Abstract
Very preterm birth (<32 weeks’ gestation) occurs in approximately 2% of live births but is a leading cause of infant mortality and morbidity in the United States. African-American women have a 2-fold to 3-fold elevated risk compared with non-Hispanic white women for reasons that are incompletely understood. This paper reviews the evidence for the biologic and social patterning of very preterm birth, with attention to leading hypotheses regarding the etiology of the racial disparity. A systematic review of the literature in the MEDLINE, CINAHL, PsycInfo, and EMBASE indices was conducted. The literature to date suggests a complex, multifactorial causal framework for understanding racial disparities in very preterm birth, with maternal inflammatory, vascular, or neuroendocrine dysfunction as proximal pathways and maternal exposure to stress, racial differences in preconceptional health, and genetic, epigenetic, and gene-environment interactions as more distal mediators. Interpersonal and institutionalized racism are mechanisms that may drive racially patterned differences. Current literature is limited in that research on social determinants and biologic processes of prematurity has been generally disconnected. Improved etiologic understanding and the potential for effective intervention may come with better integration of these research approaches.
Keywords: continental population groups, health status disparities, pregnancy complications, infectious, premature birth
INTRODUCTION
Premature birth is an intermediate cause of infant mortality and morbidity. It is intermediate in that birth too early in gestation results from proximate dysfunction in the uterine milieu; it is likely causal in that the harm to the fetus or infant is not solely from these dysfunctions but also from the consequences of shortened gestation on respiratory and neurologic function in the extrauterine world. While prematurity is a continuum, with the whole population of births before 37 weeks’ gestation experiencing some increased risk compared with term birth at 37 weeks or more, it is the extreme of the distribution, very preterm (VPT) birth (defined here as birth before 32 weeks’ gestation), that accounts for the lion’s share of harm attributable to prematurity. VPT birth occurred in 20.3 of 1,000 US births in 2006 (1). This relatively small percentage of births accounts for a third of all infant deaths, 67% of neonatal mortality (2, 3), and a significant prevalence of acquired developmental disabilities such as cerebral palsy, mental retardation, and less severe cognitive disability (4, 5).
While VPT birth is a poor outcome for any pregnancy, African-American women experience nearly 3 times the risk of VPT birth when compared with non-Hispanic white women (1). This magnitude of disparity persists for the closely related outcome of very low birth weight (<1,500 g) even among college-educated black women married to college-educated black men, who are delivering their first- or second-born infant in their twenties or thirties after having received prenatal care beginning in the first trimester (6).
This long-observed pattern of correlation between the social construct of race and the biologic outcome of VPT birth has been discussed and studied for decades in 2 largely separate literatures: social determinants of health and biologic/clinical processes resulting in prematurity. In this review, we seek to synthesize these bodies of literature, with particular attention to linkages between the 2, with the intention of advancing a research agenda to understand and diminish the population health burden embodied by the racial disparity in VPT birth.
To accomplish this goal, we systematically searched MEDLINE, CINAHL, PsycINFO, EMBASE, and the Cochrane Database using the Medical Subject Heading (MeSH) term “premature birth” as well as “preterm” and “premature” as keywords and crossing them with the MeSH term “continental population groups” or “race” as a keyword. This search resulted in 1,459 citations between 1960 and February 2009. With the stated goal of understanding the etiology of the black-white racial disparities in preterm birth, each author independently selected relevant abstracts and papers, with disagreement favoring retaining the additional citation. References from selected citations were also reviewed to identify other relevant papers. An initial inductive review of relevant literature resulted in a general conceptual framework. With this framework, we reviewed the entire list again, and relevant papers were examined and coded according to the framework.
The inductive review highlighted 2 important caveats worth noting: the choice of perinatal outcomes and measure is important; and the conceptual framework in play can drive the inferences from any given study. Each is briefly discussed in the following sections.
MEASURES AND OUTCOMES
Perinatal epidemiologists have described many similarities (and some differences) across pregnancy outcomes such as low birth weight (<2,500 g), very low birth weight (<1,500 g), preterm birth (<37 weeks), and VPT birth (variably defined as <34, <32, or <28 weeks’ gestation). The many similarities in risk factors for these outcomes make it tempting to lump the entire body of literature together, but the differences are important to note. Birth weight as an outcome is very reliably measured in vital statistics data sets, but the common low birth weight definition of <2,500 g mixes data for preterm and growth-retarded babies and is not likely a causal intermediary between proximate insult and subsequent mortality or morbidity (7, 8). Although the metric of gestational age more directly connects intrauterine processes to extrauterine consequences, it is notoriously challenging to measure well, particularly in commonly used vital statistics data sets, and particularly when comparing across demographic groups such as race or socioeconomic status. Specifically, there is evidence for differential misclassification of gestational age calculated from last menstrual period for poor and minority women, although use of ultrasound dating has reduced this error (9).
For this review, we preferentially sought studies using ultrasound-based dating or data-cleaning approaches that reduce misclassification (10, 11). Because of the high correlation between the outcome very low birth weight (<1,500 g) and VPT birth (the gestational ages of only 9.8% of black births and 12.8% of white births classified as <1,500 g were more than 32 weeks (12)), we included studies using either outcome to address our primary aim. Because so much more literature uses the broader categories of low birth weight (<2,500 g) and preterm birth (<37 weeks), we reference this as suggestive of hypotheses, acknowledging that these less extreme outcomes may be etiologically and epidemiologically distinct from VPT birth. For the remainder of this review, we specifically refer to studies that explicitly or implicitly test a hypothesis for explaining the observed association of black/white race with either very low birth weight or VPT birth as an outcome. Those studies are also summarized in Table 1, indicating, where possible, the mediating pathway evaluated.
Table 1.
Summary of Studies Evaluating Intermediate Variables in the Association Between Black/White Race and Occurrence of Very Preterm Birth or Very Low Birth Weight
| Author, Year, (Reference No.) |
Outcome | Pathwaya | Results |
|---|---|---|---|
| Simhan, 2003 (156) | VPT birthb | Genotype/epigenetics | The interleukin-6 polymorphism was protective against VPT birth in white women, but single-nucleotide polymorphism was not present in black women cases or controls in the study. |
| Catov, 2007 (138) | VPT birthb | Preconceptional health | Periconceptional multivitamin use was protective against VPT birth and was less prevalent among black women than white women. |
| Ehrenthal, 2007 (102) | VPT birthc VLBWd | Preconceptional health | Although black women had a higher prevalence of preconceptional hypertension and diabetes than white women did, control for preconceptional health status did not appreciably reduce the racial disparity. |
| Khoshnood, 1998 (136) | VLBWd | Preconceptional health | Black women and white women with an IPI of <6 months had a higher risk of VLBWthan those with an IPI of ≥12 months; black women had a higher prevalence of an IPI of <6 months than white women did. |
| Berg, 2001 (163) | VLBWd | Preconceptional health; substance abuse; SES; acute, chronic, or early- life stress | A significant racial difference in the risk profile for VLBW was found. SES markers were associated with white, but not black, VLBW. Only unmarried status, smoking, and no vitamin use during pregnancy were associated with VLBW for black women. |
| Andrews, 2006 (117) | VPT birthc | Preconceptional health, inflammation | Interconceptional antibiotic treatment in high-risk black women and white women did not change the risk of VPT birth. |
| Catov, 2007 (164) | VPT birthe | Preconceptional health, inflammation | Early-pregnancy elevated C-reactive protein and dyslipidemia levels double the risk of VPT birth for black women and white women (adjusted for but not stratified on race). |
| Jeffcoat, 2001 (53) | VPT birthc | Preconceptional health, inflammation | Periodontitis at 24 weeks increases the risk of VPT birth 7-fold and is more prevalent in black women than white women. |
| Offenbacher, 2006 (114) | VPT birthc | Preconceptional health, inflammation | Progression of periodontal disease during pregnancy doubles the risk of VPT birth for black women and white women (adjusted for but not stratified on race). |
| Dunlop, 2008 (120) | VLBWd | Preconceptional health, social support | A pilot trial of interconceptional primary medical and dental care with social support for black women with a prior VLBW birth reduced the risk of subsequent VLBW birth. |
| Ickovics, 2003 (144) | VPT birthc VLBWd | Preconceptional health, social support | Group-centered prenatal care for high-risk women resulted in a nonsignificant reduction in VLBW/VPT birth risk compared with standard prenatal care in a largely black study population. |
| Andrews, 2006 (112) | VPT birthc | Inflammation | Histologic evidence of inflammation in the placenta was more common in black women than white women with VPT birth. |
| Goldenberg, 1998 (46) | VPT birthc | Inflammation | Bacterial vaginosis and fetal fibronectin were more prevalent and were stronger predictors of VPT birth for black women compared with white women. |
| Adams, 2000 (89) | VPT birthc | Life course (SES? acute, chronic, or early-life stress? genotype/ epigenetics?) | Recurrent VPT birth is more common in black women than in white women. |
| Kistka, 2007 (88) | VPT birthb | Life course (SES, acute, chronic, or early-life stress? genotype/ epigenetics?) | VPT birth recurrence is greater for black women than for white women. |
| David, 1997 (165) | VLBWd | Life course (acute, chronic, or early-life stress?) | Among high-SES mothers, African-born blacks had a VLBW risk equivalent to that for whites, but US-born blacks had 3.3 times the risk compared with whites. |
| Shen, 2008 (166, 167) | VPT birthc | Life course (vascular dysfunction? genotype/ epigenetics?) | Black women were 2–3 times as likely as white women to have a VPT birth and PPROM or placental abruption; also found was a higher PPROM recurrence risk for black women compared with white women. |
| Collins, 2000 (77) | VLBWd | Interpersonal racism | Threefold elevated odds of VLBW were found for low-income black women reporting an experience of perceived racism during pregnancy. |
| Collins and Lespinasse, 2004 (76 and 78) | e, VLBWd | Interpersonal racism | Among black mothers, lifetime exposure to interpersonal racism was associated with VLBW. |
| Aveyard, 2002 (168) | VPT birthf | SES | SES accounts for a large portion of the Afro-Caribbean:white, but not African:white, disparity in the United Kingdom. |
| Kleinman, 1987 (169) | VLBWd | SES | The protective effect of a higher level of maternal education was greater for white women compared with black women. |
| Reagan, 2005 (170) | VPT birthe | SES; acute, chronic, or early-life stress; substance abuse; preconceptional health | Neighborhood poverty was associated with VPT birth in black women but not white or Hispanic women; tobacco, alcohol, and cocaine use was associated with VPT birth in white women but not black women. |
| Geronimus, 1996 (84) | VLBWd | SES; acute, chronic, or early-life stress–age | For black women but not white women, increasing maternal age was associated with VLBW risk, which was stronger for low-SES than high-SES black mothers. |
| Rauh, 2001 (86) | VLBWd | SES; acute, chronic, or early-life stress–age | Increasing maternal age increases VLBW risk for black women but not white women, a pattern exaggerated among Medicaid-eligible compared with -noneligible black women. |
| Schempf, 2007 (85) | VPT birthc | SES; acute, chronic, or early-life stress—age | Older, multiparous, black women have a higher risk of VPT birth than similar white women do, and this risk is not modified by SES. |
| Kramer, 2008 (171) | VPT birthc | Social environment | Black:white disparities vary significantly across US cities independent of individual SES, explained in part by degree of residential segregation and proportion of black women living in poverty. |
| Khashan, 2009 (71) | VPT birthe | Acute, chronic, or early-life stress | Risk of VPT birth for white Danish women in the index pregnancy increased if the mother experienced death or major illness of an older child in the 6 months prior to conception of the index pregnancy. This association persisted when analyses were restricted to mothers with a prior preterm birth. |
| Neggers, 2006 (61) | VPT birthc | Acute, chronic, or early-life stress | In a predominantly black population, women below the median on a psychosocial stress scale had a nonsignificant increased risk of VPT birth compared with those above the median on the stress scale. |
| Sable, 2000 (62) | VLBWd | Acute, chronic, or early-life stress | Perceived stress and major life events were associated with increased risk of VLBW for black women and white women (not stratified on race). |
| Nabukera, 2009 (172) | VPT birthc VLBWd | Acute, chronic, or early-life stress–age; preconceptional health | The black:white racial disparity in VLBW/VPT birth persists among women postponing their first pregnancy until after age 30 years. An IPI of <6 months is associated with VLBW/VPT birth in the second pregnancy. |
| Goldenberg, 2007 (173) | VPT birthc | Vascular dysfunction | Black women and white women with VPT birth had a similar prevalence of placental lesion, suggesting vasculopathy. |
| Yang, 2001 (174) | VPT birthc | Vascular dysfunction | Vaginal bleeding was associated with increased risk of VPT birth; a greater magnitude of effect was found in white women compared with black women. |
Abbreviations: IPI, interpregnancy interval; PPROM, preterm premature rupture of membranes; SES, socioeconomic status; VLBW, very low birth weight; VPT, very preterm.
Entries in this column refer to nodes in Figure 2 that were evaluated in each study.
VPT birth was defined as <34 weeks’ gestation.
VPT birth was defined as <32 weeks’ gestation.
VLBW was defined as weight <1,500 g.
VPT birth was defined as <33 weeks’ gestation.
VPT birth was defined as <28 weeks’ gestation.
CONCEPTUAL FRAMEWORK
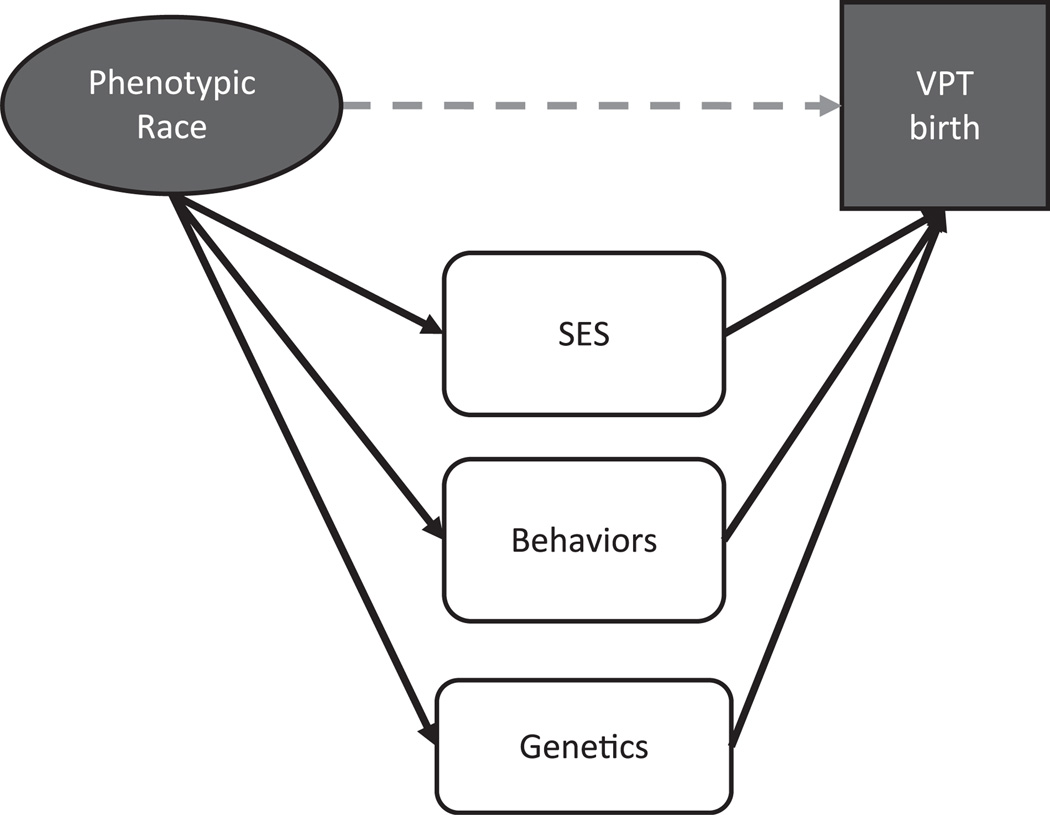
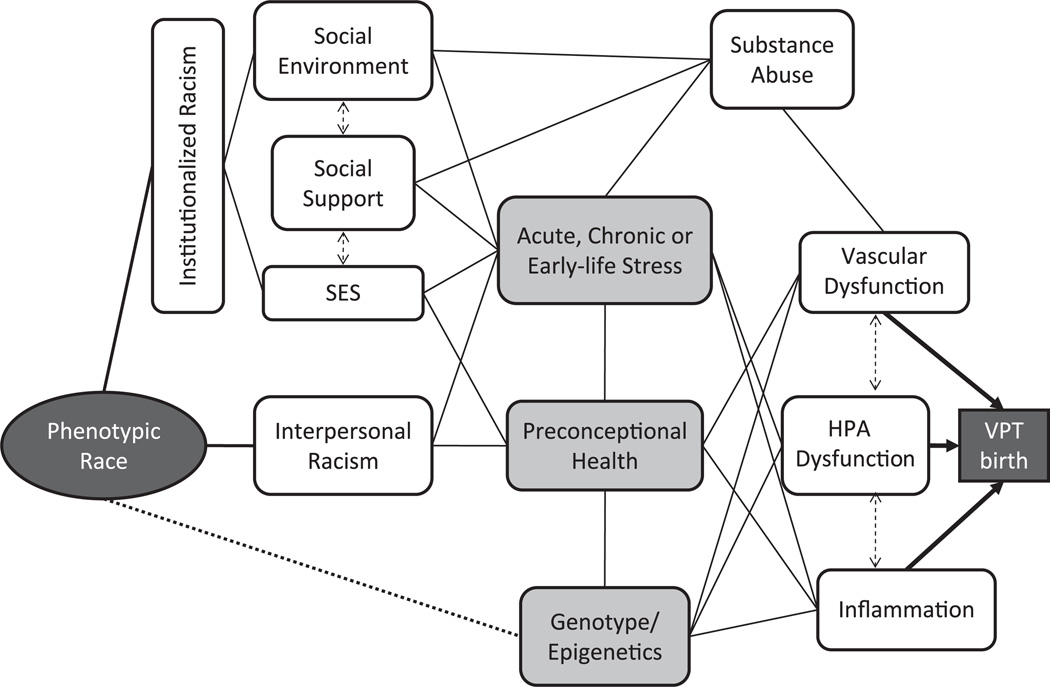
The conceptual framework used for investigating the etiology of a disparity in VPT birth affects what is included as potential causal agents. The bulk of the reviewed literature sought to explain the observed association between the socially constructed category of race and the outcome of prematurity (represented by the dotted line in Figure 1) as mediated by socioeconomic status, individual behaviors, and/or genetics. Residual association after statistical control for proxies of socioeconomic status or known risk factors has often been assumed to illuminate the role of genetic predisposition in explaining the disparity (13, 14). However, it has become increasingly clear that prematurity is a complex disease, and attribution of residual variation to an unmeasured variable may not adequately represent this complexity. Our initial review of the literature gave rise to an alternate conceptual framework explaining the observed association between phenotypic or socially constructed race and the outcome of VPT birth (Figure 2).
Figure 1.
Traditional conceptual framework for explaining the association of race with very preterm (VPT) birth (<32 weeks’ gestation). SES, socioeconomic status.
Figure 2.
Alternative conceptual framework for understanding the association of race with very preterm (VPT) birth (<32 weeks’ gestation). HPA, hypothalamic-pituitary-adrenal axis; SES, socioeconomic status.
Briefly, this conceptual framework suggests that 3 primary proximate biologic pathways mediate the racial disparity in VPT birth: uteroplacental vascular dysfunction, placental and maternal hypothalamic-pituitary-adrenal–axis dysfunction, and maternal-fetal inflammation (refer to Challis (15), Lockwood and Kuczynski (16), and Behrman and Butler (17) for further discussion of these biologic pathways). At the most distal end of the framework, there are 3 possible linkages between phenotypic race and subsequent biologic variations: the latent variable institutionalized racism, which explains the racial differences in a wide variety of socioeconomic exposures; interpersonal racism; and possibly a correlation between phenotypic race in the United States and genotype or epigenetic gene expression. Between these distal and proximal nodes are a wide variety of hypothetical intermediary pathways. To our knowledge, no study to date has attempted to describe the entirety of this complexity. However, we identified 3 frequently hypothesized intermediaries between race and VPT birth, indicated in light grey in Figure 2: maternal experiences of psychosocial stress, periconceptional maternal health status, and genetic predisposition or gene-environment/epigenetic modification of risk. We use these 3 nodes to structure the review of evidence for various pathways.
THE STRESS HYPOTHESIS
The stress hypothesis for perinatal outcomes tends to focus on 2 types and time periods of psychosocial stress: acute stress during pregnancy or life-course exposure to stressors prior to conception. Acute perinatal stressors may be pregnancy-related anxiety or major life events such as divorce, major illness, or death of a loved one. Preconceptional stressors could also be major life events but may have been temporally distant from the pregnancy, as in the case of adverse childhood events (physical or sexual abuse, death of a parent). Alternatively, preconceptional stressors could be conceptualized as chronic over a significant portion of the woman’s life, as would occur from long-time exposure to poverty, perceived discrimination, high-crime neighborhoods, or ongoing abuse.
The most proximate mechanisms commonly used to explain the effects of maternal stress on prematurity are dysfunction of the maternal-placental-fetal hypothalamic-pituitary-adrenal axis or alteration of maternal susceptibility and immune response to infection. A broader explanation first articulated by Geronimus is termed the “weathering” hypothesis (18). This idea posits that chronic exposure to psychosocial and environmental stressors prematurely ages exposed women, shifting age-related risk to the peak of reproductive activity. In the following paragraphs, evidence for each of these hypotheses is discussed and then applied to VPT birth.
Biologic pathways from stress to preterm birth
Hypothalamic-pituitary-adrenal dysfunction
Because the hypothalamic-pituitary-adrenal axis is so relevant in both normal physiologic responses to psychosocial stress (19) and the regulation and timing of birth, this system has garnered a great deal of attention in terms of understanding preterm birth (20–22). In the normal pregnancy, both the absolute levels of placentally produced corticotropin-releasing hormone (CRH) and its rate of increase throughout pregnancy may trigger the eventual cascade resulting in cervical softening, weakening of the fetal membranes, uterine contraction, and birth (23). The question of a neuroendocrine role in premature birth is raised by the observation that measured maternal serum CRH is elevated in women who subsequently deliver prematurely (24–26). Data from prospective cohorts note increases in both absolute CRH and the trajectory of CRH increase as early as the midsecond trimester in women who subsequently deliver preterm compared with those who deliver at term (27–29). Thus, CRH may be a marker of a “placental clock” that is set early in gestation and determines length of gestation (30–32).
Observed racial differences in maternal median CRH have been reported, but with differing findings. Herrmann et al. (33) reported an overrepresentation of black women in the “high CRH” group compared with white women. Holzman et al. (34) found differences in maternal CRH measured at 15–19 weeks’ gestation in a multiracial, nested case-control study. Black women who delivered both at term and preterm had lower median CRH levels compared with white women in either group; however, within each racial group, CRH was negatively associated with length of gestation, and this association was stronger for black women than white women. Wadhwa et al. (35) also reported lower CRH and cortisol levels in black compared with white pregnant women but noted elevated adrenocorticotrophic hormone in black women at all gestational ages and a lower gradient of CRH increase across gestational ages.
Hobel et al. (36) reported that maternal perceptions of stress at 18–20 weeks’ gestation explained a significant proportion of the difference in CRH at 28–30 weeks between women who subsequently delivered preterm rather than at term. Maternal stress has also been linked in pregnancy to elevated levels of several other components of the hypothalamic-pituitary-adrenal axis, including adrenocorticotrophic hormone, β-endorphin, and cortisol (37). Although some general patterns of maternal stress, elevated CRH, and spontaneous preterm birth are consistent across racial groups, differences do exist. Glynn et al. (38) reported patterns of CRH, cortisol, and adrenocorticotrophic hormone for black women that are similar to the hypothalamic-pituitary-adrenal dysregulation seen in posttraumatic stress disorder and other chronic stress syndromes and are distinct from those patterns seen in acute stress responses.
Immune and vascular dysfunction
Psychosocial stress may also lead to preterm birth by altering immune system function either independently or in interaction with the neuroendocrine dysfunction (39). A review of 300 studies of the relation between psychological stress and immune response found that the impact varied depending on the chronicity of the stress (40). Acute stressors tended to up-regulate immune responses, whereas chronic stressors tended to suppress both cellular and humoral immune activity. Evidence from both pregnant and nonpregnant populations supports the connection of stress and immune status.
C-reactive protein is a general marker of inflammation that may be predictive of preterm birth (41). One study in a nonpregnant cohort found that the count of adverse life events was the strongest predictor of C-reactive protein and that black women reported approximately twice as many such experiences as white women did (42).
Bacterial vaginosis is a common vaginal infection implicated as a risk factor for VPT birth and is more prevalent in black compared with white women (43–46). In a longitudinal follow-up study of 3,614 nonpregnant women, stress was associated with both overall prevalence and incidence of bacterial vaginosis (47). When a case-crossover analysis was conducted, each point on a 5-point calculated stress scale conferred a 2-fold (95% confidence interval: 1.1, 3.6) elevated risk of bacterial vaginosis. In a smaller cross-sectional study of nonpregnant black women in New York City, the odds ratio for the effect of stress on bacterial vaginosis was 1.4 (95% confidence interval: 0.95, 2.1) (48). Culhane et al. (49) found that moderate to high levels of perceived stress conferred an independent 2.2-fold (95% confidence interval: 1.1, 4.2) increased risk of bacterial vaginosis in low-income pregnant women, but Trabert and Misra (50) found no association between hassles, anxiety, or social support and the prevalence of bacterial vaginosis in a sample of black pregnant women. Stress may also be associated with inflammatory periodontal disease, which itself has been associated with preterm birth and is more prevalent in black compared with white women (51–53).
In addition to directly impacting neuroendocrine or immune function in pregnancy, stress could also alter vascular function, resulting in altered placental blood flow or increased exposure to risk factors such as hypertension (54, 55). In a study of stress and cardiovascular reactivity during pregnancy in active-duty military women, black women had significantly greater reactivity than white women did, which was associated with higher risk of preterm birth (56). In a prospective study of maternal hostility or anomie (alienation from social norms), higher anomie and hostility were associated with increased systolic and diastolic blood pressures and increased heart rate (57). For black women, anomie was associated with medically indicated preterm delivery, and, for black women and white women, hostility was associated with spontaneous preterm birth.
Epidemiology of stress and prematurity
Stress as an exposure is a challenging construct for epidemiologic research because it is difficult to categorize and measure (58). Stress has been conceived of as anxiety trait, acutely stressful insults, or chronic and persistent hassle. Savitz and Pastore’s review (59) of 20 studies of stress and preterm delivery suggested that anxiety states were not associated with preterm birth, whereas general stress was, with relative risk estimates ranging from 1.2 to 1.8. Subsequent to the Savitz and Pastore review, the 2007 Institute of Medicine report (17) on preterm birth identified 11 new studies and suggested that anxiety might be more important in terms of preterm birth for white women and that depression and posttraumatic stress disorder might be more important for black women. One study of black women reported little impact of anxiety but a significant association between intrusive thoughts and preterm birth (60), suggesting a possible role for preexisting traumatic life experiences in the perception of stress during pregnancy. A study with predominantly black participants found a nonsignificant increased risk of VPT birth for women with psychosocial stress scores below the median (indicating “poor psychosocial health”) compared with above the median (odds ratio = 1.3, 95% confidence interval: 0.9, 2.0) (61). A population-based case-control study found that women who “almost always felt stress” during pregnancy had 60% higher odds of delivering a very low birth weight infant when compared with women reporting some or no stress (62).
Stress has also been conceptualized as exposure to adverse life events such as homelessness, death of a loved one, or being a victim of crime or physical and sexual abuse. A small study of black women found that a higher number of adverse life events in the year prior to and during pregnancy was associated with shortened gestational age (60). Two studies in racially mixed and one in all-black populations found approximately 2-fold elevated risks of preterm birth associated with number of stressful major life events (63–65), but 2 others found no such association (66, 67). Adverse childhood events, including sexual abuse, were also associated with preterm birth in 2 studies (68, 69) but not in a third (70) (as reviewed in Cammack, A, unpublished manuscript, Emory University). A Danish population-based study reported elevated risk of VPT birth for mothers who experienced the death of an older child in the 6 months preceding conception of the index pregnancy (odds ratio = 1.7, 95% confidence interval: 1.2, 2.4) (71).
Another characterization of stressful experiences is perceptions of discrimination or interpersonal racism throughout the life course or during pregnancy (72–74). A 2007 poll by the Pew Research Center found that 81% of blacks in the United States report “frequent” experiences of discrimination in at least one of the following categories: applying for a job, eating in a restaurant, renting or buying a house, or applying to a university or college, suggesting that perceptions of discrimination and racism were prevalent exposures among blacks as recently as 2007 (75).
A handful of studies have evaluated experiences of racism in relation to preterm birth. Collins et al. (76–78) reported similar findings in 2 hospital-based case-control studies of a low-income, inner-city population. In each study— restricted to black mothers and their infants—mothers of very low birth weight infants were approximately 3 times as likely as mothers of normal birth weight infants to have experienced racial discrimination. In the CARDIA prospective cohort, Mustillo et al. (79) found that experiences of discrimination doubled the risk of self-reported preterm birth. In this study, control for experiences of discrimination reduced the black-white disparity by 50%. Dominguez et al. (80) reported a negative association for black women but not white women between maternal perceived racism and birth weight after controlling for medical risk factors and other types of stress. Finally, the authors of 2 additional prospective studies found a 40%–80% elevated risk of preterm birth associated with perceived racism or discrimination (63, 81, 82).
Although each of the above-mentioned classes of stress could be measured solely for the duration of the pregnancy, other authors propose a life-course model in which stress, resilience, and social support interact to impact reproductive health prior to conception (22, 83). Geronimus’ weathering hypothesis contributes to the life-course perspective by suggesting that preconceptional exposures lead to premature aging and dysfunction in endocrine and immune function for some women. Circumstantial support for this hypothesis comes from racial and socioeconomic differences in maternal age and the risk profile for preterm birth. In general, risk of VPT birth is highest at the extremes of maternal age (<18 or >35 years of age), with the lowest risk in the mid-twenties. However, when stratified by race, the entire U-shaped curve for black women, and particularly for poor, black women, shifts to the younger ages compared with that for white women (84). This pattern has been replicated for preterm and VPT after adjusting for parity (85) and for very low birth weight, where the left shift was exacerbated for women of lower socioeconomic status (86). However, Ananth et al. (87) found no support for the weathering effect on preterm birth in an age-period-cohort analysis of births from 1975 to 1990. The observation that the risk of recurrent VPT birth is higher for black compared with white women also points to a life-course explanation, although whether it is genetic, epigenetic, or evidence of weathering is unclear (88, 89).
Distal sources of stress and prematurity
In addition to stress being mediated by individual-level characteristics (anxiety state, experience of abuse or violence), it has also been suggested that differential population patterns of stress exposure could result from distal social and economic contextual exposures (90). This pathway suggests that living in an area of higher crime, neighborhood deprivation, or concentrated poverty may increase the chronic stress experiences of all inhabitants net of other individual-level risk factors such as smoking, maternal education, and medical conditions. As an extension of the weathering hypothesis, Cerda´ et al. (91) looked at the slope of the association between maternal age and birth weight in 342 Chicago, Illinois, neighborhoods. They reported significant neighborhood-level variation in this slope, with neighborhoods characterized by a negative slope (e.g., birth weights decline with increased maternal age) having a higher proportion of black mothers and a greater poverty concentration. The association of concentrated poverty with a negative maternal age–birth weight slope persisted with control for race and other individual-level risk factors, and the age–birth weight slopes for neighborhoods without such a concentration of poverty were positive.
Whether such contextual exposures act through a stress pathway is unclear. One study utilized direct observation of neighborhoods combined with census data to characterize the social context of low-income pregnant women in terms of psychosocial stressors versus material deprivation (92). They reported notably different environments for poor white compared with poor black pregnant women, with black women more likely to live in environments with greater physical incivility and fewer amenities such as sidewalks. In evaluating the association of psychosocial stress with bacterial vaginosis, Paul et al. (93) also explored the role of neighborhood context. They found that although neighborhood socioeconomic status was associated with prevalent bacterial vaginosis in white women, it was not for black women; only the number of stressful life events was significant. On the other hand, Culhane et al. (94) reported that accounting for both individual as well as contextual stressors such as crime rate or prevalence of homelessness significantly reduced the black-white disparity in bacterial vaginosis for low-income pregnant women.
Living in neighborhoods with high levels of violent crime could also be a source of chronic stress. For example, increased rates of violent crime are associated with decreased birth weight for both black women and white women (95), and Messer et al. (96) reported similar findings for preterm birth, although the association was statistically significant for only black women. While these and other contextual exposures appear to increase the risk of poor pregnancy outcomes, evidence also exists that some neighborhood characteristics reduce the risk for black women. In a study of the effect of residential segregation on preterm birth, one type of segregation (racial clustering) was protective against preterm birth after controlling for degree of racial-isolation segregation (97). The authors suggested that social ties and networks resulting from an ethnic enclave effect offset some of the negative stressors of neighborhood environment. Similarly, 2 studies reported that, for poor black women, living in a relatively wealthier neighborhood is protective against preterm birth if that neighborhood is predominantly black; in racially mixed neighborhoods, there is no longer a protective effect of this income incongruity between individual and median neighborhood income (98, 99).
Finally, the stress pathway may interact with known risk behaviors in a way that leads to preterm birth. For example, smoking is a modest risk factor for prematurity and is generally less prevalent among black compared with white pregnant women (67, 100–103). Bell et al. (104) looked at the prevalence of smoking among black pregnant women as a function of metropolitan area residential segregation. They report a U-shaped association between segregation and smoking, concluding that, in highly segregated cities, urban stressors and targeted tobacco marketing in black neighborhoods result in increased prevalence, whereas, in cities with the lowest amount of segregation, smoking prevalence increases because of equivalent behaviors of white women and black women. In another study, increased psychosocial stress and lower social network support was predictive of smoking, alcohol, and substance use (marijuana and “hard drugs”) for low-income pregnant black women (105). For white women, physical abuse, but not social support, was predictive of these risky pregnancy behaviors.
PRE- AND PERICONCEPTIONAL MATERNAL HEALTH
Although acute or chronic psychosocial stress is hypothesized to interact with maternal health, there is also evidence that other aspects of preconceptional and prenatal health status could transmit racially disparate pregnancy outcomes. Identifying modifiable health status indicators of preterm birth risk could allow implementation of effective interventions through delivery of health services such as primary care, prenatal care, family planning services, and health education.
Preconceptional health
Many facets of a woman’s health prior to conception could possibly influence the probability of carrying the pregnancy to term (106). Data from 26 states’ Pregnancy Risk Assessment Monitoring Systems suggest that, prior to conception of an index pregnancy, black women are more likely than white women to have prevalent diabetes, hypertension, and anemia and are less likely to be taking multivitamins or to have had prepregnancy health counseling or recent dental care (107). The following paragraphs review evidence for the role of systemic inflammation, altered vascular function, and general access to well-woman health care.
Infection and inflammation
Maternal genital tract infection and the resulting host inflammatory response are associated with as many as 30%–70% of preterm births, with an inverse association between prevalence of culture-positive amniotic fluid and gestational age (108–111). Culture-confirmed infection is not only highly prevalent in VPT birth but also more common among VPT births to black women compared with white women (46, 112). Although genital tract infections such as bacterial vaginosis are presumably the leading culprit (113), growing evidence for an association between periodontal gum disease and VPT birth suggests that host inflammatory response distinct from a site-specific infectious organism may be relevant (53, 114).
Attempts to reduce preterm birth risk by treating prevalent genital tract or dental disease during pregnancy have proved disappointingly ineffective (115, 116). What remains unclear is whether preconceptional treatment of such infections would reduce subsequent pregnancy risk. One trial of interconceptional antibiotic treatment for women with a prior preterm birth failed to demonstrate reduced risk in the subsequent pregnancy (117, 118). Some evidence exists that prenatal treatment of severe periodontal disease reduces subsequent risk of preterm birth (119), and one pilot trial of interconceptional care (including primary care and dental treatment) for women with a prior very low birth weight infant showed a reduced risk of subsequent very low birth weight delivery for black women (120).
Chronic disease
The observation that women who deliver an infant preterm have a subsequently increased risk of cardiovascular disease suggests linkage between pregnancy outcome and life-course chronic disease (121, 122). Preexisting maternal hypertension or diabetes mellitus can lead to placental insufficiency, heightened risk of preeclampsia, and indicated preterm birth. The rising population prevalence of hypertension and type 2 diabetes at younger and younger ages may make these causes more important in pregnancy outcomes. Chronic and gestational diabetes increase the risk of preterm birth by 30%–90%, and chronic hypertension can increase the risk approximately 2-fold (123–125). Even though the magnitude of the effect is similar between races, the preconceptional prevalence of both diabetes and hypertension is higher among black women than white women (107, 125). However, population studies do not show an appreciably diminished magnitude of the racial disparity in VPT birth after control for preconceptional health status (102, 123).
Grady and Ramirez (126) attempted to estimate the role of preconceptional chronic disease as a mediating factor between distal social exposures and subsequent low birth weight. They concluded that, for black women, some of the association between neighborhood residential segregation in New York City and low birth weight is mediated by the increased prevalence of chronic and pregnancy-related hypertension among women in those neighborhoods.
Well-woman care
Perhaps one of the biggest challenges for the traditional approach to pregnancy care is the notion that pregnancy is not an isolated island of health or lack of health, but rather a period integrally connected to the life-course health of women (and men) (127). Factors that may be relevant to this life-course perspective include access to primary health care and issues of family planning, including pregnancy intention and interpregnancy interval.
Having health insurance is associated with use of effective contraception and preconceptional use of multivitamins, but adequate coverage outside of pregnancy is unavailable to many, particularly low-income women of color (128–130). Pregnancy intention is closely linked to access to and use of effective contraception (131), and conceiving an unintended or unwanted pregnancy is associated with preterm delivery (132–134). The length of the inter-pregnancy interval is also tied to effective access to primary health care service, and it appears to be strongly related to the occurrence of VPT birth, with shortened intervals of less than 6 months doubling the risk compared with an ideal interval of 18–23 months (135–137). Finally, in one study, users of preconceptional multivitamins appeared to be at lower risk of VPT birth, and black women were half as likely to use multivitamins (138).
While there are racial differences in access to primary health services, there is also evidence that perceived racial discrimination in seeking health care is associated with poor self-reported health for black adults, irrespective of health insurance status (139). Whether this finding is relevant for reproductive health specifically is unknown.
Prenatal health
Intuitively, pregnancy complications could best be prevented by application of pregnancy-related health care. Unfortunately, prenatal care is notable for its lack of expected effect on preterm birth. Early observational research found a protective effect of adequate prenatal care on the risk of preterm birth and low birth weight (140, 141). These results were likely a product of selection bias because women at low risk of preterm birth are also high users of prenatal care. A randomized trial of aggressive prenatal education and care for high-risk women found no difference in preterm birth risk between the intervention and control groups, nor any appreciable difference between study participants (who all received care) and population estimates for preterm birth (142). Similarly, a population-based study limited to participants who all entered prenatal care in the first trimester reported rates and disparities of preterm birth and perinatal mortality similar to those for nonrestricted populations (143). Whether specialized, risk-targeted prenatal care will prove more useful remains to be shown, although some models are promising (144, 145).
GENETIC AND EPIGENETIC PATHWAYS TO PREMATURITY
The body of literature that mentions genetics as an explanation for the racial disparity in preterm birth is large, but notable for rarely measuring genes; most studies suggest that residual racial variation in preterm birth risk is genetic after statistical control for measured socioeconomic and behavioral risk factors. This could be problematic because of the persistence of residual socioeconomic confounding in even the richest of data sets commonly available for epidemiologic research (146), as well as the complex causal web suggested by Figure 2. Only relatively recently have specific hypotheses of variation in measured genetic and epigenetic (e.g., variation in gene expression in the absence of varying DNA sequence) traits been proposed and tested. While these early studies are promising, they suffer from the same problems of multiple testing, reproducibility, sample size, and general “needle-in-a-haystack” phenomena seen in other areas of genetic research in a post–Human Genome Project era (147).
The utility of considering genetics at all in understanding racial disparities in health is being vigorously debated (refer to Ioannidis et al. (148), Kaufman and Cooper (149), and Frank (150) for further discussion). However, there are 3 scenarios in which genetic research could be helpful in understanding the racial disparity in prematurity: 1) identification of genetic polymorphisms that are causally linked to risk of preterm birth and are differentially prevalent among black women and white women; 2) identification of genetic polymorphisms that are equally prevalent by race but that interact with social or environmental exposures that are differentially prevalent by race; or 3) identification of social or environmental exposures that are differentially prevalent by race and that alter normal genetic expression, resulting in an epigenetic racial variation in protein synthesis or function. Of the small body of literature seeking specific genetic mechanisms responsible for preterm birth, most assume that the first scenario is true, although arguably the second and third offer the most opportunity for intervention.
In one of the largest-scale candidate gene studies to date, Velez et al. (151) evaluated the association between 1,432 single nucleotide polymorphisms in 130 candidate genes from the inflammatory, vascular, and neuroendocrine pathways and related them to gestational age for pregnant black women. Their findings are consistent with some previous literature, in that the strongest associations between genotype and the phenotype of preterm birth were in the infection and inflammation pathway. The most commonly studied genes in this pathway are those regulating interleukin-1-beta, interleukin-6, and tumor necrosis factor-alpha. Findings regarding racial differences in prevalence of candidate gene polymorphisms vary, even for the same polymorphism, with a few reports of difference for tumor necrosis factor (152– 154) and interleukin-6 genes (152, 155). In only one of these studies (155) is the observed racial difference in polymorphism prevalence explanatory of preterm case-control status (P = 0.04), an association not corrected for multiple comparisons. Simhan et al. (156) found that a polymorphism of the interleukin-6 promoter region was protective against VPT birth for white women but was completely absent in the 58 black women cases or controls in the study.
Studies that jointly consider genetic polymorphisms and the presence of bacterial vaginosis suggest that gene-environment interactions may be important. Macones et al. (157) reported a similar prevalence of tumor necrosis factor alleles in blacks and whites but noted (for both blacks and whites) a 6-fold elevated risk of preterm delivery for carriers of the rarer variant who also had bacterial vaginosis. Similar interactions were reported for tumor necrosis factor (153, 158, 159) and interleukin-1 (158, 160). A single report of an interaction between maternal smoking and a mitochondrial DNA variant suggests that other gene-environment interactions may be worth examining as well (161). Fortunato et al. (162) reported different racial patterns of gene-gene interactions between tumor necrosis factor-alpha and interleukin-6 genes in relation to preterm birth but did not evaluate whether these different patterns account for any of the disparity.
While promising, genetic studies to date have been limited by small sample sizes and the near absence of research on births of <32 weeks’ gestation compared with <37 weeks’ gestation. If VPT births are at all etiologically distinct from the more common late-preterm births, this exclusion could mean a heterogeneous mix in the case population, reducing power to identify important pathways.
DISCUSSION
Simple approaches have thus far failed to explain the long-standing problem of racial disparities in prematurity. Evidence that the biologic pathways, maternal risk profiles, and magnitude of the racial disparity all vary between extreme and moderate categories of prematurity means that pooling all preterm births obscures patterns that may be informative. Wilcox (8) notes that a birth weight of <1,500 g (an outcome highly correlated with VPT birth) is a crude measure of the size of the residual distribution of births composed of truly high-risk infants, emphasizing again the etiologic and epidemiologic distinction between extreme and moderate outcomes.
We found growing evidence to support the role of socially patterned maternal stress, possibly over the life course, as a cause of racial disparities in VPT birth. We also found evidence for racial differences in the propensity for a pregnant woman to deliver prematurely because of host inflammatory response or vascular and neuroendocrine dysfunction. What is largely missing in the literature, with a few exceptions, are studies that informatively combine these 2 patterns in ways that increase our understanding and illuminate opportunities for effective intervention. Figure 2 is an attempt to depict how these patterns could be studied, although it just begins to represent the complex causal pathways that may link the social construct of race to the serious biologic outcome of VPT birth. Future studies should incorporate a rich causal framework while attending to outcome specificity, study design, and data sources.
When possible, a broader selection of potential factors should be incorporated into the study design. For example, social and environmental factors that are measured precisely with established instruments should be included routinely in studies primarily focused on finding genetic differences. Likewise, hypothesized biologic markers should be routinely incorporated into all preterm birth studies of racial disparities.
Epidemiologists have long critiqued the overuse of readily available, large-scale vital statistics data sets. It is clear that vital statistics data sets have many limitations in terms of testing hypotheses, even when they appear to have a variable that closely proxies the exposure or outcome of interest. Nonetheless, for questions of racial disparities in a relatively rare outcome, the benefits are undeniable. Understanding geographic variation, racial differences in maternal age-related risk, and differences in mortality by gestational age would be cost prohibitive without use of vital statistics.
However, if researchers are to explore the more complicated linkages between race and VPT birth suggested by Figure 2, new approaches will be needed. Creative ways to combine vital statistics (e.g., maternally linked or inter-generationally linked birth records) with richer clinical and social information, or approaches that identify cohorts of prepregnant women and follows them for risk, may allow exploration of some of the life-course questions raised.
Clearly, one of the biggest challenges in addressing the racial disparity in VPT birth is our incomplete understanding of what the causal web looks like. Whereas, on some level, all disease may boil down to a combination of genes, environment, and individual behaviors, understanding the reason for population differences in health requires more sophistication, or at least a very broad view of how each of these interact. At a minimum, researchers should articulate the causal thinking that underlies their research, including identifying the section(s) of the causal network represented in their work and addressing how the missing section(s) limits their results. Eventually, results from these studies that individually answer only a small part of the puzzle may be pieced together to form a coherent explanation that will lead to meaningful interventions and elimination of the racial disparity in VPT birth.
ACKNOWLEDGMENTS
Author affiliation: Women’s and Children’s Center, Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, Georgia (Michael R. Kramer, Carol R. Hogue).
This work was supported by a Health Resource and Service Administration Maternal and Child Health training grant (T03MC07651 to M. K.) and a National Institute of Health Reproductive, Perinatal, and Pediatric Health Training grant (T32 HD052460 to M. K.).
Abbreviations
- CRH
corticotropin-releasing hormone
- VPT
very preterm
Footnotes
Conflict of interest: none declared.
REFERENCES
- 1.Hamilton BE, Martin JA, Ventura SJ. Births: preliminary data for 2006. Natl Vital Stat Rep. 2007;56(7) [PubMed] [Google Scholar]
- 2.Callaghan WM, MacDorman MF, Rasmussen SA, et al. The contribution of preterm birth to infant mortality rates in the United States. Pediatrics. 2006;118(4):1566–1573. doi: 10.1542/peds.2006-0860. [DOI] [PubMed] [Google Scholar]
- 3.MacDorman MF, Callaghan WM, Mathews TJ, et al. Trends in preterm-related infant mortality by race and ethnicity, United States, 1999–2004. Int J Health Serv. 2007;37(4):635–641. doi: 10.2190/HS.37.4.c. [DOI] [PubMed] [Google Scholar]
- 4.Colvin M, McGuire W, Fowlie PW. Neurodevelopmental outcomes after preterm birth. BMJ. 2004;329(7479):1390–1393. doi: 10.1136/bmj.329.7479.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saigal S. Follow-up of very low birth weight babies to adolescence. Semin Neonatol. 2000;5(2):107–118. doi: 10.1053/siny.1999.0003. [DOI] [PubMed] [Google Scholar]
- 6.Schoendorf KC, Hogue CJ, Kleinman JC, et al. Mortality among infants of black as compared with white college-educated parents. N Engl J Med. 1992;326(23):1522–1526. doi: 10.1056/NEJM199206043262303. [DOI] [PubMed] [Google Scholar]
- 7.Basso O, Wilcox AJ, Weinberg CR. Birth weight and mortality: causality or confounding? Am J Epidemiol. 2006;164(4):303–311. doi: 10.1093/aje/kwj237. [DOI] [PubMed] [Google Scholar]
- 8.Wilcox AJ. On the importance—and the unimportance—of birthweight. Int J Epidemiol. 2001;30(6):1233–1241. doi: 10.1093/ije/30.6.1233. [DOI] [PubMed] [Google Scholar]
- 9.Dietz PM, England LJ, Callaghan WM, et al. A comparison of LMP-based and ultrasound-based estimates of gestational age using linked California livebirth and prenatal screening records. Paediatr Perinat Epidemiol. 2007;21(suppl 2):62–71. doi: 10.1111/j.1365-3016.2007.00862.x. [DOI] [PubMed] [Google Scholar]
- 10.Ananth CV. Menstrual versus clinical estimate of gestational age dating in the United States: temporal trends and variability in indices of perinatal outcomes. Paediatr Perinat Epidemiol. 2007;21(suppl 2):22–30. doi: 10.1111/j.1365-3016.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- 11.Haglund B. Birthweight distributions by gestational age: comparison of LMP-based and ultrasound-based estimates of gestational age using data from the Swedish Birth Registry. Paediatr Perinat Epidemiol. 2007;21(suppl 2):72–78. doi: 10.1111/j.1365-3016.2007.00863.x. [DOI] [PubMed] [Google Scholar]
- 12.Alexander GR, Kogan M, Bader D, et al. US birth weight/ gestational age-specific neonatal mortality: 1995–1997 rates for whites, Hispanics, and blacks [electronic article] Pediatrics. 2003;111(1):e61–e66. doi: 10.1542/peds.111.1.e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esplin MS. Preterm birth: a review of genetic factors and future directions for genetic study. Obstet Gynecol Surv. 2006;61(12):800–806. doi: 10.1097/01.ogx.0000248747.52343.5f. [DOI] [PubMed] [Google Scholar]
- 14.DeFranco E, Teramo K, Muglia L. Genetic influences on preterm birth. Semin Reprod Med. 2007;25(1):40–51. doi: 10.1055/s-2006-956774. [DOI] [PubMed] [Google Scholar]
- 15.Challis JRG. Mechanism of parturition and preterm labor. Obstet Gynecol Surv. 2000;55(10):650–660. doi: 10.1097/00006254-200010000-00025. [DOI] [PubMed] [Google Scholar]
- 16.Lockwood CJ, Kuczynski E. Risk stratification and pathological mechanisms in preterm delivery. Paediatr Perinat Epidemiol. 2001;15(suppl 2):78–89. doi: 10.1046/j.1365-3016.2001.00010.x. [DOI] [PubMed] [Google Scholar]
- 17.Behrman RE, Butler AS, editors. Preterm Birth: Causes, Consequences, and Prevention. Washington, DC: National Academy Press, Institute of Medicine; 2007. [PubMed] [Google Scholar]
- 18.Geronimus AT. The weathering hypothesis and the health of African-American women and infants: evidence and speculations. Ethn Dis. 1992;2(3):207–221. [PubMed] [Google Scholar]
- 19.de Weerth C, Buitelaar JK. Physiological stress reactivity in human pregnancy—a review. Neurosci Biobehav Rev. 2005;29(2):295–312. doi: 10.1016/j.neubiorev.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Rich-Edwards JW, Grizzard TA. Psychosocial stress and neuroendocrine mechanisms in preterm delivery. Am J Obstet Gynecol. 2005;192(5 suppl):S30–S35. doi: 10.1016/j.ajog.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 21.Hobel CJ, Goldstein A, Barrett ES. Psychosocial stress and pregnancy outcome. Clin Obstet Gynecol. 2008;51(2):333–348. doi: 10.1097/GRF.0b013e31816f2709. [DOI] [PubMed] [Google Scholar]
- 22.Hogue CJ, Bremner JD. Stress model for research into pre-term delivery among black women. Am J Obstet Gynecol. 2005;192(5 suppl):S47–S55. doi: 10.1016/j.ajog.2005.01.073. [DOI] [PubMed] [Google Scholar]
- 23.Challis JR, Matthews SG, Gibb W, et al. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev. 2000;21(5):514–550. doi: 10.1210/edrv.21.5.0407. [DOI] [PubMed] [Google Scholar]
- 24.Warren WB, Patrick SL, Goland RS. Elevated maternal plasma corticotropin-releasing hormone levels in pregnancies complicated by preterm labor. Am J Obstet Gynecol. 1992;166(4):1198–1204. doi: 10.1016/s0002-9378(11)90606-1. discussion 1204–1207. [DOI] [PubMed] [Google Scholar]
- 25.Wolfe CD, Patel SP, Linton EA, et al. Plasma corticotrophin-releasing factor (CRF) in abnormal pregnancy. Br J Obstet Gynaecol. 1988;95(10):1003–1006. doi: 10.1111/j.1471-0528.1988.tb06504.x. [DOI] [PubMed] [Google Scholar]
- 26.Erickson K, Thorsen P, Chrousos G, et al. Preterm birth: associated neuroendocrine, medical, and behavioral risk factors. J Clin Endocrinol Metab. 2001;86(6):2544–2552. doi: 10.1210/jcem.86.6.7607. [DOI] [PubMed] [Google Scholar]
- 27.Moawad AH, Goldenberg RL, Mercer B, et al. The Preterm Prediction Study: the value of serum alkaline phosphatase, alpha-fetoprotein, plasma corticotropin-releasing hormone, and other serum markers for the prediction of spontaneous preterm birth. Am J Obstet Gynecol. 2002;186(5):990–996. doi: 10.1067/mob.2002.121727. [DOI] [PubMed] [Google Scholar]
- 28.McLean M, Smith R. Corticotropin-releasing hormone in human pregnancy and parturition. Trends Endocrinol Metab. 1999;10(5):174–178. doi: 10.1016/s1043-2760(98)00146-5. [DOI] [PubMed] [Google Scholar]
- 29.Wadhwa PD, Garite TJ, Porto M, et al. Placental corticotropin-releasing hormone (CRH), spontaneous preterm birth, and fetal growth restriction: a prospective investigation. Am J Obstet Gynecol. 2004;191(4):1063–1069. doi: 10.1016/j.ajog.2004.06.070. [DOI] [PubMed] [Google Scholar]
- 30.McLean M, Bisits A, Davies J, et al. A placental clock controlling the length of human pregnancy. Nat Med. 1995;1(5):460–463. doi: 10.1038/nm0595-460. [DOI] [PubMed] [Google Scholar]
- 31.Smith R, Mesiano S, McGrath S. Hormone trajectories leading to human birth. Regul Pept. 2002;108(2–3):159–164. doi: 10.1016/s0167-0115(02)00105-2. [DOI] [PubMed] [Google Scholar]
- 32.Sandman CA, Glynn L, Schetter CD, et al. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides. 2006;27(6):1457–1463. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Herrmann TS, Siega-Riz AM, Hobel CJ, et al. Prolonged periods without food intake during pregnancy increase risk for elevated maternal corticotropin-releasing hormone concentrations. Am J Obstet Gynecol. 2001;185(2):403–412. doi: 10.1067/mob.2001.115863. [DOI] [PubMed] [Google Scholar]
- 34.Holzman C, Jetton J, Siler-Khodr T, et al. Second trimester corticotropin-releasing hormone levels in relation to preterm delivery and ethnicity. Obstet Gynecol. 2001;97(5 pt 1):657–663. doi: 10.1016/s0029-7844(00)01209-6. [DOI] [PubMed] [Google Scholar]
- 35.Wadhwa PD. Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology. 2005;30(8):724–743. doi: 10.1016/j.psyneuen.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Hobel CJ, Dunkel-Schetter C, Roesch SC, et al. Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks’ gestation in pregnancies ending in preterm delivery. Am J Obstet Gynecol. 1999;180(1 pt 3):S257–S263. doi: 10.1016/s0002-9378(99)70712-x. [DOI] [PubMed] [Google Scholar]
- 37.Wadhwa PD, Dunkel-Schetter C, Chicz-DeMet A, et al. Prenatal psychosocial factors and the neuroendocrine axis in human pregnancy. Psychosom Med. 1996;58(5):432–446. doi: 10.1097/00006842-199609000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Glynn LM, Schetter CD, Chicz-DeMet A, et al. Ethnic differences in adrenocorticotropic hormone, cortisol and corticotropin-releasing hormone during pregnancy. Peptides. 2007;28(6):1155–1161. doi: 10.1016/j.peptides.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 39.Culhane JF, Rauh VA, Goldenberg RL. Stress, bacterial vaginosis, and the role of immune processes. Curr Infect Dis Rep. 2006;8(6):459–464. doi: 10.1007/s11908-006-0020-x. [DOI] [PubMed] [Google Scholar]
- 40.Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130(4):601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pitiphat W, Gillman MW, Joshipura KJ, et al. Plasma C-reactive protein in early pregnancy and preterm delivery. Am J Epidemiol. 2005;162(11):1108–1113. doi: 10.1093/aje/kwi323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paul K, Boutain D, Agnew K, et al. The relationship between racial identity, income, stress and C-reactive protein among parous women: implications for preterm birth disparity research. J Natl Med Assoc. 2008;100(5):540–546. doi: 10.1016/s0027-9684(15)31300-6. [DOI] [PubMed] [Google Scholar]
- 43.Leitich H, Bodner-Adler B, Brunbauer M, et al. Bacterial vaginosis as a risk factor for preterm delivery: a meta-analysis. Am J Obstet Gynecol. 2003;189(1):139–147. doi: 10.1067/mob.2003.339. [DOI] [PubMed] [Google Scholar]
- 44.Ness RB, Hillier S, Richter HE, et al. Can known risk factors explain racial differences in the occurrence of bacterial vaginosis? J Natl Med Assoc. 2003;95(3):201–212. [PMC free article] [PubMed] [Google Scholar]
- 45.O’Brien RF. Bacterial vaginosis: many questions—any answers? Curr Opin Pediatr. 2005;17(4):473–479. doi: 10.1097/01.mop.0000170516.35272.45. [DOI] [PubMed] [Google Scholar]
- 46.Goldenberg RL, Iams JD, Mercer BM, et al. The preterm prediction study: the value of new vs standard risk factors in predicting early and all spontaneous preterm births. NICHD MFMU Network. Am J Public Health. 1998;88(2):233–238. doi: 10.2105/ajph.88.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nansel TR, Riggs MA, Yu KF, et al. The association of psychosocial stress and bacterial vaginosis in a longitudinal cohort. Am J Obstet Gynecol. 2006;194(2):381–386. doi: 10.1016/j.ajog.2005.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harville EW, Hatch MC, Zhang J. Perceived life stress and bacterial vaginosis. J Womens Health (Larchmt) 2005;14(7):627–633. doi: 10.1089/jwh.2005.14.627. [DOI] [PubMed] [Google Scholar]
- 49.Culhane JF, Rauh V, McCollum KF, et al. Maternal stress is associated with bacterial vaginosis in human pregnancy. Matern Child Health J. 2001;5(2):127–134. doi: 10.1023/a:1011305300690. [DOI] [PubMed] [Google Scholar]
- 50.Trabert B, Misra DP. Risk factors for bacterial vaginosis during pregnancy among African-American women [electronic article] Am J Obstet Gynecol. 2007;197(5):477.e1–477.e8. doi: 10.1016/j.ajog.2007.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Borrell LN, Burt BA, Warren RC, et al. The role of individual and neighborhood social factors on periodontitis: the third National Health and Nutrition Examination Survey. J Periodontol. 2006;77(3):444–453. doi: 10.1902/jop.2006.050158. [DOI] [PubMed] [Google Scholar]
- 52.Page RC. The pathobiology of periodontal diseases may affect systemic diseases: inversion of a paradigm. Ann Perio-dontol. 1998;3(1):108–120. doi: 10.1902/annals.1998.3.1.108. [DOI] [PubMed] [Google Scholar]
- 53.Jeffcoat MK, Geurs NC, Reddy MS, et al. Periodontal infection and preterm birth: results of a prospective study. J Am Dent Assoc. 2001;132(7):875–880. doi: 10.14219/jada.archive.2001.0299. [DOI] [PubMed] [Google Scholar]
- 54.Wadhwa PD, Culhane JF, Rauh V, et al. Stress and preterm birth: neuroendocrine, immune/inflammatory, and vascular mechanisms. Matern Child Health J. 2001;5(2):119–125. doi: 10.1023/a:1011353216619. [DOI] [PubMed] [Google Scholar]
- 55.Fiscella K. Racial disparity in infant and maternal mortality: confluence of infection, and microvascular dysfunction. Matern Child Health J. 2004;8(2):45–54. doi: 10.1023/b:maci.0000025726.53515.65. [DOI] [PubMed] [Google Scholar]
- 56.Hatch M, Berkowitz G, Janevic T, et al. Race, cardiovascular reactivity, and preterm delivery among active-duty military women. Epidemiology. 2006;17(2):178–182. doi: 10.1097/01.ede.0000199528.28234.73. [DOI] [PubMed] [Google Scholar]
- 57.Tiedje L, Holzman CB, De Vos E, et al. Hostility and anomie: links to preterm delivery subtypes and ambulatory blood pressure at mid-pregnancy. Soc Sci Med. 2008;66(6):1310–1321. doi: 10.1016/j.socscimed.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O’Campo P, Schempf A. Racial inequalities in preterm delivery: issues in the measurement of psychosocial constructs. Am J Obstet Gynecol. 2005;192(5 suppl):S56–S63. doi: 10.1016/j.ajog.2005.01.074. [DOI] [PubMed] [Google Scholar]
- 59.Savitz DA, Pastore LM. Causes of prematurity. In: McCormick MC, Siegel JE, editors. Prenatal Care: Effectiveness and Implementation. Cambridge, United Kingdom: Cambridge University Press; 1999. pp. 63–104. [Google Scholar]
- 60.Dominguez TP, Schetter CD, Mancuso R, et al. Stress in African American pregnancies: testing the roles of various stress concepts in prediction of birth outcomes. Ann Behav Med. 2005;29(1):12–21. doi: 10.1207/s15324796abm2901_3. [DOI] [PubMed] [Google Scholar]
- 61.Neggers Y, Goldenberg R, Cliver S, et al. The relationship between psychosocial profile, health practices, and pregnancy outcomes. Acta Obstet Gynecol Scand. 2006;85(3):277–285. doi: 10.1080/00016340600566121. [DOI] [PubMed] [Google Scholar]
- 62.Sable MR, Wilkinson DS. Impact of perceived stress, major life events and pregnancy attitudes on low birth weight. Fam Plann Perspect. 2000;32(6):288–294. [PubMed] [Google Scholar]
- 63.Dole N, Savitz DA, Hertz-Picciotto I, et al. Maternal stress and preterm birth. Am J Epidemiol. 2003;157(1):14–24. doi: 10.1093/aje/kwf176. [DOI] [PubMed] [Google Scholar]
- 64.Whitehead N, Hill HA, Brogan DJ, et al. Exploration of threshold analysis in the relation between stressful life events and preterm delivery. Am J Epidemiol. 2002;155(2):117–124. doi: 10.1093/aje/155.2.117. [DOI] [PubMed] [Google Scholar]
- 65.Barbosa GA. The association of life events to gestational age at delivery among low-income, urban, African American women. J Perinatol. 2000;20(7):438–442. doi: 10.1038/sj.jp.7200423. [DOI] [PubMed] [Google Scholar]
- 66.Lu MC, Chen B. Racial and ethnic disparities in preterm birth: the role of stressful life events. Am J Obstet Gynecol. 2004;191(3):691–699. doi: 10.1016/j.ajog.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 67.Goldenberg RL, Cliver SP, Mulvihill FX, et al. Medical, psychosocial, and behavioral risk factors do not explain the increased risk for low birth weight among black women. Am J Obstet Gynecol. 1996;175(5):1317–1324. doi: 10.1016/s0002-9378(96)70048-0. [DOI] [PubMed] [Google Scholar]
- 68.Noll JG, Schulkin J, Trickett PK, et al. Differential pathways to preterm delivery for sexually abused and comparison women. J Pediatr Psychol. 2007;32(10):1238–1248. doi: 10.1093/jpepsy/jsm046. [DOI] [PubMed] [Google Scholar]
- 69.Stevens-Simon C, Kaplan DW, McAnarney ER. Factors associated with preterm delivery among pregnant adolescents. J Adolesc Health. 1993;14(4):340–342. doi: 10.1016/1054-139x(93)90185-r. [DOI] [PubMed] [Google Scholar]
- 70.Benedict MI, Paine LL, Paine LA, et al. The association of childhood sexual abuse with depressive symptoms during pregnancy, and selected pregnancy outcomes. Child Abuse Negl. 1999;23(7):659–670. doi: 10.1016/s0145-2134(99)00040-x. [DOI] [PubMed] [Google Scholar]
- 71.Khashan AS, McNamee R, Abel KM, et al. Rates of preterm birth following antenatal maternal exposure to severe life events: a population-based cohort study. Hum Reprod. 2009;24(2):429–437. doi: 10.1093/humrep/den418. [DOI] [PubMed] [Google Scholar]
- 72.Kessler RC, Mickelson KD, Williams DR. The prevalence, distribution, and mental health correlates of perceived discrimination in the United States. J Health Soc Behav. 1999;40(3):208–230. [PubMed] [Google Scholar]
- 73.Paradies Y. A systematic review of empirical research on self-reported racism and health. Int J Epidemiol. 2006;35(4):888–901. doi: 10.1093/ije/dyl056. [DOI] [PubMed] [Google Scholar]
- 74.Clark R, Anderson NB, Clark VR, et al. Racism as a stressor for African Americans. A biopsychosocial model. Am Psychol. 1999;54(10):805–816. doi: 10.1037//0003-066x.54.10.805. [DOI] [PubMed] [Google Scholar]
- 75.Kohut A, Taylor P, Keeter S. Optimism About Black Progress Declines: Blacks See Growing Values Gap Between Poor and Middle Class. Washington, DC: Pew Research Center; 2007. [Accessed August 2008]. http://pewresearch.org/pubs/634/black-public-opinion. [Google Scholar]
- 76.Collins JW, David RJ, Handler A, et al. Very low birthweight in African American infants: the role of maternal exposure to interpersonal racial discrimination. Am J Public Health. 2004;94(12):2132–2138. doi: 10.2105/ajph.94.12.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Collins JW, David RJ, Symons R, et al. Low-income African-American mothers’ perception of exposure to racial discrimination and infant birth weight. Epidemiology. 2000;11(3):337–339. doi: 10.1097/00001648-200005000-00019. [DOI] [PubMed] [Google Scholar]
- 78.Lespinasse AA, David RJ, Collins JW, et al. Maternal support in the delivery room and birthweight among African-American women. J Natl Med Assoc. 2004;96(2):187–195. [PMC free article] [PubMed] [Google Scholar]
- 79.Mustillo S, Krieger N, Gunderson EP, et al. Self-reported experiences of racial discrimination and Black-White differences in preterm and low-birthweight deliveries: the CARDIA study. Am J Public Health. 2004;94(12):2125–2131. doi: 10.2105/ajph.94.12.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dominguez TP, Dunkel-Schetter C, Glynn LM, et al. Racial differences in birth outcomes: the role of general, pregnancy, and racism stress. Health Psychol. 2008;27(2):194–203. doi: 10.1037/0278-6133.27.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rosenberg L, Palmer JR, Wise LA, et al. Perceptions of racial discrimination and the risk of preterm birth. Epidemiology. 2002;13(6):646–652. doi: 10.1097/00001648-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 82.Dole N, Savitz DA, Siega-Riz AM, et al. Psychosocial factors and preterm birth among African American and White women in central North Carolina. Am J Public Health. 2004;94(8):1358–1365. doi: 10.2105/ajph.94.8.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lu MC, Halfon N. Racial and ethnic disparities in birth outcomes: a life-course perspective. Matern Child Health J. 2003;7(1):13–30. doi: 10.1023/a:1022537516969. [DOI] [PubMed] [Google Scholar]
- 84.Geronimus AT. Black/white differences in the relationship of maternal age to birthweight: a population-based test of the weathering hypothesis. Soc Sci Med. 1996;42(4):589–597. doi: 10.1016/0277-9536(95)00159-x. [DOI] [PubMed] [Google Scholar]
- 85.Schempf AH, Branum AM, Lukacs SL, et al. Maternal age and parity-associated risks of preterm birth: differences by race/ ethnicity. Paediatr Perinat Epidemiol. 2007;21(1):34–43. doi: 10.1111/j.1365-3016.2007.00785.x. [DOI] [PubMed] [Google Scholar]
- 86.Rauh VA, Andrews HF, Garfinkel RS. The contribution of maternal age to racial disparities in birthweight: a multilevel perspective. Am J Public Health. 2001;91(11):1815–1824. doi: 10.2105/ajph.91.11.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ananth CV, Misra DP, Demissie K, et al. Rates of preterm delivery among Black women and White women in the United States over two decades: an age-period-cohort analysis. Am J Epidemiol. 2001;154(7):657–665. doi: 10.1093/aje/154.7.657. [DOI] [PubMed] [Google Scholar]
- 88.Kistka ZA, Palomar L, Lee KA, et al. Racial disparity in the frequency of recurrence of preterm birth [electronic article] Am J Obstet Gynecol. 2007;196(2):131.e1–131.e6. doi: 10.1016/j.ajog.2006.06.093. [DOI] [PubMed] [Google Scholar]
- 89.Adams MM, Elam-Evans LD, Wilson HG, et al. Rates of and factors associated with recurrence of preterm delivery. JAMA. 2000;283(12):1591–1596. doi: 10.1001/jama.283.12.1591. [DOI] [PubMed] [Google Scholar]
- 90.Culhane JF, Elo IT. Neighborhood context and reproductive health. Am J Obstet Gynecol. 2005;192(5 suppl):S22–S29. doi: 10.1016/j.ajog.2005.01.071. [DOI] [PubMed] [Google Scholar]
- 91.Cerda´ M, Buka SL, Rich-Edwards JW. Neighborhood influences on the association between maternal age and birth-weight: a multilevel investigation of age-related disparities in health. Soc Sci Med. 2008;66(9):2048–2060. doi: 10.1016/j.socscimed.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Laraia BA, Messer L, Kaufman JS, et al. Direct observation of neighborhood attributes in an urban area of the US south: characterizing the social context of pregnancy [electronic article] Int J Health Geogr. 2006;5:11. doi: 10.1186/1476-072X-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Paul K, Boutain D, Manhart L, et al. Racial disparity in bacterial vaginosis: the role of socioeconomic status, psychosocial stress, and neighborhood characteristics, and possible implications for preterm birth. Soc Sci Med. 2008;67(5):824–833. doi: 10.1016/j.socscimed.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 94.Culhane JF, Rauh V, McCollum KF, et al. Exposure to chronic stress and ethnic differences in rates of bacterial vaginosis among pregnant women. Am J Obstet Gynecol. 2002;187(5):1272–1276. doi: 10.1067/mob.2002.127311. [DOI] [PubMed] [Google Scholar]
- 95.Masi CM, Hawkley LC, Piotrowski ZH, et al. Neighborhood economic disadvantage, violent crime, group density, and pregnancy outcomes in a diverse, urban population. Soc Sci Med. 2007;65(12):2440–2457. doi: 10.1016/j.socscimed.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 96.Messer LC, Kaufman JS, Dole N, et al. Neighborhood crime, deprivation, and preterm birth. Ann Epidemiol. 2006;16(6):455–462. doi: 10.1016/j.annepidem.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 97.Bell JF, Zimmerman FJ, Almgren GR, et al. Birth outcomes among urban African-American women: a multilevel analysis of the role of racial residential segregation. Soc Sci Med. 2006;63(12):3030–3045. doi: 10.1016/j.socscimed.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 98.Pickett KE, Collins JW, Masi CM, et al. The effects of racial density and income incongruity on pregnancy outcomes. Soc Sci Med. 2005;60(10):2229–2238. doi: 10.1016/j.socscimed.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 99.Vinikoor LC, Kaufman JS, MacLehose RF, et al. Effects of racial density and income incongruity on pregnancy outcomes in less segregated communities. Soc Sci Med. 2008;66(2):255–259. doi: 10.1016/j.socscimed.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 100.Morken NH, Kallen K, Jacobsson B. Outcomes of preterm children according to type of delivery onset: a nationwide population-based study. Paediatr Perinat Epidemiol. 2007;21(5):458–464. doi: 10.1111/j.1365-3016.2007.00823.x. [DOI] [PubMed] [Google Scholar]
- 101.Robson S, Cameron CA, Roberts CL. Birth outcomes for teenage women in New South Wales, 1998–2003. Aust N Z J Obstet Gynaecol. 2006;46(4):305–310. doi: 10.1111/j.1479-828X.2006.00597.x. [DOI] [PubMed] [Google Scholar]
- 102.Ehrenthal DB, Jurkovitz C, Hoffman M, et al. A population study of the contribution of medical comorbidity to the risk of prematurity in blacks [electronic article] Am J Obstet Gynecol. 2007;197(4):409.e1–409.e6. doi: 10.1016/j.ajog.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 103.Savitz DA, Dole N, Terry JW, et al. Smoking and pregnancy outcome among African-American and white women in central North Carolina. Epidemiology. 2001;12(6):636–642. doi: 10.1097/00001648-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 104.Bell JF, Zimmerman FJ, Mayer JD, et al. Associations between residential segregation and smoking during pregnancy among urban African-American women. J Urban Health. 2007;84(3):372–388. doi: 10.1007/s11524-006-9152-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Elizabeth Jesse D, Graham M, Swanson M. Psychosocial and spiritual factors associated with smoking and substance use during pregnancy in African American and White low-income women. J Obstet Gynecol Neonatal Nurs. 2006;35(1):68–77. doi: 10.1111/j.1552-6909.2006.00010.x. [DOI] [PubMed] [Google Scholar]
- 106.Johnson K, Posner SF, Biermann J, et al. Recommendations to improve preconception health and health care—United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. MMWR Recomm Rep. 2006;55(RR-6):1–23. [PubMed] [Google Scholar]
- 107.D’Angelo D, Williams L, Morrow B, et al. Preconception and interconception health status of women who recently gave birth to a live-born infant—Pregnancy Risk Assessment Monitoring System (PRAMS), United States, 26 reporting areas, 2004. MMWR Surveill Summ. 2007;56(10):1–35. [PubMed] [Google Scholar]
- 108.Mueller-Heubach E, Rubinstein DN, Schwarz SS. Histologic chorioamnionitis and preterm delivery in different patient populations. Obstet Gynecol. 1990;75(4):622–626. [PubMed] [Google Scholar]
- 109.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342(20):1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 110.Watts DH, Krohn MA, Hillier SL, et al. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol. 1992;79(3):351–357. doi: 10.1097/00006250-199203000-00005. [DOI] [PubMed] [Google Scholar]
- 111.McElrath TF, Hecht JL, Dammann O, et al. Pregnancy disorders that lead to delivery before the 28th week of gestation: an epidemiologic approach to classification. Am J Epidemiol. 2008;168(9):980–989. doi: 10.1093/aje/kwn202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Andrews WW, Goldenberg RL, Faye-Petersen O, et al. The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. Am J Obstet Gynecol. 2006;195(3):803–808. doi: 10.1016/j.ajog.2006.06.083. [DOI] [PubMed] [Google Scholar]
- 113.Andrews WW, Hauth JC, Goldenberg RL. Infection and preterm birth. Am J Perinatol. 2000;17(7):357–365. doi: 10.1055/s-2000-13448. [DOI] [PubMed] [Google Scholar]
- 114.Offenbacher S, Boggess KA, Murtha AP, et al. Progressive periodontal disease and risk of very preterm delivery. Obstet Gynecol. 2006;107(1):29–36. doi: 10.1097/01.AOG.0000190212.87012.96. [DOI] [PubMed] [Google Scholar]
- 115.Xiong X, Buekens P, Vastardis S, et al. Periodontal disease and pregnancy outcomes: state-of-the-science. Obstet Gyne-col Surv. 2007;62(9):605–615. doi: 10.1097/01.ogx.0000279292.63435.40. [DOI] [PubMed] [Google Scholar]
- 116.Leitich H, Brunbauer M, Bodner-Adler B, et al. Antibiotic treatment of bacterial vaginosis in pregnancy: a meta-analysis. Am J Obstet Gynecol. 2003;188(3):752–758. doi: 10.1067/mob.2003.167. [DOI] [PubMed] [Google Scholar]
- 117.Andrews WW, Goldenberg RL, Hauth JC, et al. Intercon-ceptional antibiotics to prevent spontaneous preterm birth: a randomized clinical trial. Am J Obstet Gynecol. 2006;194(3):617–623. doi: 10.1016/j.ajog.2005.11.049. [DOI] [PubMed] [Google Scholar]
- 118.Tita AT, Cliver SP, Goepfert AR, et al. Clinical trial of interconceptional antibiotics to prevent preterm birth: subgroup analyses and possible adverse antibiotic-microbial interaction [electronic article] Am J Obstet Gynecol. 2007;197(4):367.e1–367.e6. doi: 10.1016/j.ajog.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 119.Lopez NJ, Smith PC, Gutierrez J. Periodontal therapy may reduce the risk of preterm low birth weight in women with periodontal disease: a randomized controlled trial. J Perio-dontol. 2002;73(8):911–924. doi: 10.1902/jop.2002.73.8.911. [DOI] [PubMed] [Google Scholar]
- 120.Dunlop AL, Dubin C, Raynor BD, et al. Interpregnancy primary care and social support for African-American women at risk for recurrent very-low-birthweight delivery: a pilot evaluation. Matern Child Health J. 2008;12(4):461–468. doi: 10.1007/s10995-007-0279-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Davey Smith G, Hypponen E, Power C, et al. Offspring birth weight and parental mortality: prospective observational study and meta-analysis. Am J Epidemiol. 2007;166(2):160–169. doi: 10.1093/aje/kwm054. [DOI] [PubMed] [Google Scholar]
- 122.Lawlor DA, Davey Smith G, Whincup P, et al. Association between offspring birth weight and atherosclerosis in middle aged men and women: British Regional Heart Study. J Epidemiol Community Health. 2003;57(6):462–463. doi: 10.1136/jech.57.6.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Graham J, Zhang L, Schwalberg R. Association of maternal chronic disease and negative birth outcomes in a non-Hispanic Black-White Mississippi birth cohort. Public Health Nurs. 2007;24(4):311–317. doi: 10.1111/j.1525-1446.2007.00639.x. [DOI] [PubMed] [Google Scholar]
- 124.Ray JG, Burrows RF, Burrows EA, et al. MOS HIP: McMaster outcome study of hypertension in pregnancy. Early Hum Dev. 2001;64(2):129–143. doi: 10.1016/s0378-3782(01)00181-5. [DOI] [PubMed] [Google Scholar]
- 125.Rosenberg TJ, Garbers S, Lipkind H, et al. Maternal obesity and diabetes as risk factors for adverse pregnancy outcomes: differences among 4 racial/ethnic groups. Am J Public Health. 2005;95(9):1545–1551. doi: 10.2105/AJPH.2005.065680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Grady SC, Ramirez IJ. Mediating medical risk factors in the residential segregation and low birthweight relationship by race in New York City. Health Place. 2008;14(4):661–677. doi: 10.1016/j.healthplace.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 127.Misra DP, Grason H. Achieving safe motherhood: applying a life course and multiple determinants perinatal health framework in public health. Womens Health Issues. 2006;16(4):159–175. doi: 10.1016/j.whi.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 128.Nearns J. Health insurance coverage and prescription contraceptive use among young women at risk for unintended pregnancy. Contraception. 2009;79(2):105–110. doi: 10.1016/j.contraception.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 129.Adams EK, Gavin NI, Ayadi MF, et al. The State Children’s Health Insurance Program (SCHIP) and prepregnancy coverage of teenage mothers. Med Care. 2008;46(10):1071–1078. doi: 10.1097/MLR.0b013e31818888de. [DOI] [PubMed] [Google Scholar]
- 130.Rosenbaum S. Women and health insurance: implications for financing preconception health. Womens Health Issues. 2008;18(6 suppl):S26–S35. doi: 10.1016/j.whi.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 131.Culwell KR, Feinglass J. The association of health insurance with use of prescription contraceptives. Perspect Sex Reprod Health. 2007;39(4):226–230. doi: 10.1363/3922607. [DOI] [PubMed] [Google Scholar]
- 132.Afable-Munsuz A, Braveman P. Pregnancy intention and preterm birth: differential associations among a diverse population of women. Perspect Sex Reprod Health. 2008;40(2):66–73. doi: 10.1363/4006608. [DOI] [PubMed] [Google Scholar]
- 133.Mohllajee AP, Curtis KM, Morrow B, et al. Pregnancy intention and its relationship to birth and maternal outcomes. Obstet Gynecol. 2007;109(3):678–686. doi: 10.1097/01.AOG.0000255666.78427.c5. [DOI] [PubMed] [Google Scholar]
- 134.Orr ST, Miller CA, James SA, et al. Unintended pregnancy and preterm birth. Paediatr Perinat Epidemiol. 2000;14(4):309–313. doi: 10.1046/j.1365-3016.2000.00289.x. [DOI] [PubMed] [Google Scholar]
- 135.Zhu BP, Haines KM, Le T, et al. Effect of the interval between pregnancies on perinatal outcomes among white and black women. Am J Obstet Gynecol. 2001;185(6):1403–1410. doi: 10.1067/mob.2001.118307. [DOI] [PubMed] [Google Scholar]
- 136.Khoshnood B, Lee KS, Wall S, et al. Short interpregnancy intervals and the risk of adverse birth outcomes among five racial/ethnic groups in the United States. Am J Epidemiol. 1998;148(8):798–805. doi: 10.1093/oxfordjournals.aje.a009701. [DOI] [PubMed] [Google Scholar]
- 137.Smith GC, Pell JP, Dobbie R. Interpregnancy interval and risk of preterm birth and neonatal death: retrospective cohort study [electronic article] BMJ. 2003;327(7410):313. doi: 10.1136/bmj.327.7410.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Catov JM, Bodnar LM, Ness RB, et al. Association of periconceptional multivitamin use and risk of preterm or small-for-gestational-age births. Am J Epidemiol. 2007;166(3):296–303. doi: 10.1093/aje/kwm071. [DOI] [PubMed] [Google Scholar]
- 139.Hausmann LR, Jeong K, Bost JE, et al. Perceived discrimination in health care and health status in a racially diverse sample. Med Care. 2008;46(9):905–914. doi: 10.1097/MLR.0b013e3181792562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kotelchuck M. The Adequacy of Prenatal Care Utilization Index: its US distribution and association with low birth-weight. Am J Public Health. 1994;84(9):1486–1489. doi: 10.2105/ajph.84.9.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kramer MS. Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Organ. 1987;65(5):663–737. [PMC free article] [PubMed] [Google Scholar]
- 142.Multicenter randomized, controlled trial of a preterm birth prevention program. Collaborative Group on Preterm Birth Prevention. Am J Obstet Gynecol. 1993;169(2 pt 1):352–366. doi: 10.1016/0002-9378(93)90087-y. [DOI] [PubMed] [Google Scholar]
- 143.Healy AJ, Malone FD, Sullivan LM, et al. Early access to prenatal care: implications for racial disparity in perinatal mortality. Obstet Gynecol. 2006;107(3):625–631. doi: 10.1097/01.AOG.0000201978.83607.96. [DOI] [PubMed] [Google Scholar]
- 144.Ickovics JR, Kershaw TS, Westdahl C, et al. Group prenatal care and preterm birth weight: results from a matched cohort study at public clinics. Obstet Gynecol. 2003;102(5 pt 1):1051–1057. doi: 10.1016/s0029-7844(03)00765-8. [DOI] [PubMed] [Google Scholar]
- 145.Iams JD, Romero R, Culhane JF, et al. Primary, secondary, and tertiary interventions to reduce the morbidity and mortality of preterm birth. Lancet. 2008;371(9607):164–175. doi: 10.1016/S0140-6736(08)60108-7. [DOI] [PubMed] [Google Scholar]
- 146.Kaufman JS, Cooper RS, McGee DL. Socioeconomic status and health in blacks and whites: the problem of residual confounding and the resiliency of race. Epidemiology. 1997;8(6):621–628. [PubMed] [Google Scholar]
- 147.Khoury MJ, Gwinn M, Burke W, et al. Will genomics widen or help heal the schism between medicine and public health? Am J Prev Med. 2007;33(4):310–317. doi: 10.1016/j.amepre.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 148.Ioannidis JP, Ntzani EE, Trikalinos TA. ‘Racial’ differences in genetic effects for complex diseases. Nat Genet. 2004;36(12):1312–1318. doi: 10.1038/ng1474. [DOI] [PubMed] [Google Scholar]
- 149.Kaufman JS, Cooper RS. Race in epidemiology: new tools, old problems. Ann Epidemiol. 2008;18(2):119–123. doi: 10.1016/j.annepidem.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 150.Frank R. What to make of it? The (Re)emergence of a biological conceptualization of race in health disparities research. Soc Sci Med. 2007;64(10):1977–1983. doi: 10.1016/j.socscimed.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 151.Velez DR, Fortunato S, Thorsen P, et al. Spontaneous preterm birth in African Americans is associated with infection and inflammatory response gene variants [electronic article] Am J Obstet Gynecol. 2009;200(2):209.e1–209.e27. doi: 10.1016/j.ajog.2008.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hassan MI, Aschner Y, Manning CH, et al. Racial differences in selected cytokine allelic and genotypic frequencies among healthy, pregnant women in North Carolina. Cytokine. 2003;21(1):10–16. doi: 10.1016/s1043-4666(02)00489-1. [DOI] [PubMed] [Google Scholar]
- 153.Genc MR, Vardhana S, Delaney ML, et al. TNFA-308G>A polymorphism influences the TNF-alpha response to altered vaginal flora. Eur J Obstet Gynecol Reprod Biol. 2007;134(2):188–191. doi: 10.1016/j.ejogrb.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 154.Menon R, Velez DR, Thorsen P, et al. Ethnic differences in key candidate genes for spontaneous preterm birth: TNF-alpha and its receptors. Hum Hered. 2006;62(2):107–118. doi: 10.1159/000096301. [DOI] [PubMed] [Google Scholar]
- 155.Velez DR, Menon R, Thorsen P, et al. Ethnic differences in interleukin 6 (IL-6) and IL6 receptor genes in spontaneous preterm birth and effects on amniotic fluid protein levels. Ann Hum Genet. 2007;71(pt 5):586–600. doi: 10.1111/j.1469-1809.2007.00352.x. [DOI] [PubMed] [Google Scholar]
- 156.Simhan HN, Krohn MA, Roberts JM, et al. Interleukin-6 promoter −174 polymorphism and spontaneous preterm birth. Am J Obstet Gynecol. 2003;189(4):915–918. doi: 10.1067/s0002-9378(03)00843-3. [DOI] [PubMed] [Google Scholar]
- 157.Macones GA, Parry S, Elkousy M, et al. A polymorphism in the promoter region of TNF and bacterial vaginosis: preliminary evidence of gene-environment interaction in the etiology of spontaneous preterm birth. Am J Obstet Gynecol. 2004;190(6):1504–1508. doi: 10.1016/j.ajog.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 158.Engel SA, Erichsen HC, Savitz DA, et al. Risk of spontaneous preterm birth is associated with common proinflammatory cytokine polymorphisms. Epidemiology. 2005;16(4):469–477. doi: 10.1097/01.ede.0000164539.09250.31. [DOI] [PubMed] [Google Scholar]
- 159.Menon R, Velez DR, Morgan N, et al. Genetic regulation of amniotic fluid TNF-alpha and soluble TNF receptor concentrations affected by race and preterm birth. Hum Genet. 2008;124(3):243–253. doi: 10.1007/s00439-008-0547-z. [DOI] [PubMed] [Google Scholar]
- 160.Genc MR, Onderdonk AB, Vardhana S, et al. Polymorphism in intron 2 of the interleukin-1 receptor antagonist gene, local midtrimester cytokine response to vaginal flora, and subsequent preterm birth. Am J Obstet Gynecol. 2004;191(4):1324–1330. doi: 10.1016/j.ajog.2004.05.074. [DOI] [PubMed] [Google Scholar]
- 161.Velez DR, Menon R, Simhan H, et al. Mitochondrial DNA variant A4917G, smoking and spontaneous preterm birth. Mitochondrion. 2008;8(2):130–135. doi: 10.1016/j.mito.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 162.Fortunato SJ, Menon R, Velez DR, et al. Racial disparity in maternal-fetal genetic epistasis in spontaneous preterm birth [electronic article] Am J Obstet Gynecol. 2008;198(6):666.e1–666.e9. doi: 10.1016/j.ajog.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 163.Berg CJ, Wilcox LS, d’Almada PJ. The prevalence of socioeconomic and behavioral characteristics and their impact on very low birth weight in black and white infants in Georgia. Matern Child Health J. 2001;5(2):75–84. doi: 10.1023/a:1011344914802. [DOI] [PubMed] [Google Scholar]
- 164.Catov JM, Bodnar LM, Ness RB, et al. Inflammation and dyslipidemia related to risk of spontaneous preterm birth. Am J Epidemiol. 2007;166(11):1312–1319. doi: 10.1093/aje/kwm273. [DOI] [PubMed] [Google Scholar]
- 165.David RJ, Collins JW., Jr Differing birth weight among infants of U.S.-born blacks, African-born blacks, and U.S.-born whites. N Engl J Med. 1997;337(17):1209–1214. doi: 10.1056/NEJM199710233371706. [DOI] [PubMed] [Google Scholar]
- 166.Shen TT, DeFranco EA, Stamilio DM, et al. A population-based study of race-specific risk for preterm premature rupture of membranes [electronic article] Am J Obstet Gynecol. 2008;199(4):373.e1–373.e7. doi: 10.1016/j.ajog.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 167.Shen TT, DeFranco EA, Stamilio DM, et al. A population-based study of race-specific risk for placental abruption [electronic article] BMC Pregnancy Childbirth. 2008;8:43. doi: 10.1186/1471-2393-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Aveyard P, Cheng KK, Manaseki S, et al. The risk of preterm delivery in women from different ethnic groups. BJOG. 2002;109(8):894–899. doi: 10.1111/j.1471-0528.2002.01197.x. [DOI] [PubMed] [Google Scholar]
- 169.Kleinman JC, Kessel SS. Racial differences in low birth weight. Trends and risk factors. N Engl J Med. 1987;317(12):749–753. doi: 10.1056/NEJM198709173171207. [DOI] [PubMed] [Google Scholar]
- 170.Reagan PB, Salsberry PJ. Race and ethnic differences in determinants of preterm birth in the USA: broadening the social context. Soc Sci Med. 2005;60(10):2217–2228. doi: 10.1016/j.socscimed.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 171.Kramer MR, Hogue CR. Place matters: variation in the black/ white very preterm birth rate across U.S. metropolitan areas, 2002–2004. Public Health Rep. 2008;123(5):576–585. doi: 10.1177/003335490812300507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nabukera SK, Wingate MS, Owen J, et al. Racial disparities in perinatal outcomes and pregnancy spacing among women delaying initiation of childbearing. Matern Child Health J. 2009;13(1):81–89. doi: 10.1007/s10995-008-0330-8. [DOI] [PubMed] [Google Scholar]
- 173.Goldenberg RL, Faye-Petersen O, Andrews WW, et al. The Alabama Preterm Birth Study: diffuse decidual leukocytoclastic necrosis of the decidua basalis, a placental lesion associated with preeclampsia, indicated preterm birth and decreased fetal growth. J Matern Fetal Neonatal Med. 2007;20(5):391–395. doi: 10.1080/14767050701236365. [DOI] [PubMed] [Google Scholar]
- 174.Yang J, Savitz DA. The effect of vaginal bleeding during pregnancy on preterm and small-for-gestational-age births: US National Maternal and Infant Health Survey, 1988. Paediatr Perinat Epidemiol. 2001;15(1):34–39. doi: 10.1046/j.1365-3016.2001.00318.x. [DOI] [PubMed] [Google Scholar]