT-cell subpopulations, as defined by CD3, CD16, and CD56 surface antigen expression. We found that groups B and C patients had absolute NK-cell counts, and CD3-CD56dim and CD3-CD56dimCD16+ subpopulations almost had 2-fold lower, and group D had 3-fold lower compared to controls, yet these differences were not statistically significant. Group D patients exhibited significantly lower absolute CD3-CD56+high and CD3-CD56+highCD16+ cell counts (p = 0.036 and p < 0.001, respectively, data not shown).
Intracellular Expression of Type 1 and Type 2 Cytokines
In all patient groups, stimulation with PMA/ionomycin resulted in a significant increase of IFNγ-producing CD4+ T cells compared to controls. The same was true for IFNγ-producing CD8+ T cells, in patient groups II and IV (Fig. 2). Also, the MFI of IFNγ on CD4+ cells (but not on CD8+ cells) was significantly higher for all patient groups. In contrast, the percentage of IL13-, IL4-, and IL10-producing CD4+ T cells, following mitogenic stimulation, did not differ significantly between any patient group and controls, implying a strong TH1 direction of lymphocyte activation. Intracellular cytokine expression in the unstimulated CD4+ and CD8+ cells was almost zero in both groups (Table 2).
Fig. 2.
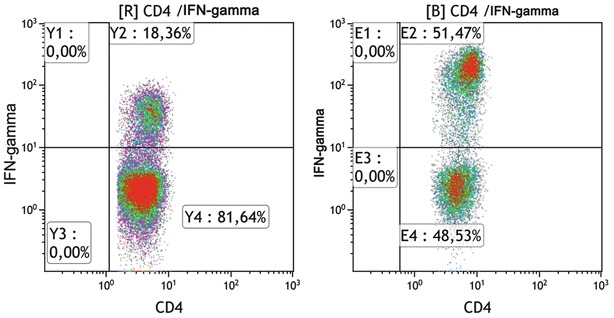
Typical scattergram of IFN-γ-producing CD4+ populations, following PMA/ionomycin stimulation of a patient with Gaucher disease (left) and a healthy control subject (right)
Table 2.
Intracellular cytokine expression following mitogenic stimulation
| % on CD4+ or CD8+ cells | Control Gr I | PT Gr II | PT Gr III | PT Gr IV | p value (log transformed) | ||
|---|---|---|---|---|---|---|---|
| N = 10 (mean ± SD) | N = 15 (mean ± SD) | N = 9 (mean ± SD) | N = 6 (mean ± SD) | I vs. II | I vs. III | I vs. IV | |
| CD4+IFNγ+ | 22.2 ± 7.10 | 39.3 ± 12.0 | 34.4 ± 11.5 | 46.8 ± 8.4 | <0.001 | 0.016 | <0.001 |
| MFI of IFNγ on CD4+ cells | 3004 ± 53.2 | 3144 ± 122.7 | 3122 ± 136 | 3177 ± 89.3 | 0.005 | 0.036 | <0.001 |
| CD8+IFNγ+ | 46.0 ± 14.2 | 65.3 ± 22.8 | 53.4 ± 21.4 | 83.2 ± 8.9 | 0.023 | n.s. | <0.001 |
| MFI of IFNγ on CD8+ cells | 3044 ± 110 | 3064 ± 54.5 | 3059 ± 46.4 | 3071 ± 64.2 | n.s. | n.s. | n.s. |
| CD4+IL13+ | 1.21 ± 0.95 | 1.26 ± 0.79 | 0.91 ± 0.75 | 1.62 ± 0.69 | n.s. | n.s. | n.s. |
| CD4+IL4+ | 0.84 ± 0.32 | 1.03 ± 0.54 | 1.05 ± 0.54 | 1.13 ± 0.54 | n.s. | n.s. | n.s. |
| CD4+IL10+ | 1.03 ± 0.49 | 1.15 ± 0.71 | 1.21 ± 0.84 | 1.14 ± 0.41 | n.s. | n.s. | n.s. |
Discussion
This study confirmed that type 1 GD patients exhibit mainly quantitative, but also functional abnormalities of the adaptive immunity. The CD4+ cell compartment is the most commonly and almost universally affected population, resulting in a significant proportional and absolute count decrease of T-helper cells. Impairment of T-helper cell function appears to be a constitutive feature of GD, since it is not affected by disease severity, effectiveness of treatment, previous splenectomy, or other features. Low CD4+ cell count is a hallmark of chronic inflammatory diseases, such as HIV infection (Catalfamo et al. 2011) and chronic parasitic infestations (Kalinkovich et al. 1998), and may result from an increased rate of apoptosis in the CD4+ cell compartment, something already described in chronic immune stimulation-associated diseases and conditions (Jelley-Gibbs et al. 2005).
Our study also showed that in patients with GD (excluding osteopathic patients), the CD8+ cell subset has an increased proportional representation. We did not find disturbed absolute CD8+ cell counts, either in patients with or in those without bone involvement. This finding argues with what other studies have reported, namely, that GD patients with bone involvement have reduced absolute CD8+ cell counts (Lacerda et al. 1999). This discrepancy might be attributed to the relatively small number of osteopathic patients included in our study (n = 5).
To estimate the direction of lymphocyte activation, we investigated the intracellular cytokine expression, following mitogenic stimulation. We found that IFNγ-producing helper T cells were significantly higher among patients than controls, although helper T cells producing the Th2 cytokines IL-4 and IL-13 and the immunoregulatory cytokine IL-10 were not significantly different. The same was also true for the IFNγ-producing CD8+ cells, except of the non-osteopathic patient group. Therefore, it appears that patients with type 1 GD have a TH1 polarization of their CD4+ cells and an increased activity of IFNγ-producing cytotoxic CD8+ cells. Similar findings have been observed in other diseases and conditions, associated with chronic immune stimulation (Smolenska et al. 2012; Berner et al. 2000). The high frequencies of IFNγ-producing T cells could result from the increased expression of CD1d and MHC class II molecules (LeibundGut-Landmann et al. 2004) that has been reported on the monocytes of these patients (Balreira et al 2005). Additionally, patients with GD produce more IFNγ per CD4+ cell compared to controls, as it has been shown by the MFI values. This may indicate that the Th1 CD4+ cells also demonstrate alterations in the IFNγ production at the cellular level, and this issue would be interesting to be further investigated.
To our knowledge, increased IFNγ expression in the T cells of patients with GD has not yet been reported. However, upregulation of the IFNγ gene expression in CBE-treated mouse microglia (Hong et al. 2006) or increased serum IFNγ levels in GBA-deficient mice has been reported (Pandey et al. 2012; Liu et al. 2012). Therefore, the cause of increased presence of TH1 IFNγ-producing cells, possibly resulting in increased serum IFNγ levels of patients with GD, needs to be explored.
On the other hand, excluding CD8+ cells of splenectomized patients, neither CD4+ nor CD8+ lymphocytes displayed significantly increased HLA-DR expression. This was rather unexpected, since TH1 polarization should lead to HLA-DR+ phenotype of T cells (Volk et al. 1986). A possible explanation might be that almost all studied patients were under ERT, which could have resulted in downregulation of the HLA-DR expression, as this has been reported, following treatment of chronic inflammatory conditions (Wilson et al. 1994; Bass et al. 1992). To ascertain this, further study of HLA-DR expression before and after therapy in a larger group of patients is designed by our group.
The expression of another activation marker, CD25, was found reduced on the CD4+ T cells of all patient groups, with the exception of osteopathic patients. CD25 is the α-chain of the IL-2 receptor that promotes T-helper cell proliferation. In vitro studies have shown that activated CD4+ lymphocytes exhibit increased CD25 expression, which is released into the culture medium and is detected as sIL-2R (Brusko et al. 2009). It has been reported that type 1 GD patients have increased serum levels of sIL-2R (Barak et al. 1999). Thus, we could assume that the reduced CD25 expression in CD4+ cells of GD patients implies an activation and proliferation state of the CD4+ cells.
Low NK-cell counts have been reported in patients with GD. In our study, although the cytotoxic CD3-CD56+dimNK-cell subset was almost 2-fold lower, it was not significantly reduced compared to controls. However, we found a significant decrease in the absolute immunoregulatory CD3-CD56+high cell count among patients with osteopenia, i.e., in patients, more severely affected by GD. An anticipated exception was observed in previously splenectomized patients, for whom it is well known that exhibit increased NK-cell subpopulations. Among splenectomized GD patients the CD3-CD56+high cell population was not significantly different, compared to controls Reduced NK-cell function may, at least partially, explain the increased sensitivity to infections and neoplasias, which has been reported in patients with GD.
We have shown for the first time that patients with GD exhibit significantly decreased T-regs. Numerical T-reg impairment was a global phenomenon in our patient population, and it was not affected by age, disease severity, and years of treatment. This finding implies that there is a significant deficiency in the immunoregulatory ability of GD patients, since the low number of T-regs cannot effectively control the overactive T-helper cell compartment (Sakaguchi et al. 2009). Therefore, the quantitative deficiency of T-regs might contribute to the establishment of the TH1 polarization (Shevach et al. 2001).
Taken together, we have demonstrated a heterogeneous immunophenotypic profile among patients with type 1 GD, depending on whether they have bone disease/osteopenia or previous splenectomy. Non-osteopathic patients did not exhibit higher IFNγ-producing CD8+ cells, in contrast to the other patient groups. We have confirmed that splenectomized patients have higher T-, B-, CD4+HLA-DR+, and CD8+HLA-DR+ lymphocyte counts and NK cells compared to controls. These variations might influence the course of the disease, and it is unclear whether they can be reversed by an effective treatment program.
There are some limitations in our study. First, we included low numbers of osteopathic and splenectomized patients. It is therefore important to confirm these data in a larger patient cohort exhibiting these features. Second, we were unable to investigate the impact of treatment on the immune abnormalities, since almost all patients were under treatment long before their evaluation, and they were well responders. We have studied only two treatment-naïve patients, who did not show significant differences compared to the remaining. Third, although we have shown numerical impairment of T-regs, we did not investigate their functionality. This could be accessed through the measurement of suppressive activity of purified T-regs in vitro.
In conclusion, our study has shown that patients with GD exhibit an important deregulation of the immune system, characterized by a profile of uncontrolled immune response (reversal of the CD4/CD8 cell ratio, low regulatory T cells, and increased IFNγ-producing T cells) (Belkaid and Rouse 2005), and this possibly contributes to the establishment of low-grade chronic inflammatory status that has been observed in these patients.
Acknowledgments
Argiris Symeonidis designed and conducted the study. Christos Sotiropoulos performed the research as part of his PhD thesis work. George Theodorou and Eugenia Verigou contributed to the flow cytometric evaluation of regulatory T cells and NK cells. Constantina Repa and Theodore Marinakis contributed to the design of the study and provided clinical data on patient information and follow-up. Elena Solomou and Marina Karakantza supervise lab work and supported the appropriate analysis of the data.
We would like to thank Dr Alexandra Kourakli and Dr Panayiotis Tsaftaridis for their substantial contribution in the clinical management of the patients, in providing patients’ clinical data, and for their general participation in the designing and fulfilling of the project. We also wish to thank Mrs Angeliki Anagnostopoulou, MScN, for the excellent clinical care of the patients and her assistance in blood sample collection.
Take-Home Message
Patients with Gaucher disease have severe numerical impairment of their regulatory T-cell counts and exhibit a strong TH1 direction of lymphocyte activation.
Compliance with Ethics Guidelines
Conflict of Interest
Christos Sotiropoulos, George Theodorou, Constantina Repa, Theodoros Marinakis, Eugenia Verigou, Elena Solomou, Marina Karakantza, and Argiris Symeonidis have no conflict of interest to disclose in relation to this piece of scientific work.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients and controls for being included in this study.
Footnotes
Competing interests: None declared
Contributor Information
Argiris Symeonidis, Email: argiris.symeonidis@yahoo.gr.
Collaborators: Johannes Zschocke, Matthias Baumgartner, K Michael Gibson, Marc Patterson, and Shamima Rahman
References
- Aker M, Zimran A, Abrahamov A, Horowitz M, Matzner Y. Abnormal neutrophil chemotaxis in Gaucher disease. Brit. J. Haematol. 1993;83:187–191. doi: 10.1111/j.1365-2141.1993.tb08270.x. [DOI] [PubMed] [Google Scholar]
- Allen MJ, Myer BJ, Khokher AM, Rushton N, Cox TM. Pro-inflammatory cytokines and the pathogenesis of Gaucher’s disease: increased release of interleukin-6 and interleukin-10. QJM. 1997;90:19–25. doi: 10.1093/qjmed/90.1.19. [DOI] [PubMed] [Google Scholar]
- Arends M, van Dussen L, Biegstraaten M, Hollak CE. Malignancies and monoclonal gammopathy in Gaucher disease; a systematic review of the literature. Br J Haematol. 2013;161:832–842. doi: 10.1111/bjh.12335. [DOI] [PubMed] [Google Scholar]
- Balreira A, Lacerda L, Miranda CS, Arosa FA. Evidence for a link between sphingolipid metabolism and expression of CD1d and MHC-class II: monocytes from Gaucher disease patients as a model. Br J Haematol. 2005;129:667–676. doi: 10.1111/j.1365-2141.2005.05503.x. [DOI] [PubMed] [Google Scholar]
- Barak V, Acker M, Nisman B, Kalickman I, Abrahamov A, Zimran A, Yatziv S. Cytokines in Gaucher’s disease. Eur Cytokine Network. 1999;10:205–210. [PubMed] [Google Scholar]
- Bass HZ, Hardy WD, Mitsuyasu RT, Wang YX, Cumberland W, Fahey JL. Eleven lymphoid phenotypic markers in HIV infection: selective changes induced by zidovudine treatment. J Acquir Immune Defic Syndr. 1992;5:890–897. doi: 10.1097/00126334-199203000-00001. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6:353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- Berner B, Akça D, Jung T, Muller GA, Reuss-Borst MA. Analysis of Th1 and Th2 cytokines expressing CD4+ and CD8+ T cells in rheumatoid arthritis by flow cytometry. J Rheumatol. 2000;27:1128–1135. [PubMed] [Google Scholar]
- Braudeau C, Graveleau J, Rimbert M, Néel A, Hamidou M, Grosbois B, Besançon A, et al. Altered innate function of plasmacytoid dendritic cells restored by enzyme replacement therapy in Gaucher disease. Blood Cells Mol Dis. 2013;50:281–288. doi: 10.1016/j.bcmd.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Brusko T, Wasserfall CH, Hulme MA, Cabrera R, Schatz D, Atkinson MA. Influence of membrane CD25 stability on T lymphocyte activity: implications for immunoregulation. PloS One. 2009;4:e7980. doi: 10.1371/journal.pone.0007980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein Y, Zakuth V, Rechavi G, Spirer Z. Abnormalities of cellular immunity and natural killer cells in Gaucher’s disease. J Clin Lab Immunol. 1987;23:149–151. [PubMed] [Google Scholar]
- Catalfamo M, Wilhelm C, Tcheung L, Proschan M, Friesen T, Park JH, Adelsberger J, et al. CD4 and CD8 T cell immune activation during chronic HIV infection-role of homeostasis, HIV, Type I IFN, and IL-7. J Immunol. 2011;186:2106–2116. doi: 10.4049/jimmunol.1002000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Fost M, Out TA, de Wilde FA, Tjin EP, Pals ST, van Oers MH, Boot RG et al (2008) Immunoglobulin and free light chain abnormalities in Gaucher disease type I: data from an adult cohort of 63 patients and review of the literature. Ann Hematol 87:439–449 [DOI] [PMC free article] [PubMed]
- Deegan PB, Moran MT, McFarlane I, Schofield JP, Boot RG, Aerts JM, Cox TM. Clinical evaluation of chemokine and enzymatic biomarkers of Gaucher disease. Blood Cells Mol Dis. 2005;35:259–267. doi: 10.1016/j.bcmd.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Hong Y, Kim EY, Jung SC. Upregulation of proinflammatory cytokines in the fetal brain of the Gaucher mouse. J Korean Med Sci. 2006;21:733–748. doi: 10.3346/jkms.2006.21.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelley-Gibbs DM, Dibble JP, Filipson S, Haynes L, Kemp RA, Swain SL. Repeated stimulation of CD4 effector T cells can limit their protective function. J Exp Med. 2005;201:1101–1112. doi: 10.1084/jem.20041852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinkovich A, Weisman Z, Greenberg Z, Nahmias J, Eitan S, Stein M, Bentwich Z. Decreased CD4 and increased CD8 counts with T cell activation is associated with chronic helminth infection. Clin Exp Immunol. 1998;114:414–421. doi: 10.1046/j.1365-2249.1998.00736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakantza M, Theodorou GL, Mouzaki A, Theodori E, Vagianos C, Maniatis A. In vitro study of the long-term effects of post-traumatic splenectomy on cellular immunity. Scand J Immunol. 2004;59:209–219. doi: 10.1111/j.0300-9475.2004.01379.x. [DOI] [PubMed] [Google Scholar]
- Lacerda L, Arosa FA, Lacerda R, Cabeda J, Porto G, Amaral O, Fortuna A, et al. T cell numbers relate to bone involvement in Gaucher disease. Blood Cells Mol Dis. 1999;25:130–138. doi: 10.1006/bcmd.1999.0237. [DOI] [PubMed] [Google Scholar]
- LeibundGut-Landmann S, Waldburger JM, Krawczyk M, Otten LA, Suter T, Fontana A, Acha-Orbea H, Reith W. Mini-review: specificity and expression of CIITA, the master regulator of MHC class II genes. Eur J Immunol. 2004;34:1513–1525. doi: 10.1002/eji.200424964. [DOI] [PubMed] [Google Scholar]
- Liu J, Halene S, Yang M, Iqbal J, Yang R, Mehal WZ, Chuang WL, et al. Gaucher disease gene GBA functions in immune regulation. PNAS. 2012;109:10018–10023. doi: 10.1073/pnas.1200941109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maródi L, Káposzta R, TóthJ LA. Impaired microbicidal capacity of mononuclear phagocytes from patients with GD type I disease: partial correction by enzyme replacement therapy. Blood. 1995;86:4645–4649. [PubMed] [Google Scholar]
- Michelakakis H, Spanou C, Kondyli A, Dimitriou E, Van Weely S, Hollak CE, Van Oers MH, Aerts JM. TNF-α levels in Gaucher disease. Biochim Biophys Acta Mol Basis Dis. 1996;1317:219–222. doi: 10.1016/S0925-4439(96)00056-7. [DOI] [PubMed] [Google Scholar]
- Micheva I, Marinakis T, Repa C, Kouraklis-Symeonidis A, Vlacha V, Anagnostopoulos N, Zoumbos N, et al. Dendritic cells in patients with type I Gaucher disease are decreased in number but functionally normal. Blood Cells Mol Dis. 2006;36:298–307. doi: 10.1016/j.bcmd.2005.12.029. [DOI] [PubMed] [Google Scholar]
- Pandey M, Rani R, Zhang W, Setchell K, Grabowski GA. Immunological cell type characterization and Th1-Th17 cytokine production in a mouse model of Gaucher disease. Mol Genet Metab. 2012;106:310–320. doi: 10.1016/j.ymgme.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez Calvo J, Iñigo-Gil P, Giraldo-Castellano P, Torralba-Cabeza MA, Civeira F, Lario-García S, Pocoví M. (2000) Transforming growth factor beta (TGF-beta) in Gaucher’s disease. Preliminary results in a group of patients and their carrier and non-carrier relatives (Article in Spanish). Med Clin (Barc) 115:601–604 [DOI] [PubMed]
- Rogowski O, Shapira I, Zimran A, Zeltser D, ElsteinD AD, Bashkin A, et al. Automated system to detect low-grade underlying inflammatory profile: Gaucher disease as a model. Blood Cells Mol Dis. 2005;34:26–29. doi: 10.1016/j.bcmd.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int Immunol. 2009;21:1105–1111. doi: 10.1093/intimm/dxp095. [DOI] [PubMed] [Google Scholar]
- Shevach EM, McHugh RS, Piccirillo CA, Thornton AM (2001) Control of T-cell activation by CD4+CD25+ suppressor T cells. Immunol Rev 182:58–67 [DOI] [PubMed]
- Shoenfeld Y, Gallant LA, Shaklai M, Livni E, Djaldetti M, Pinkhas J. Gaucher’s disease: a disease with chronic stimulation of the immune system. Arch Pathol Lab Med. 1982;106:388–391. [PubMed] [Google Scholar]
- Shoenfeld Y, Beresovski A, Zharhary D, Tomer Y, Swissa M, Sela E, Zimran A, et al. Natural autoantibodies in sera of patients with Gaucher’s disease. J Clin Immunol. 1995;15:363–372. doi: 10.1007/BF01541326. [DOI] [PubMed] [Google Scholar]
- Smolenska Z, Pawłowska J, Zdrojewski Z, Daca A, Bryl E. Increased percentage of CD8+CD28- T cells correlates with clinical activity in primary Sjögren’s syndrome. Cell Immunol. 2012;278:143–151. doi: 10.1016/j.cellimm.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Symeonidis A, Liga M, Triantafyllou E, Kouraklis A, Tiniakou M, Karakantza M, Spyridonidis A, Zoumbos N. Successful mobilization with plerixafor and autologous hematopoietic stem cell transplantation in a patient with refractory Hodgkin’s lymphoma and Gaucher disease. Bone Marrow Transplant. 2011;46:1161–1162. doi: 10.1038/bmt.2010.263. [DOI] [PubMed] [Google Scholar]
- Volk HD, Kopp J, Körner IJ, Jahn S, Grunow R, Barthelmes H, Fiebig H. Correlation between the phenotype and the functional capacity of activated T cells in patients with active systemic lupus erythematosus. Scand J Immunol. 1986;24:109–114. doi: 10.1111/j.1365-3083.1986.tb02074.x. [DOI] [PubMed] [Google Scholar]
- Wilson JW, Djukanović R, Howarth PH, Holgate ST. Inhaled beclomethasone dipropionate downregulates airway lymphocyte activation in atopic asthma. Am J Resp Crit Care Med. 1994;1994(149):86–90. doi: 10.1164/ajrccm.149.1.8111605. [DOI] [PubMed] [Google Scholar]


