Abstract
Introduction
To evaluate long-term overall survival (OS), progression- free survival (PFS), and outcomes in pathologically proven brainstem low-grade gliomas (BS-LGG) in children.
Methods
The Mayo Clinic tumor registry identified 48 consecutive children (≤20 y, 52% female) with biopsy-proven BS-LGG treated at Mayo Clinic between January 1971 and December 2004. Medical records were retrospectively reviewed. For analysis, patients were censored at the time of recurrence, death, or last follow-up.
Results
The median age at diagnosis was 12 years with a median follow-up of 6.0 years. The majority of tumors were grade I (69%) and pathology was consistent with an astrocytoma in the majority of patients (98%). Gross total resection was obtained in 4, subtotal in 17, and 27 patients were biopsied only. Postoperative radiotherapy (RT) was used in 29 patients. Median OS for the entire group was 14.8 years with a 1-, 5-, and 10-year OS of 85%, 67% and 59%, respectively. Median PFS for the entire group was 7.3 years. Improved survival was associated with undergoing resection versus biopsy-only with 5-year OS rates of 85% and 50% (P = 0.002), respectively. A high proportion of patients (42%) had diffuse tumors and 13 patients (27%) had diffuse pontine gliomas (DPGs). DPGs had an OS of 1.8 years with a worse median PFS than non-DPGs (1.8 vs. 11.1 y; P = 0.009). RT was used preferentially in patients with poor prognosis such as those who had a biopsy-only procedure (19/27) and DPGs (9/13).
Conclusions
OS in this single institution retrospective study in pathologically proven BS-LGG with extensive follow-up displayed favorable long-term outcomes. Improved outcomes were associated with nondiffuse classification.
Keywords: pediatric brainstem gliomas, diffuse pontine gliomas, diffuse brainstem gliomas, focal brainstem gliomas
Brainstem gliomas encompass a heterogenous group of tumors with widely varying prognosis dependent primarily on tumor location. For example, tectal gliomas are considered benign and often never require treatment except ventriculoperitoneal shunting for symptomatic hydrocephalus.1 Diffuse pontine gliomas (DPGs), however, are associated with a median overall survival (OS) of only 12 months, even with aggressive combined modality therapy.2 The most recent classification system developed by Choux et al3 classifies brainstem gliomas into diffuse, intrinsic focal, extrinsic focal, and cervicomedullary.
Because of the uniformly poor outcomes in DPGs and risks associated with biopsy, diagnosis of DPGs has become largely based on clinical and radiographic evidence. As treatment and outcomes have been thought to be similar regardless of histology, this approach seems justifiable. Even after over 20 years of cooperative group research efforts, no significant progress has been made in outcomes for patients with DPGs.2 With advances in targeted therapy occurring in other brain tumors, neurooncologists have begun to reconsider the potential role of biopsy in DPGs. In addition, advances in neurosurgical techniques have significantly reduced the risk of morbidity and mortality from the biopsy procedure.4–7
The purpose of this retrospective review is to better understand the role of both histologic grade and tumor location in childhood brainstem tumors. The review was restricted to biopsy-proven tumors to ensure accurate tumor grade and only low-grade histologies to allow comparison between DPGs and the more favorable prognosis of tumors in other locations.
METHODS
After the approval from Mayo Clinic Institutional Review Board, the Mayo Clinic Tumor Registry was used to identify children (≤20 y) with biopsy-proven brainstem low-grade (grade I or II) gliomas (BS-LGG) treated between January 1971 and December 2004 and followed until time of analysis in March 2011. The definition of a brainstem tumor was limited to intra-axial lesions within the midbrain, pons, or the medulla and the classification system developed by Choux et al3 was used to classify tumors in this study. Medical records were retrospectively reviewed for demographics, symptoms at the time of diagnosis, character, location of tumor, treatment, survival, and recurrence.
The extent of resection deemed as gross total resection (GTR), subtotal resection (STR), or radical subtotal resection (rSTR) was determined by assessment of the operative report, the neurosurgeon’s impression, and imaging. Resection was determined to be a rSTR if the operative report stated “radical subtotal resection,” if GTR was the operative intent but minimal tumor was known to be left in situ, or if imaging reports indicated minimal, questionable amounts of residual tumor after GTR. Tumors were classified by World Health Organization histologic type and the Kernohan grading system.8 All specimens were reviewed by neuropathologists at the Mayo Clinic.
Descriptive statistics were used to summarize the cohort including median and range for continuous variables or counts and percentages for categorical variables. The OS and progression-free survival (PFS) rates were calculated from the date of diagnosis to the date of death or progression using the Kaplan-Meier Method.9 The log-rank test was used to test differences in survival or PFS rate.10 Patients who did survive or did not progress were censored at last follow-up. Radiologic data was reviewed to determine tumor progression and extent of disease. A 2-tailed P < 0.05 was considered statistically significant. Statistical analyses were performed using SAS 9.2 and JMP 7 (SAS Institute Inc., Cary, NC).
RESULTS
Patient, Tumor, and Symptom Characteristics
A total of 662 patients underwent brainstem biopsies during this time period. The most common histologies were high-grade gliomas (230), grade II gliomas (187), pilocytic astrocytomas (61), and ependymomas (17 grade III/IV, 15 grade 2). From the low-grade cohort, 48 brainstem gliomas were identified in children (≤20 y) by review of imaging and/or the medical record. The mean age of patients in this study was 12.0 ± 5.2 years (range, 2.5 to 19.5 y) with a median follow-up of 6.0 years. Twenty-five patients (52%) were female. The majority were grade I (69%) tumors and the remaining cases were grade II. At the time of diagnosis, 1 patient experienced seizures, 25 experienced headaches, 41 reported motor symptoms, and 35 reported sensory symptoms. Table 1 lists tumor location, grade, and histology as determined by radiographic analysis.
TABLE 1.
Patient and Tumor Characteristics
| Diffuse | Focal Exophytic | |||||
|---|---|---|---|---|---|---|
| Diffuse Pontine | Diffuse Other | Cervicomedullary | Focal Intrinsic | Dorsal Exophytic | Tectal Glioma | |
| Grade | ||||||
| I | 7 | 5 | 9 | 9 | 2 | 1 |
| II | 6 | 2 | 4 | 3 | — | — |
| Histology | ||||||
| Astrocytoma | 9 | 4 | 11 | 4 | — | — |
| Pilocytic astrocytoma | 4 | 3 | 2 | 7 | 2 | 1 |
| Oligodendroglioma | — | — | — | 1 | — | — |
| Location | ||||||
| Pons only | 2 | — | — | — | — | — |
| Pons/medulla | 2 | 2 | — | — | — | — |
| Pons/medulla/midbrain | 1 | 2 | — | — | — | — |
| Pons/midbrain | 8 | 2 | — | 2 | — | — |
| Medulla only | — | — | 13 | 3 | 2 | — |
| Midbrain only | — | 1 | — | 7 | — | 1 |
Surgery, Treatment, and Histology
Four patients underwent GTR, 5 underwent rSTR, and 12 underwent STR. The remaining patients underwent biopsy-only. Twenty-nine patients underwent radiotherapy (RT) treatment. Details regarding dose and field were available for 22 patients. Patients were treated to a median dose of 54 Gy (range, 16.2 to 78 Gy) in a median of 31 fractions (range, 21 to 78 Gy) over a median of 41 days (range, 34 to 62 d). Four patients received chemotherapy and 2 patients received both radiation and chemotherapy. Chemotherapy consisted of regimens of procarbazine, lomustine, and vincristine. Table 1 shows the classification of tumors. Histologic subtype analysis revealed 19 patients to have pilocytic astrocytomas (all grade I), 28 patients with astrocytomas (14 grade I and 14 grade II), and 1 patient with an oligodendroglioma (grade II).
Survival Outcomes
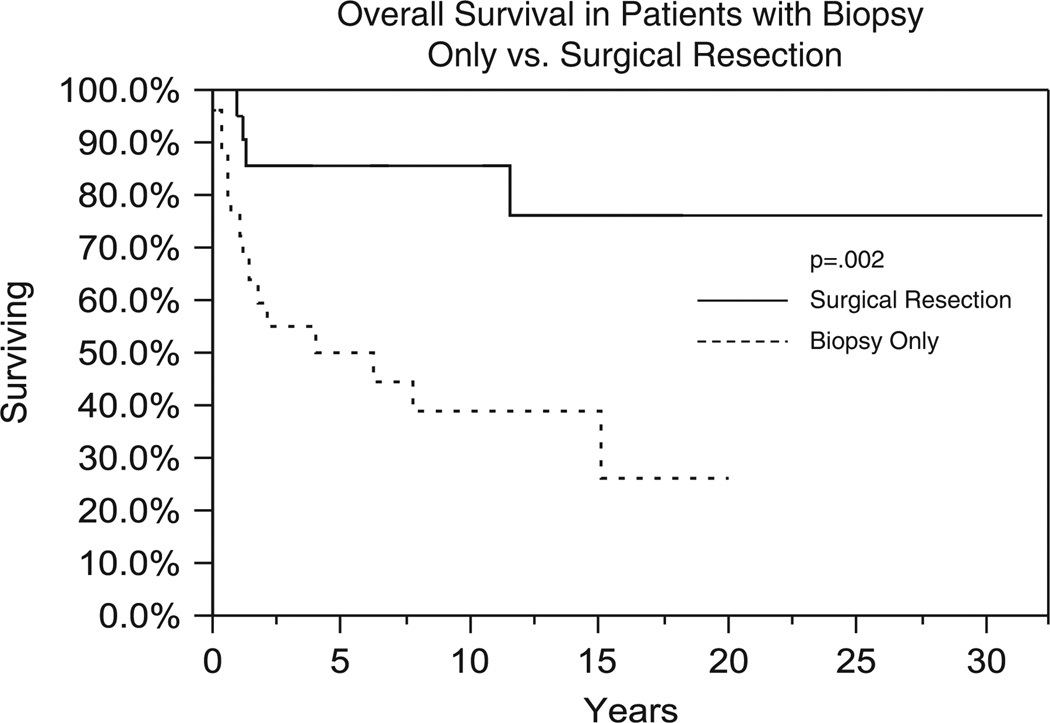
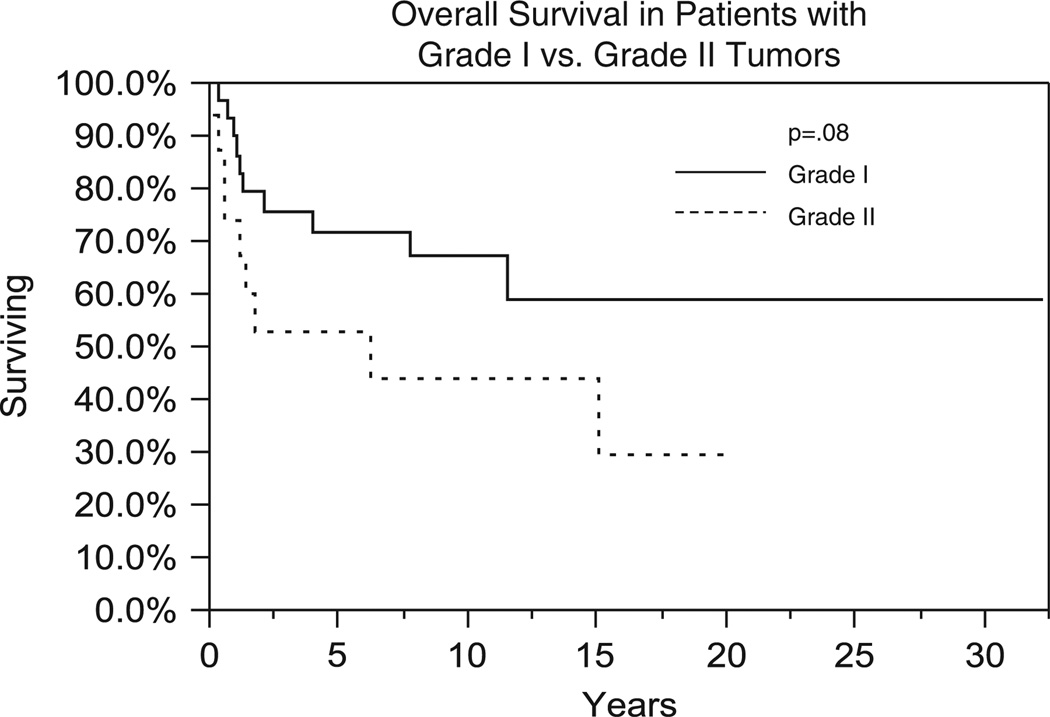
Nineteen patients (40%) died with or due to the disease. Of the remaining 29 patients who were alive at last follow-up, 16 patients had no evidence of disease, 7 had unknown disease status, and 8 were alive with disease. Median OS for the entire group was 14.8 years with 1-, 5-, and 10-year OS of 85%, 67%, and 59%, respectively (Fig. 1). GTR was associated with an improvement in OS in comparison to biopsy, 85% versus 50% at 5 years, respectively (P = 0.002) (Fig. 2). Improved OS was also associated with grade I versus grade II histology with 5-year survival of 71% versus 52%, respectively (P = 0.08) (Fig. 3). RT was not associated with improved OS outcomes; however, RT was used preferentially in patients with poor prognosis such as those who had a biopsy-only procedure (19/27), DPGs (9/13), and grade 2 tumors (13/15).
FIGURE 1.
Overall survival (Kaplan-Meier method) for all brainstem lesions.
FIGURE 2.
Comparison of overall survival (Kaplan-Meier method) between patients undergoing biopsy versus surgical resection.
FIGURE 3.
Comparison of overall survival (Kaplan-Meier method) between patients with grade I versus grade II lesions.
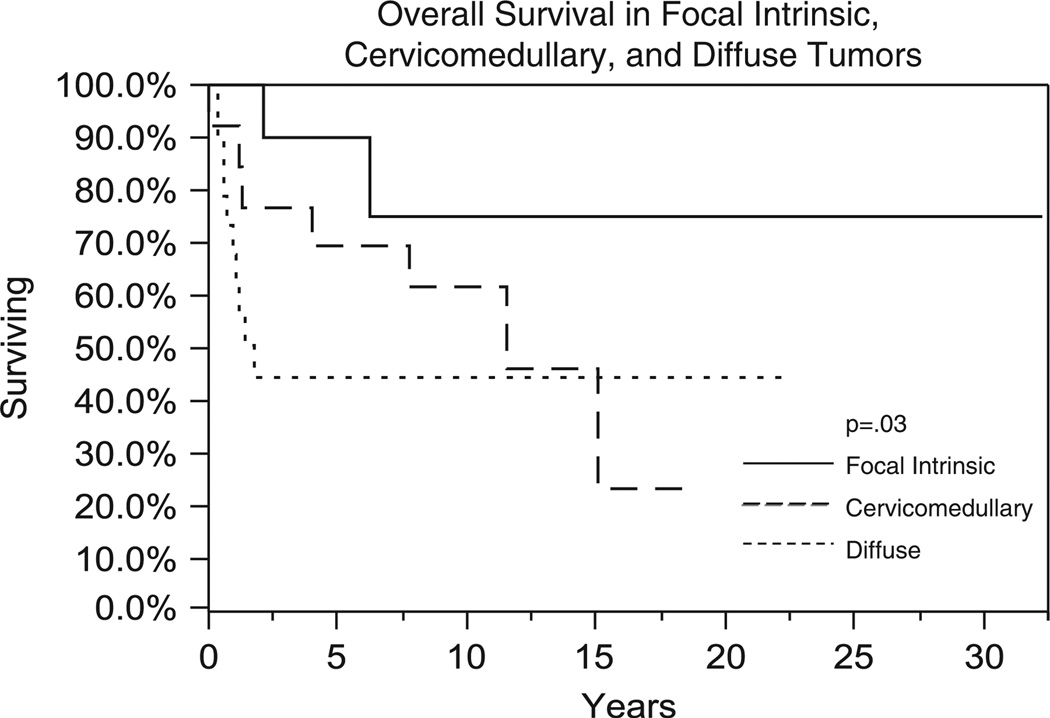
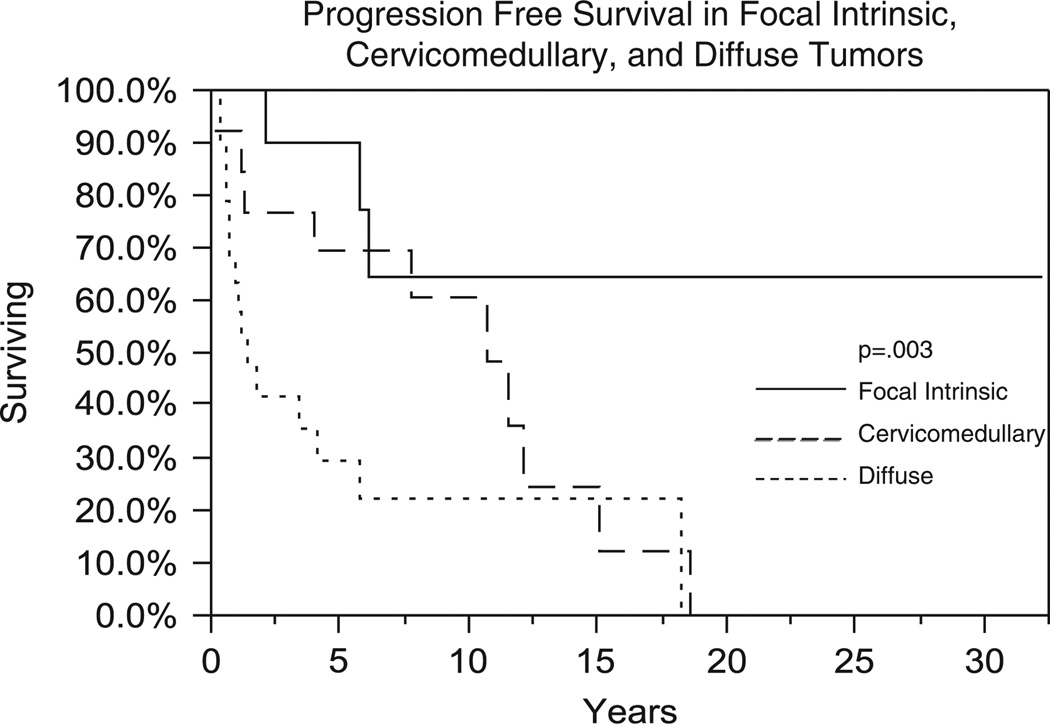
There were significant differences in survival by tumor classification. Figure 4 shows OS between focal intrinsic, cervicomedullary, and diffuse tumors. Five-year OS was 90%, 69%, and 41% for focal intrinsic, cervicomedullary, and diffuse tumors, respectively (P = 0.03). PFS also showed similar trends (5-y PFS rates of 90%, 69%, and 30% were obtained for focal intrinsic, cervicomedullary, and diffuse gliomas, respectively) (P = 0.003) (Fig. 5). OS was 70.7% in grade I (n = 12) and 62.5% in grade II (n = 8) diffuse tumors at 1 year (P = 0.52). Intrinsic focal grade I tumors had 5-year OS of 85.7% (n = 9) and 66.7% in grade II tumors (n = 3) (P = 0.77). Cervicomedullary grade I (n = 9) tumors had an OS of 77.7% and grade II (n = 4) tumors had an OS of 50% at 5 years (P = 0.34). Three patients had grade I focal exophytic tumors with a median OS of 9.6 years (range, 6.8 to 25.2 y).
FIGURE 4.
Comparison of overall survival (Kaplan-Meier method) between patients with focal intrinsic, cervicomedullary, and diffuse tumors.
FIGURE 5.
Comparison of progression-free survival (Kaplan-Meier method) between patients with focal intrinsic, cervicomedullary, and diffuse tumors.
Recurrence and Progression
The median length of follow-up was 8.7 years for surviving patients. Recurrences were documented in 20 patients with a median PFS of 7.3 years. The 1-, 5-, and 10-year PFS rates for all patients were 84.7%, 59%, and 48%, respectively. RT was not associated with improved PFS.
Recurrences were documented in 20 patients radio-graphically. Among these, symptoms were documented in 19 patients. Ten patients underwent at least a biopsy with a confirmation of recurrence. In 1 patient, the tumor was found to have progressed to a higher grade than recorded at diagnosis. Treatment of recurrence occurred in 14 patients with 9 patients undergoing surgery, 4 undergoing radiation, and 6 treated with chemotherapy. Median survival after recurrence was 6.8 years (range, 0.2 to 30.8 y).
DPGs
Median follow-up for patients with DPGs was 5.5 years (range, 0.2 to 22.3 y). The characteristics of each of the 13 DPG patients are shown in Table 2. Postoperative RT was used in 9 patients and postoperative chemotherapy in 1 patient. Patients were treated to a median dose of 55.6 Gy (range, 45 to 72 Gy) in a median of 30 fractions (range, 25 to 71 Gy). Median OS was 1.8 years (range, 0.3 to 21.2 y). One-year OS was 73% and 44% at 2 years. Neither the extent of resection nor the use of RT treatment was associated with OS. Grade I DPGs had a poorer OS at 1 year when compared with all other grade I tumors, 60% and 95.6%, respectively (P = 0.05). There was a trend toward poorer OS of DPGs compared with focal pontine tumors at 1 year (73.3% and 100%) and 5 years (44% and 90%) (P = 0.10).
TABLE 2.
Diffuse Pontine Glioma Characteristics
| Age (y)/ Sex |
Medulla | Midbrain | Histologic Subtype |
Grade | Extent Resection |
Postoperative Radiotherapy |
Postoperative Chemotherapy |
Survival (y) |
|---|---|---|---|---|---|---|---|---|
| 12.8/M | Y | N | Astrocytoma | II | Biopsy only | Y | N | 1.4 |
| 3/F | N | Y | Astrocytoma | I | Biopsy only | N | N | 0.7 |
| 6/M | N | Y | Pilocytic Astrocytoma | I | Radical subtotal | N | N | 19.9 |
| 19.1/F | N | N | Astrocytoma | II | Biopsy only | Y | N | 0.4 |
| 17.4/M | N | N | Pilocytic astrocytoma | I | Biopsy only | N | N | 5.9 |
| 17.4/M | N | Y | Pilocytic astrocytoma | I | Subtotal | N | N | 5.5 |
| 9.1/F | N | Y | Astrocytoma | II | Biopsy only | Y | Y | 0.9 |
| 13.4/M | Y | N | Astrocytoma | II | Biopsy only | Y | N | 1.8 |
| 9.2/M | N | Y | Astrocytoma | II | Biopsy only | Y | N | 0.2 |
| 13.6/M | N | Y | Astrocytoma | II | Biopsy only | Y | N | 1.7 |
| 6.1/M | N | Y | Astrocytoma | I | Subtotal | Y | N | 0.9 |
| 14.4/F | N | Y | Pilocytic astrocytoma | I | Subtotal | Y | N | 11.4 |
| 19.1/F | Y | Y | Astrocytoma | I | Biopsy only | Y | N | 1.1 |
Neuroimaging of DPGs
For each of these 13 patients, initial imaging was obtained prior to 1998 and thus images were no longer available for review as films obtained prior to 1998 were purged from the records. However, the preoperative neuroimaging written reports for each patient were available for review and are summarized. In 10 cases, the primary site of the tumor was noted to be pontine, whereas 3 were described as centered in the middle cerebellar peduncle. For 10 of the patients, a preoperative computed tomography (CT) report was available for review. In 3 of the patients, a preoperative CT did not seem to have been performed. In 11 patients, a preoperative magnetic resonance imaging (MRI) report was available for review, whereas in 2 patients, a preoperative MRI did not seem to have been performed. On the basis of the CT reports, 5 tumors demonstrated enhancement. In another 5, the tumor was specifically described as nonenhancing. In the remaining 3, no enhancement characteristics were reported in the CT report. For the 11 patients that underwent MRI, 4 reportedly demonstrated enhancement and 3 specifically did not. In the remaining 4, enhancing characteristics were not described. Of all patients, 5 were reportedly cystic and another 5 were reportedly not. In 3 of the patients, cystic characteristics were not described. Of all the patients, 6 reportedly had no hydrocephalus at the time of preoperative imaging. Another 3 had a hydrocephalus that was described as moderate and 3 had hydrocephalus that was described as marked. In 1 case, the size of the ventricular system was not described. Calcification was not described in any imaging report for these 13 tumors. In summary, based upon the preoperative imaging reports, all of these tumors were radiographically nonspecific, as enhancement, cystic change, and hydrocephalus were all present or absent to varying degrees. Given the lack of specificity and the clinical practice at the time, biopsy was performed to confirm the radiographic suspicion of glioma.
DISCUSSION
This single institution retrospective study in pathologically proven BS-LGG with extensive follow-up reveals several findings. First, the study shows favorable long-term outcomes for patients with BS-LGG. Second, outcomes were better for patients with nondiffuse classifications. Third, improved survival was associated with undergoing resection versus biopsy-only. Fourth, low-grade DPGs seem to have better OS than historical controls.
DPGs have been considered to have a uniformly poor prognosis with no significant improvement in outcomes after over 20 years of cooperative group research.2 Because the treatment recommendations, typically RT, are not influenced by histology, and because of the risks of morbidity from biopsy in this sensitive location, biopsy confirmation of these tumors has largely been abandoned in favor of radiographic diagnosis.
Because of the aggressive nature of DPGs, they are often treated with chemotherapy regimens used in high-grade gliomas.11–13 It is becoming increasingly clear, however, that high-grade and low-grade tumors have distinctly different sensitivities to available chemotherapies. In addition, many treatment regimens are adapted from promising agents in adult gliomas and it is becoming evident that adult and pediatric gliomas have distinct molecular and genetic alterations, despite the similar histologic appearance.14–19
Because of the increasing awareness of molecular and genetic differences in childhood gliomas, the role for histologic confirmation of DPGs is once again under debate. Proponents argue that biopsy confirmation is necessary to allow for individualized, targeted therapy both now and through molecular characterization that may benefit future patients. In addition, the risks of biopsy have decreased such that biopsy may be safer than previous studies report.4–7
Generally, biopsy is reserved for tumors with an atypical appearance, those that have more focal or exophytic components and/or enhance.
Current practice at our facility is consistent with the current standard of radiographic diagnosis for DPGs. However, prior to MRI availability in 1990, biopsy was performed more frequently for brainstem tumors. For example, biopsy for DPGs in this series occurred between 1971 and 1996 and 11 of the 13 biopsies were performed before 1990. Although in general, biopsy of brainstem tumors is only performed for tumors with “atypical” appearances, in this series the biopsies are largely reflective of a change in treatment practice and are less likely due to selection bias than more modern series. Although some selection bias would be expected still to be present, this bias would be similar for tumors at all locations such that this cohort of biopsy-proven tumors may be more appropriate for comparison between grade and location.
Overall, our results are consistent with other series confirming the prognostic role of radiographic classification of Choux and colleagues. In our series, OS for focal tumors was 90% at 5 years. This compares favorably to a report by Sandri and colleagues. This group found in a cohort of 17 patients with focal brainstem gliomas the 4-year overall and disease-free survival rates to be 87.4% and 58.8%, respectively.20 Patients were treated with a combination of surgery, RT, and chemotherapy. Similar to our results, the study found the extent of resection to be an important prognostic factor (P = 0.012).20 Our study found the OS at 5 and 10 years to be 90% and 75%, respectively, in patients with focal brainstem gliomas. In addition, our study confirmed the prognostic significance of tumor location, even when controlling for similar low-grade histology, with pontine tumors fairing the worst. In contrast to other reports, however, tumor grade would suggest differences in prognosis.
Outcomes of brainstem gliomas differ based on location and pattern of growth. The most recent classification system developed by Choux et al3 classifies brainstem gliomas into diffuse, intrinsic focal, extrinsic focal, and cervicomedullary. Many focal tumors are considered benign and handled surgically, whereas diffuse processes are considered malignant and treated nonoperatively. Our results showed the most favorable OS and PFS rates to be in focal intrinsic tumors with poor prognosis in diffuse tumors. There was a trend toward grade I tumors having an improved OS compared with grade II tumors as shown in Figure 3.
Our study found median OS and PFS to be 1.8 years for low-grade DPGs, in contrast to the <1-year OS given for DPGs in historical controls.2,21 The study by Sandri et al20 found the median time to progression and to death to be 8 (range, 3 to 13 mo) and 13 months (range, 4 to 25 mo), respectively, with a 2-year OS rate of 12.3% in diffuse brainstem gliomas. The concentration of low-grade tumors in our study may account for the differences in OS in DPGs compared with previous reports. In a study of 34 patients treated with hyperfractionated RT with concurrent carboplatin, median OS at 1 year was 12 months (range, 5 to 104 + mo).22 At the last follow-up, there were 5 long-term survivors (15%) after a mean follow-up period of 79 months.22 Other studies analyzing chemotherapy in DPG patients have not shown differences to historical controls. Topotecan used as a radiosensitizer administered concurrently with radiation in 17 patients with these tumors showed a 1-year survival of 53% with a median survival time of 15 months (range, 9.6 to 19 mo).23 In addition, a COG phase II study did not show an improvement in OS using vincristine with oral VP16 along with radiation therapy with a median OS of 9 months (range, 3 to 36 mo).24 Although median OS is only slightly improved in our cohort, 2- and 5-year OS seems to be different in this low-grade cohort. The better longer term survival in this study may be largely attributed to 4 patients whose survival surpassed 5 years. These patients all had grade I, pilocytic astrocytomas on histology and all but 1 tumor was partially resected. The low-grade histology of these DPGs may have led to these tumors improved survival profiles. Many studies have shown a few patients to survive months or years past the median survival. It is quite possible that these patients had a low-grade glioma at diagnosis, which placed them at an increased likelihood for improved survival prior to beginning therapy. This may be important both in counseling parents and families and developing more targeted therapies in the future.
Because of the extended study period in this series, many diagnoses predated MRI evaluation. Thus, a significant limitation of this study is the difficulty assessing focal and diffuse patterns on CT and the correlation between those descriptions on MRI. In addition, classification of tumors was conducted retrospectively based on radiographic appearance and clinical note descriptions. As such, classification may have been influenced by the known outcomes of these patients. The number of patients in this study also limits our ability to draw firm conclusions. This is most apparent for our cohort of patients with DPGs. Despite these limitations, this series is unique in its restriction to low-grade and biopsy-proven brainstem tumors. Based on the different outcomes in pontine and DPGs by grade and histology compared with nonbiopsy series, we believe that histologic confirmation should be considered in DPGs both for its value in providing potential prognostic information for families and for an opportunity to recommend targeted individualized treatment based on molecular and genetic tumor profiles as well as histologic diagnosis.
CONCLUSIONS
This single institution, retrospective series confirms the prognostic significance of tumor location and radiologic classification. In contrast to other reports, the confirmation of low-grade histology was associated with differences in OS compared with historical controls. This suggests a role for histologic confirmation of brainstem tumors for prognostic implications. Further tissue availability could also help in the understanding of molecular and genetic alterations that will allow for targeted therapies in the future.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Stark AM, Fritsch MJ, Claviez A, et al. Management of tectal glioma in childhood. Pediatr Neurol. 2004;33:33–38. doi: 10.1016/j.pediatrneurol.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Freeman CR, Farmer JP. Pediatric brain stem gliomas: a review. Int J Radiat Oncol Biol Phys. 1998;40:265–271. doi: 10.1016/s0360-3016(97)00572-5. [DOI] [PubMed] [Google Scholar]
- 3.Choux M, Lena G, Do L. Brain stem tumors. In: Choux M, Di Rocco C, Hockley A, editors. Pediatric Neurosurgery. New York: Churchill Livingstone; 2000. pp. 471–491. [Google Scholar]
- 4.Steck J, Friedman WA. Stereotactic biopsy of brainstem mass lesions. Surg Neurol. 1995;43:563–567. doi: 10.1016/0090-3019(95)00156-5. [DOI] [PubMed] [Google Scholar]
- 5.Rajshekhar V, Chandy MJ. Computerized tomography-guided stereotactic surgery for brainstem masses: a risk-benefit analysis in 71 patients. J Neurosurg. 1995;82:976–981. doi: 10.3171/jns.1995.82.6.0976. [DOI] [PubMed] [Google Scholar]
- 6.Albright AL, Price RA, Guthkelch AN. Brain stem gliomas of children. A clinicopathological study. Cancer. 1983;52:2313–2319. doi: 10.1002/1097-0142(19831215)52:12<2313::aid-cncr2820521226>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 7.Amundson EW, McGirt MJ, Olivi A. A contralateral, transfrontal, extraventricular approach to stereotactic brainstem biopsy procedures. Technical note. J Neurosurg. 2005;102:565–570. doi: 10.3171/jns.2005.102.3.0565. [DOI] [PubMed] [Google Scholar]
- 8.Rosenblum MK. The 2007 WHO Classification of Nervous System Tumors: newly recognized members of the mixed glioneuronal group. Brain Pathol. 2007;17:308–313. doi: 10.1111/j.1750-3639.2007.00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 10.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. Analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sirachainan N, Pakakasama S, Visudithbhan A, et al. Concurrent radiotherapy with temozolomide followed by adjuvant temozolomide and cis-retinoic acid in children with diffuse intrinsic pontine glioma. Neuro Oncol. 2008;10:577–582. doi: 10.1215/15228517-2008-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim CY, Kim SK, Phi JH, et al. A prospective study of temozolomide plus thalidomide during and after radiation therapy for pediatric diffuse pontine gliomas: preliminary results of the Korean Society for Pediatric Neuro-Oncology study. J Neurooncol. 2010;100:193–198. doi: 10.1007/s11060-010-0157-1. [DOI] [PubMed] [Google Scholar]
- 13.Bouffet E, Raquin M, Doz F, et al. Radiotherapy followed by high dose busulfan and thiotepa: a prospective assessment of high dose chemotherapy in children with diffuse pontine gliomas. Cancer. 2000;88:685–692. doi: 10.1002/(sici)1097-0142(20000201)88:3<685::aid-cncr27>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 14.Newcomb EW, Alonso M, Sung T, et al. Incidence of p14ARF gene deletion in high-grade adult and pediatric astrocytomas. Hum Pathol. 2000;31:115–119. doi: 10.1016/s0046-8177(00)80207-5. [DOI] [PubMed] [Google Scholar]
- 15.Gallia GL, Rand V, Siu IM, et al. PIK3CA gene mutations in pediatric and adult glioblastoma multiforme. Mol Cancer Res. 2006;4:709–714. doi: 10.1158/1541-7786.MCR-06-0172. [DOI] [PubMed] [Google Scholar]
- 16.Pollack IF, Hamilton RL, James CD, et al. Rarity of PTEN deletions and EGFR amplification in malignant gliomas of childhood: results from the Children’s Cancer Group 945 cohort. J Neurosurg. 2006;105:418–424. doi: 10.3171/ped.2006.105.5.418. [DOI] [PubMed] [Google Scholar]
- 17.Faury D, Nantel A, Dunn SE, et al. Molecular profiling identifies prognostic subgroups of pediatric glioblastoma and shows increased YB-1 expression in tumors. J Clin Oncol. 2007;25:1196–1208. doi: 10.1200/JCO.2006.07.8626. [DOI] [PubMed] [Google Scholar]
- 18.Rickert CH, Strater R, Kaatsch P, et al. Pediatric high-grade astrocytomas show chromosomal imbalances distinct from adult cases. Am J Pathol. 2001;158:1525–1532. doi: 10.1016/S0002-9440(10)64103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong KK, Tsang YT, Chang YM, et al. Genome-wide allelic imbalance analysis of pediatric gliomas by single nucleotide polymorphic allele array. Cancer Res. 2006;66:11172–11178. doi: 10.1158/0008-5472.CAN-06-2438. [DOI] [PubMed] [Google Scholar]
- 20.Sandri A, Sardi N, Genitori L, et al. Diffuse and focal brain stem tumors in childhood: prognostic factors and surgical outcome: Experience in a single institution. Childs Nerv Syst. 2006;22:1127–1135. doi: 10.1007/s00381-006-0083-x. [DOI] [PubMed] [Google Scholar]
- 21.Khatua S, Moore KR, Vats TS, et al. Diffuse intrinsic pontine glioma—current status and future strategies. Childs Nerv Syst. 2011;27:1391–1397. doi: 10.1007/s00381-011-1468-z. [DOI] [PubMed] [Google Scholar]
- 22.Allen J, Siffert J, Donahue B, et al. A phase I/II study of carboplatin combined with hyperfractionated radiotherapy for brainstem gliomas. Cancer. 1999;86:1064–1069. doi: 10.1002/(sici)1097-0142(19990915)86:6<1064::aid-cncr24>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 23.Sanghavi SN, Needle MN, Krailo MD, et al. A phase I study of topotecan as a radiosensitizer for brainstem glioma of childhood: first report of the Children’s Cancer Group-0952. Neuro Oncol. 2003;5:8–13. doi: 10.1093/neuonc/5.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korones DN, Fisher PG, Kretschmar C, et al. Treatment of children with diffuse intrinsic brain stem glioma with radiotherapy, vincristine and oral VP-16: a Children’s Oncology Group phase II study. Pediatr Blood Cancer. 2008;50:227–230. doi: 10.1002/pbc.21154. [DOI] [PubMed] [Google Scholar]