Abstract
Background
Autoantibodies are of growing interest in cancer research as potential biomarkers; yet the determinants of autoimmunity are not well understood. Antinuclear antibodies (ANA) are common in the general population, and are more prevalent in women and older adults. Here we examined the relationship of ANA with reproductive and hormonal factors in a representative sample of U.S. women.
Methods
We analyzed data on reproductive history and exogenous hormone use in relation to serum ANA in 2,037 females ages 12 and older from the National Health and Nutrition Examination Survey (NHANES; 1999–2004). Estimated ANA prevalences were adjusted for sampling weights. Prevalence odds ratios (POR) and 95% confidence intervals (CI) were adjusted for age, race and poverty-income-ratio, and models were stratified by menopause status.
Results
In premenopausal women ages 20 and older, ANA prevalence was associated with parity (p<0.001; parous versus nulliparous POR=2.0; 95%CI 1.2, 3.4), but in parous women ANA did not vary by number of births, age at first birth, years since last birth or breastfeeding. In postmenopausal women, ANA prevalence was associated with an older age at menarche (p=0.019; age 16–20 versus 10–12 years POR=3.0, 95%CI 1.6, 5.9), but not with parity. Oral contraceptives and estrogen therapy were not associated with a higher ANA prevalence.
Conclusions
Childbearing (having had one or more births) may explain age-associated elevations in ANA prevalence seen in premenopausal women.
Impact
These findings highlight the importance of considering reproductive history in studies of autoimmunity and cancer in women.
Keywords: Autoimmunity, Parity, Antinuclear antibodies, Epidemiology, Women’s Health
INTRODUCTION
Autoantibodies are of growing interest in cancer research based on their potential as diagnostic, therapeutic and etiologic markers across multiple types of cancers (1–4). Reactivity to tumor-associated antigens in healthy controls has been described in several studies (5–7), but the determinants of autoimmunity to tumors and other self-antigens are not well understood.
Self-reactive antibodies to ubiquitous cellular components are considered to be a hallmark of systemic autoimmune diseases, and antinuclear antibodies (ANA) are the most common assessed in clinical practice. Although ANA may precede the development of systemic autoimmune disease in some cases, ANA are also frequently detected in healthy individuals and are generally considered to be non-specific markers of autoimmunity in the absence of other clinical and laboratory features of autoimmune disease (8, 9). Similar to the female predominance in many autoimmune diseases, a higher ANA prevalence is seen in females compared to males (10–14). In a sample of the U.S. population ages 12 and older (the National Health and Nutrition Examination Survey, NHANES; 1999–2004), 17.8% of women versus 9.6% of men were identified as ANA positive (11). Notably, the female to male ratio increased during the reproductive years, peaking at ages 40–49, though the higher prevalence in females compared to males persisted throughout the later decades.
Reasons for sex differences in autoimmunity are not well understood. Possibilities include X-chromosome immune-related genes, fetal microchimerism and differential hormonal and reproductive factors (15, 16). To better understand the development of autoimmunity in women, we examined associations of reproductive and hormonal factors with ANA prevalence among female participants in the previously described NHANES study sample (11).
MATERIALS AND METHODS
Sample
The study sample included NHANES mobile exam participants ages 12 and older with blood specimens, enrolled during 1999–2004, and were a subset of participants selected for a study of ANA as previously described (11). The present sample (N=2,037) was limited to female participants who had ever menstruated, were not pregnant at the time of blood collection, and completed the reproductive history questionnaire. The NHANES protocol was approved by a human subjects review board, and written informed consent was obtained from all participants.
Laboratory Data
Serum ANA were measured by a standardized indirect immunofluorescence assay on HEp2 cells at a 1:80 dilution as previously described (11). Intensity was graded on a scale of 0 to 4, and specimens with a score of 3 or 4 were considered positive based on findings from commercial ANA reference laboratories (12 positive and two negative controls). Controls also included 200 CDC referent sera and unknowns(17). Two independent raters agreed on over 95% of readings and repeat testing of a 2% random sample showed >98% concordance.
Exposures and covariates
Data on female reproductive factors and hormone use were collected during the mobile examination by computer assisted personal interview. Menstrual histories included age at menarche, age at last period, having regular periods (1999–2002) or at least one period (2003–4) in the past 12 months, and the reasons for not having regular periods/a period in the past 12 months (i.e., recent pregnancy, recent or current breast feeding, menopause, irregular periods, and medical conditions or treatments). Reproductive history included number of pregnancies and live births. We defined parous women as those who had at least one live birth. Because information was not collected on pregnancy losses or stillbirth, being nulliparous was defined only by reporting no live births. Questions were also asked about age at first and most recent live birth, and ever and total number of babies breastfed at least 1 month.
Women over age 20 were asked about hysterectomy with or without ovaries removed and ages at surgery or removal of ovaries. For women under age 55, we defined premenopausal status as having had a period in the past 12 months, but also considered women premenopausal if they reported not having had a period due to pregnancy or breastfeeding (n=46), irregular periods (n=2) or injectable contraceptives (n=4). We defined postmenopausal status as the cessation of periods for at least 12 months (not due to pregnancy, breast feeding, irregular periods or injectable contraceptives) or having a hysterectomy with both ovaries removed prior to or within 1 year of age at cessation of periods. Women who had a simple hysterectomy (retaining at least one ovary) at the cessation of periods were considered premenopausal if they were younger than age 55 (n=54; 6.9% of premenopausal women in the sample) or as having had a natural menopause if they were age 55 and older (n=138; 19% of postmenopausal women in the sample). Women with both ovaries removed prior to age 55 were classified as having a surgical menopause. Menopause type could not be established in some women because the reason for the absence of periods was not known or was due to unspecified medical conditions or treatments (N=35; “other” menopause type in Table 1). These were excluded from subsequent analyses of menopause and menopause-age. Menopause age, assigned based on the age at last menstrual period or when the second ovary was removed, could not be determined for a subset of women (N=121) due to missing data or surgical removal of the uterus with the ovaries left in place prior to reported cessation of periods.
Table 1.
Weighted prevalence of antinuclear antibodies (ANA) in the U.S. female population ages 12 and older in NHANES (1999 – 2004) by demographic and personal characteristics, overall and across three age groups
| Overall | Ages 12–19 | Ages 20–49 | Ages 50 and older | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | N* | N ANA+* | % ANA+ (95% CI) | N* | N ANA+* | % ANA+ (95% CI) | N* | N ANA+* | % ANA+ (95% CI) | N* | N ANA+* | % ANA+ (95% CI) |
| ANA prevalence | 2037 | 351 | 17.6 (15.5, 19.8) | 481 | 68 | 12.4 (8.3, 18.0) | 770 | 137 | 17.4 (14.8, 20.4) | 786 | 146 | 19.6 (16.0, 23.8) |
|
| ||||||||||||
| Race/Ethnicity | ||||||||||||
| Non-Hispanic White | 916 | 159 | 17.7 (15.1–20.7) | 119 | 12 | 10.8 (6.0–18.8) | 342 | 64 | 18.6 (15.3–22.5) | 455 | 83 | 18.6 (14.3–24.0) |
| Non-Hispanic Black | 420 | 75 | 19.2 (15.9–22.9) | 139 | 22 | 16.7 (10.9–24.7) | 167 | 29 | 17.5 (12.6–23.8) | 114 | 24 | 23.4 (17.1–31.1) |
| Mexican American | 529 | 90 | 17.5 (13.8–21.9) | 179 | 29 | 15.1 (10.3–21.7) | 189 | 35 | 17.4 (12.1–24.2) | 161 | 26 | 20.7 (13.9–29.7) |
| Other | 172 | 27 | 15.0 (9.2–23.5) | 44 | 5 | 13.3 (4.6–32.8) | 72 | 9 | 10.1 (4.5–21.0) | 56 | 13 | 23.6 (13.0–38.9) |
| P Value† | 0.830 | 0.596 | 0.235 | 0.718 | ||||||||
| Family PIR | ||||||||||||
| <1 (Below Poverty) | 437 | 61 | 15.5 (11.4–20.1) | 169 | 19 | 7.6 (3.3–15.2) | 160 | 27 | 17.8 (11.4–26.8) | 108 | 15 | 18.5 (11.9–27.8) |
| >=1 (At or Above Poverty) | 1442 | 260 | 17.8 (15.4–20.4) | 274 | 42 | 13.2 (9.4–18.4) | 556 | 99 | 17.0 (14.1–20.3) | 612 | 119 | 20.0 (15.8–24.5) |
| P-value† | 0.359 | 0.079 | 0.833 | 0.756 | ||||||||
| Body Mass Index | ||||||||||||
| Not Overweight | 838 | 152 | 18.6 (15.6–22.0) | 300 | 44 | 13.6 (9.3–19.4) | 312 | 59 | 18.4 (14.4–23.2) | 226 | 49 | 22.7 (16.4–30.5) |
| Overweight/Obese | 1157 | 188 | 16.4 (14.0–19.2) | 176 | 23 | 8.2 (4.5–14.7) | 450 | 76 | 16.3 (13.0–20.3) | 531 | 89 | 18.0 (13.6–23.4) |
| P Value† | 0.249 | 0.070 | 0.458 | 0.272 | ||||||||
| Ever had a live birth | ||||||||||||
| No (Nulliparous) | 733 | 99 | 11.8 (9.3–14.7) | 442 | 59 | 11.7 (8.0–16.6) | 202 | 26 | 11.1 (7.5–16.1) | 89 | 14 | 14.3 (8.5–22.9) |
| Yes (Parous) | 1302 | 252 | 20.3 (17.5–23.4) | 39 | 9 | 23.3 (8.7–49.2) | 568 | 111 | 20.1 (16.8–23.8) | 695 | 132 | 20.4 (16.3–25.2) |
| P Value† | <0.001 | 0.253 | 0.001 | 0.180 | ||||||||
| Menopausal status‡ | ||||||||||||
| Premenopausal | 782 | 150 | 19.1 (16.1–22.6) | - | - | - | 722 | 134 | 18.3 (15.5–21.4) | 60 | 16 | 28.9 (15.9–46.5) |
| Postmenopausal (Surgical) | 138 | 27 | 17.3 (10.6–26.8) | - | - | - | 16 | 2 | 13.8 (2.5–49.8) | 122 | 25 | 18.1 (11.3–27.6) |
| Postmenopausal (Natural) | 583 | 99 | 17.6 (14.2–21.5) | - | - | - | 24 | 1 | 1.7 (0.2–12.2) | 559 | 98 | 18.7 (15.2–22.8) |
| Postmenopausal (Other) | 35 | 1 | 2.5 (0.3–16.7) | - | - | - | 4 | 0 | - | 31 | 1 | 2.9 (0.4–19.4) |
| P Value† | 0.016 | - | <0.001 | 0.043 | ||||||||
ANA = antinuclear antibodies; + = positive; CI = confidence interval.
N reflects the number of subjects within the sample, not an estimated count for the U.S. population. ; % is adjusted for sample weights; individual totals for each variable may vary due to the number of missing or ‘don’t know’ responses.
P Value for the Wald Chi-squared test of general association among ANA+ and levels of the variables (Don’t know / Missing excluded).
Menopause questions were asked of 20+ year old subjects only..
Information about birth control included use of oral contraceptives (age first used, current use, duration and age stopped) and Depo-Provera or injectable hormonal contraception. Women over age 20 were asked about other non-contraceptive female hormone use (hormone therapy, including pills, patches, or creams, suppositories, or injections), hormone type (estrogen only, progestin only, or both estrogen and progestin), age first used, current use, and total years used. Responses were combined to derive variables estimating total years use of estrogen or progestin.
Covariates included age, race/ethnicity, poverty index ratio (PIR), and overweight or obesity (based on measured height and weight used to calculate body mass index ≥25 in adults 20 and older or using age/sex-specific guidelines for ages 12–19), smoking status and pack-years (11). Participants were also asked about history of thyroid disease and rheumatoid arthritis.
Analyses
We used SAS SURVEY procedures (Version 9.3, SAS Institute Inc., Cary, NC) and SAS-callable SUDAAN (Version 11, Research Triangle Institute, Research Triangle Park, NC) to estimate ANA prevalences accounting for sampling weights to extrapolate to the US population. Tests of equality of prevalences across covariate categories were based on likelihood ratio tests. Prevalence odds ratios (PORs) and 95% confidence intervals (CI) were estimated by logistic regression, adjusting for age, race/ethnicity, and poverty index ratio (baseline model). The sample weights were not informative (i.e., not related to the outcome, given the covariates). Sample-weighted analyses with non-informative weights can reduce statistical power (18), so we set the weights to 1 and used the survey procedures to account for clustered sampling and to ensure valid variance estimation. Covariates in the base model were included to address potential confounding due to selection bias, and based on prior findings in the overall sample (11). In sensitivity analyses, we conducted sample-weighted analyses for comparison and also ran models adjusted for NHANES cycle, both of which showed reduced precision, but otherwise had little impact on our findings.
We ran additional models to rule out potential confounding; the first added overweight or obese body mass index (BMI) and an interaction term for age by BMI to the base model. Subsequent models also adjusted for age at menarche, parity, and potential confounders relevant to specific exposures (e.g., birth-related factors in parous women and menopause characteristics in postmenopausal women). Models were run also adjusting for smoking pack-years and current smoking status. Because confounding was not seen, only results from the baseline models are shown. We conducted sensitivity analyses excluding women with self-reported thyroid disease (N=190) and rheumatoid arthritis (N=109); both groups included a greater proportion of postmenopausal women than the full sample (65% versus 49%). We tested the impact of our assumption that younger (<55 years) women with simple hysterectomy (retaining one or more ovaries; n=54) were premenopausal, by considering the most extreme scenario, reclassifying them as postmenopausal. Most results were unchanged in these analyses, except where noted.
Given the expected differences in reproductive and hormonal factors across the female lifespan, we first explored the distribution of ANA and covariates stratified by age (12–19 years, 20–49 years, and 50 years and over; Table 1). We limited subsequent analyses (Tables 2 and 3) to women over age 20 and stratified by menopause status, due to differences in the questionnaire structure and the lower ANA levels seen in both younger and older postmenopausal women. Based on the observed difference in the parity-ANA association by menopause status, we tested the multiplicative interaction through inclusion of a product term (parity X menopause) in a model including parity and menopause. To explore the role of parity in the increased ANA prevalence with age, we examined ANA prevalence across age categories, stratified by parity. We then estimated the linear effect of age in women ages 12–59 and in women ages 60 and over (empirically based on the inflection point seen in Figure 1, when ANA prevalence began to increase in nulliparous women), and tested whether adjusting for parity and age at menarche might mediate age-associated increases in ANA prevalence in these two age groups.
Table 2.
Antinuclear antibodies (ANA) prevalence in the U.S. female population ages 20 and older in NHANES (1999 – 2004) by reproductive history, and prevalence odds ratios
| Premenopausal | Postmenopausal | |||||||
|---|---|---|---|---|---|---|---|---|
| N* | N ANA+* | Weighted % ANA+ (95% CI) | Prevalence Odds Ratio (95% CI)† | N* | N ANA+* | Weighted % ANA+ (95% CI) | Prevalence Odds Ratio (95% CI)† | |
| Age at menarche | ||||||||
| <10 | 29 | 3 | 16.8 (5.0–43.6) | 0.50 (0.16–1.54) | 7 | 0 | ||
| 10–12 | 344 | 68 | 18.6 (14.3–23.9) | [Reference] | 295 | 47 | 15.6 (11.3–21.2) | [Reference] |
| 13–15 | 371 | 70 | 20.0 (15.3–25.6) | 0.97 (0.65–1.45) | 364 | 60 | 17.3 (12.9–22.7) | 1.10 (0.73–1.66) |
| 16–20 | 38 | 9 | 17.1 (7.3–35.1) | 1.10 (0.45–2.69) | 54 | 19 | 35.5 (21.7–52.4) | 3.04 (1.56–5.90) |
| P Value‡ | 0.954 | 0.019 | ||||||
| Ever had a live birth | ||||||||
| No (Nulliparous) | 196 | 25 | 11.1 (7.5–16.2) | [Reference] | 88 | 14 | 14.3 (8.4–23.3) | [Reference] |
| Yes (Parous) | 586 | 125 | 22.4 (18.6–26.7) | 2.01 (1.20–3.36) | 631 | 112 | 18.0 (14.5–22.2) | 1.03 (0.53–2.02) |
| P Value‡ | <0.001 | 0.399 | ||||||
| Number of births | ||||||||
| 1 | 140 | 28 | 21.4 (13.9–31.4) | [Reference] | 81 | 14 | 19.4 (10.7–32.6) | [Reference] |
| 2 | 210 | 50 | 22.1 (17.0–28.3) | 1.20 (0.67–2.14) | 155 | 27 | 16.5 (9.9–26.3) | 1.03 (0.43–2.47) |
| 3 | 131 | 26 | 20.9 (12.7–32.3) | 0.99 (0.47–2.07) | 140 | 25 | 15.1 (9.6–22.9) | 1.10 (0.47–2.58) |
| 4+ | 105 | 21 | 27.8 (16.2–43.5) | 1.10 (0.50–2.40) | 255 | 46 | 21.3 (14.6–29.9) | 1.24 (0.52–2.92) |
| P Value‡ | 0.848 | 0.948 | 0.619 | 0.579 | ||||
| Years since last birth | ||||||||
| <=1 | 101 | 22 | 24.3 (15.7–35.7) | [Reference] | - | - | - | - |
| >1–5 | 110 | 21 | 20.0 (13.2–29.1) | 0.67 (0.33–1.36) | - | - | - | - |
| >5–10 | 117 | 28 | 25.1 (16.4–36.3) | 1.09 (0.55–2.15) | 8 | 2 | 35.1 (8.2–76.6) | [Reference] |
| >10–15 | 110 | 22 | 23.4 (13.9–36.8) | 0.82 (0.43–1.57) | 20 | 5 | 19.8 (7.0–45.0) | 0.84 (0.06–11.8) |
| >15–20 | 78 | 15 | 19.4 (10.3–33.4) | 0.76 (0.27–2.13) | 31 | 4 | 13.6 (4.6–34.2) | 0.94 (0.08–11.3) |
| >20 | 70 | 17 | 21.3 (11.7–35.4) | 1.03 (0.42–2.51) | 569 | 101 | 18.0 (13.8–23.2) | 0.81 (0.08–7.94) |
| P Value‡ | 0.967 | 0.934 | 0.820 | 0.889 | ||||
| Age at Menopause (natural; years) | ||||||||
| <35 | - | - | - | - | 17 | 5 | 22.3 (8.6–46.7) | 2.12 (0.66–6.79) |
| 35–44 | - | - | - | - | 78 | 6 | 5.0 (1.5–15.2) | 0.55 (0.18–1.67) |
| 45–49 | - | - | - | - | 112 | 24 | 21.6 (13.9–32.0) | 1.58 (0.70–3.58) |
| 50–54 | - | - | - | - | 186 | 29 | 16.1 (8.9–27.3) | [Reference] |
| 55+ | - | - | - | - | 69 | 12 | 20.9 (11.6–34.8) | 1.06 (0.48–2.34) |
| P Value‡ | 0.038 | 0.554 | ||||||
ANA = antinuclear antibodies; + = positive.
N is the number of subjects within the sample, not an estimated count for the U.S. population; % is adjusted for sample weights; individual totals for each variable may vary due to the number of missing or ‘don’t know’ responses.
Prevalence Odds Ratios (POR) and 95% Confidence Intervals (CI) were estimated by survey logistic regression, adjusting for age, race/ethnicity and poverty-index-ratio. Values were not calculated (---) where numerically infeasible.
P Value for the Wald Chi-squared test for general association (Missing / Don’t knows excluded), or linear trend test in regression models for POR.
Table 3.
Antinuclear Antibodies (ANA) prevalence in the U.S. female population ages 20 and older in NHANES (1999 – 2004) by exogenous hormone use
| Premenopausal | Postmenopausal | |||||||
|---|---|---|---|---|---|---|---|---|
| N* | ANA+* | Weighted % ANA+ (95% CI) | Prevalence Odds Ratio (95% CI)† | N* | ANA+* | Weighted % ANA+ (95% CI) | Prevalence Odds Ratio (95% CI)† | |
| Oral contraceptives | ||||||||
| Never | 209 | 46 | 19.9 (14.2–27.1) | [Reference] | 391 | 71 | 19.7 (15.0–25.6) | [Reference] |
| Current | 94 | 16 | 17.4 (11.3–25.6) | 0.70 (0.36–1.36) | 0 | 0 | ||
| Past | 478 | 87 | 19.2 (15.6–23.4) | 0.64 (0.44–0.93) | 327 | 55 | 15.6 (12.0–20.2) | 1.22 (0.77–1.94) |
| P Value‡ | 0.876 | 0.224 | ||||||
| Oral contraceptives, total years of use | ||||||||
| Never used | 209 | 46 | 19.9 (14.2–27.1) | [Reference] | 391 | 71 | 19.7 (15.0–25.6) | [Reference] |
| <1 year | 121 | 16 | 16.6 (9.5–27.5) | 0.47 (0.24–0.93) | 64 | 9 | 11.8 (6.3–21.1) | 0.96 (0.41–2.26) |
| 1–2 years | 115 | 31 | 28.8 (19.5–40.4) | 1.15 (0.63–2.09) | 78 | 9 | 9.3 (4.2–19.4) | 0.69 (0.27–1.76) |
| 3–5 years | 136 | 22 | 17.7 (10.8–27.4) | 0.60 (0.33–1.12) | 63 | 10 | 15.1 (9.8–22.5) | 1.31 (0.63–2.75) |
| 6–10 years | 124 | 21 | 14.0 (8.4–22.4) | 0.54 (0.31–0.96) | 74 | 15 | 23.2 (11.9–40.3) | 1.47 (0.78–2.78) |
| >10 years | 73 | 13 | 18.1 (10.3–29.8) | 0.59 (0.27–1.30) | 43 | 9 | 15.4 (7.9–27.9) | 1.45 (0.60–3.50) |
| P Value‡ | 0.338 | 0.232 | 0.321 | 0.134 | ||||
| Any use of estrogen pills or patch | ||||||||
| Never | 722 | 142 | 19.9 (16.8–23.4) | [Reference] | 387 | 69 | 19.4 (14.7–25.3) | [Reference] |
| Current | 30 | 4 | 9.1 (2.8–25.8) | NS | 164 | 29 | 16.3 (10.4–24.5) | 1.34 (0.75–2.37) |
| Past | 17 | 1 | 8.4 (1.1–43.0) | NS | 147 | 21 | 14.3 (9.8–20.5) | 0.89 (0.56–1.43) |
| P Value‡ | 0.073 | 0.339 | ||||||
| Estrogen pills/patch, years used | ||||||||
| Never used | 722 | 142 | 19.9 (16.8–23.4) | [Reference] | 387 | 69 | 19.4 (14.7–25.3) | [Reference] |
| <1 year | 20 | 1 | 2.4 (0.3–16.1) | NS | 47 | 8 | 19.4 (6.7–44.5) | 1.13 (0.44–2.93) |
| 1–4 years | 12 | 1 | 11.4 (1.5–51.9) | NS | 66 | 9 | 12.0 (6.5–21.2) | 0.90 (0.41–1.95) |
| 5–9 years | 10 | 1 | 10.3 (1.4–48.3) | NS | 60 | 8 | 12.1 (5.9–23.2) | 1.03 (0.47–2.25) |
| 10+ years | 5 | 2 | 29.3 (5.1–76.3) | NS | 135 | 25 | 17.5 (11.2–26.3) | 1.24 (0.72–2.16) |
| P Value‡ | <0.001 | 0.296 | 0.476 | 0.697 | ||||
ANA = antinuclear antibodies; + = positive.
N is the number of subjects within the sample, not an estimated count for the U.S. population; % is adjusted for sample weights; individual totals for each variable may vary due to the number of missing or ‘don’t know’ responses.
Prevalence Odds Ratios (POR) and 95% Confidence Intervals (CI) estimated by survey logistic regression, adjusting for age, race/ethnicity and poverty-index-ratio. Values were not calculated (---) where numerically infeasible or not shown (NS) for categories with fewer than 5 ANA positive women.
P Value for the Wald Chi-squared test for general association or linear trend test in regression models for POR.
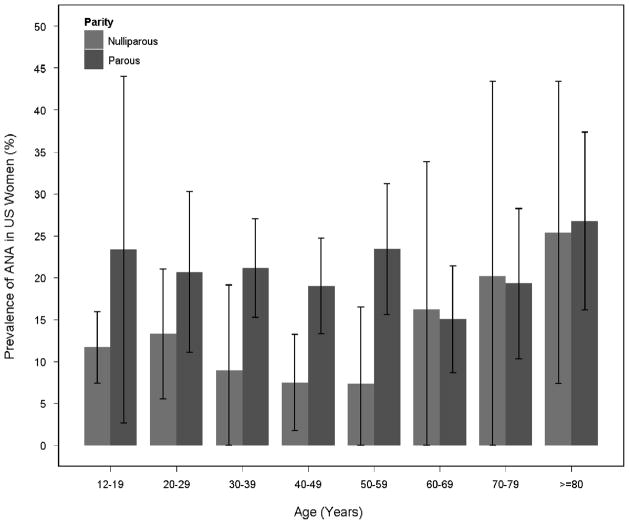
Figure 1. Prevalence of ANA by age depends on parity.
Weighted ANA prevalence estimates and 95% confidence intervals
RESULTS
The ANA test was positive in 351 women with an overall weighted prevalence of 17.6%, increasing from 12.4% for ages 12–19 to 17.4% and 19.6% for ages 20–49 and 50+, respectively (Table 1). ANA prevalence estimates did not vary significantly by demographic factors or BMI, but were higher in women who had ever given birth, including those under age 20. In adult women over age 20 with menopause data, lower ANA prevalence was seen in both younger and older postmenopausal women (20–49 and 50+ years) compared with premenopausal women, but did not vary for surgical versus natural menopause. The lower ANA prevalence associated with postmenopausal status persisted in a model adjusting for age, race/ethnicity and poverty-index ratio (POR=0.54; 95%CI 0.34, 0.87).
Associations of ANA positivity with reproductive characteristics for women ages 20 and older are shown in Table 2, stratified by menopause status. Having a positive ANA was associated with being parous in premenopausal (POR=2.0; 95%CI 1.2, 3.4 versus nulliparous), but not postmenopausal women (interaction p=0.12), and with later onset of menarche (age 16–20 versus 10–12 years: POR=3.0; 95%CI 1.6, 5.9) in postmenopausal women. Differences in ANA prevalence were not associated with age at first birth or years since last birth, or with age at first birth or breastfeeding (not shown). ANA prevalence was not associated with age at natural menopause or with overall age at menopause when surgical menopause was included (not shown). Elevated ANA prevalence in the small number of women with natural menopause before age 35 (POR=2.12; 95% CI 0.66, 6.79), was not seen after excluding those reporting RA (POR=0.85; 95% CI 0.17, 4.23).
In women over age 20, regardless of menopause status, we saw no consistent evidence that higher ANA prevalence was associated with oral contraceptives or other exogenous estrogen use (Table 3), nor with injectable contraceptives or progestin use (not shown). In premenopausal women, adjusted models suggested an inverse association with past oral contraceptive use that was not confounded by parity (not shown). But no duration effect was seen and this association was attenuated and no longer significant after excluding younger women with simple hysterectomy (not shown). Although estimated ANA prevalences did not vary by oral contraceptive use in post-menopausal women, adjusted models suggested a trend in the opposite direction from pre-menopausal women, which became more pronounced with a dose-response for more years of use after excluding women reporting RA (not shown).
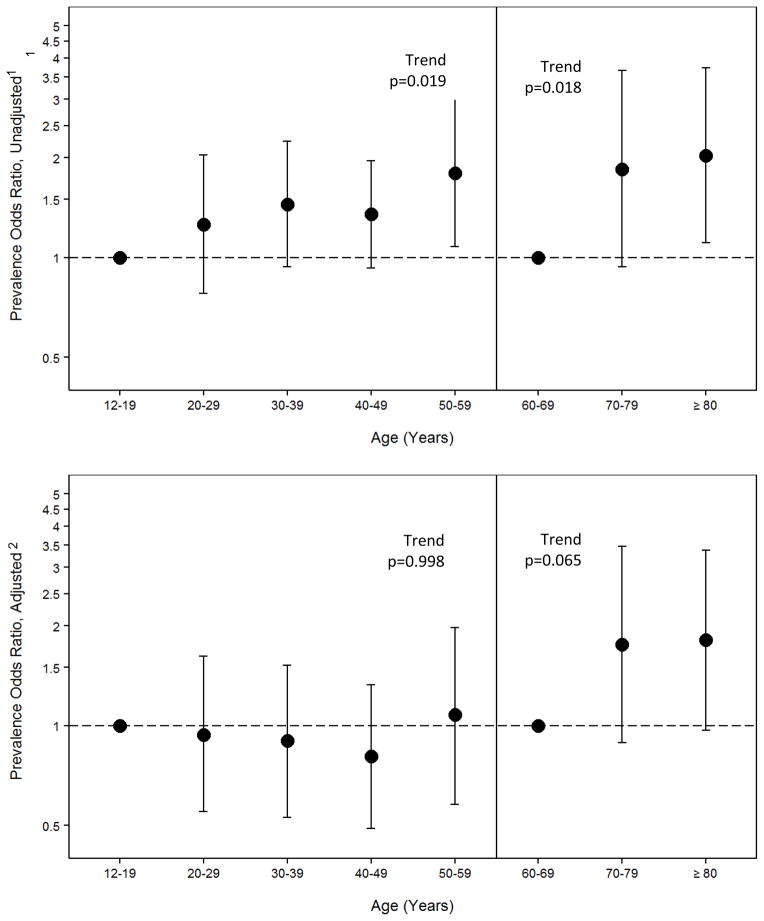
Lastly, we explored the relationship of ANA, age, and parity. Figure 1 shows ANA prevalence by age, stratified by parity. Across ages 12–19 through 50–59 years, ANA prevalence was elevated in parous compared to nulliparous women, but did not increase with age. For women age 60 and older, ANA prevalence increased steadily with age regardless of parity. Based on this inflection point and the possibility that different factors may influence ANA at different points in the lifespan, we ran separate models for younger (12–59) and older (60+) women to describe the relationship between age and ANA prevalence, with and without adjusting for parity (Figure 2). Results showed that parity completely attenuated the age-association in younger women ages (for each increasing decade versus ages 12–19, p for trend=0.019 before and p=0.998 after adjusting for parity). Adjusting for parity did not impact the age-related increases in ANA prevalence in women age 60 and older (not shown), and additional adjustment for age at menarche only slightly attenuated the age-trend in older women (p=0.018 before and p=0.065 after adjusting for parity and menarche age).
Figure 2. Parity explains age-associated increase in ANA prevalence in women ages 12–59, but not in those ages 60+.
Prevalence Odds Ratios (POR) were estimated by survey logistic regression for increasing decade of age; the trend test for age by ANA is shown.
1Estimates shown in the upper panel are adjusted for race/ethnicity and poverty-index-ratio
2Estimates shown in the lower panel are also adjusted for parity in women ages 12–59 and parity plus age at menarche in women ages 60 and older.
DISCUSSION
Our results suggest that childbearing (i.e., having had at least one live birth) is an important determinant of ANA positivity in premenopausal U.S. women, and that age-associated elevations in ANA prevalence across the reproductive years in women may be due to the increasing prevalence of parity with age. In nulliparous women, the pattern of age-specific ANA prevalence appeared strikingly similar to what we previously observed in men (11), with a slight decline in the 30s and 40s before rising with age in older adults. By contrast, ANA prevalence in parous women remained elevated from ages 12–19 up to age 59 years, after which ANA prevalence increased with age for all women regardless of parity. Our results do not support the idea that exogenous hormone use contributes to a higher ANA prevalence in women overall. Given the potential for confounding or modification by systemic autoimmunity in studies of tumor autoimmunity (6, 19–21), our findings highlight the need to understand the role of female reproductive factors and hormonal in the development of autoimmunity.
Immune self-tolerance is induced and maintained by important developmental mechanisms early in life and throughout the lifespan. Low levels of autoantibodies are normal in healthy people, but are typically low-affinity and mostly Immunoglobulin (Ig) M isotypes. The gold standard ANA assay (immunofluorescence on HEp-2 cells) detects IgG antibodies to 150 to 200 known autoantigens and others that are as yet undefined. In autoimmune disease, autoantibodies are thought to arise from a breakdown in specific immune tolerance to self-antigens involving both antibody-producing B cells and stimulation by helper T-cells. Although a causal role in disease pathogenesis is not fully established for many autoantibodies, they often play an critical role in the diagnosis of specific autoimmune diseases in the context of clinical phenotypes (22). In patients who develop systemic autoimmune diseases, ANA can be detected years prior to diagnosis, with increasing antibody levels and specific types predicting the onset of clinical disease (8, 23, 24). Little is known about the natural history of ANA in healthy individuals, or the factors contributing to the progression from ANA-positivity to an autoimmune pathology and disease. Two studies of healthy individuals showed that ANA persisted in the majority over a 5 year follow-up, with little evidence of clinical autoimmune disease (9, 25).
An association of parity with ANA prevalence has not been previously described. Studies have focused on infertility and other adverse outcomes associated with pre-pregnancy autoimmunity and autoimmune diseases (26), but little is known about the incidence and determinants of ANA after pregnancy and throughout the reproductive years in healthy women. Our findings are consistent with the idea that the post-partum period is a time of increased risk of autoimmunity and autoimmune diseases (27, 28). Pregnancy and childbirth induce profound hormonal changes, including large, transient shifts in estrogen and progesterone, which both have immune modulating properties (29). Prolactin is another pregnancy-related hormone associated with autoimmune diseases (30), but we saw no association of breast feeding with ANA.
Other explanations for why parity could increase ANA prevalence include microchimerism due to the transfer of fetal cells that can engraft and persist for decades (31). Microchimerism has been proposed as a cause of various autoimmune diseases, but the findings are inconsistent (32). Survival of fetal chimeric grafts may depend on variation in the Major Histocompatibility Complex, and existing grafts may be subject to aging, competition or replacement with grafts from subsequent births (33). Thus, lack of a dose-effect for number of births and ANA is not inconsistent with the idea of microchimerism. We also saw no waning of ANA with time since last birth, but this cannot be directly assessed in our cross-sectional analysis or disentangled from other aging-related influences across such a broad age-range. We cannot rule out the possibility that longer time since last birth might contribute to the lack of observed effect of parity on ANA in postmenopausal women. Longitudinal studies with ANA measures before, during and after pregnancy, and also through the menopausal transition, would be particularly valuable.
Interpreting an association between parity and ANA is also complicated by the possibility that autoimmunity and autoimmune diseases can impair fertility or affect childbearing choices (34). Women in our study who reported a history of pregnancy and no live births had a lower ANA prevalence (n=73; ANA 7.7%; 95% CI 3.4, 16.6; not shown in table), but we had no data on miscarriage or abortion. The association of ANA with parity might be underestimated, since the referent category is likely to include some women with no pregnancies or births and higher ANA due to autoimmune-related infertility. Many younger women in the U.S. are nulliparous by choice and the fraction of nulliparous women who are infertile should increase with age; but in our sample we saw no age-related increase in ANA among nulliparous women before age 60. Thus underestimation due to lack of data on autoimmune-related infertility may be negligible.
Parity was not associated with ANA prevalence in postmenopausal women, though evidence for a statistical interaction was modest. Different factors may contribute to the initiation and persistence of ANA across different life stages. Despite being older and more likely to be parous, postmenopausal women had a lower overall ANA prevalence than premenopausal women. This finding suggests a role for endogenous hormones on ANA production. We stratified analyses of reproductive and hormonal risk factors based on this observation as well as sample and data constraints. Stratified analyses are also supported by known effects of menopause and estrogen on immunity and autoimmunity (15, 35, 36). Estrogen receptors are expressed in most immune cells (37), but effects depend on receptor type and may be complex (38, 39). Estrogen can impact antibody-mediated immunity, including suppression of B-cell development (40). We saw no consistent patterns for exogenous estrogen use associated with ANA, with a complicated picture suggesting differences by menopause status. We noted higher ANA levels in the oldest premenopausal women (age 50+; Table 1), but a larger sample is needed to disentangle menopause status from age and hormone use, and to investigate perimenopausal factors.
Later age at menarche is associated with fewer lifetime menstrual cycles and lower estrogen levels (41, 42), so the observed association of late menarche age with ANA in postmenopausal women was unexpected. The finding was based on relatively few women with older menarche age and could be due to chance or an age-cohort effect. We did not test for interaction by menopause status. Age at menarche may be a marker for non-estrogen-mediated effects on the immune system, e.g., perinatal factors (43, 44), early life infections (45) and social hardship (46). xenobiotics, infections and stress may impact immune development (47) and evidence is growing for a role of early life factors in autoimmune diseases (48–50). Together, these findings support further study of early life factors and autoimmunity.
Our study has limitations. A lack of data on autoimmune diseases other than RA limited our ability to rule out confounding by autoimmune diagnoses, which could influence reproductive history and the use of hormones. We saw no differences in ANA associations with parity and menarche age when women reporting RA or thyroid disease were excluded from analyses. Because self-report of RA and thyroid disease is sensitive, but not specific, the number of reported cases in our sample is higher than it would have been if we classified cases based on a more rigorous definition using available data on use of disease-specific medications (51–53). We chose to exclude all self-reported cases because reliance on current medication data would have missed cases in remission or those using less specific therapies. Although our main findings were robust to confounding and sensitivity analyses, generalizing results to the full NHANES sample and US population requires cautious interpretation given the lower response rate of older women in the examination (e.g., in 1999–2001 93% of females overall and 72% of women age 80+;(54)) and exclusions for blood draws (e.g., chemotherapy within the past 4 weeks).
Cross-sectional analyses cannot address the timing of ANA occurrence relative to exposures. More than 10% of adolescent girls had a positive ANA, suggesting the importance of inherent and early life risk factors for autoimmunity. Genetic predisposition to ANA is supported by higher prevalence of ANA in family members of patients with autoimmune diseases (55–57), but family history of autoimmune diseases was not collected in NHANES. We also lacked data on infertility, pregnancy loss, mode of delivery, or other pregnancy characteristics, such as pre-eclampsia (58, 59). We did not examine the influence of circadian or monthly variation in ANA given the long half-life of IgG antibodies (2–4 weeks).
Some misclassification of menopause status and hormone use is likely, due to diminishing accuracy of recall over time and more systemic sources of bias, such as the assumptions regarding menopause status in women with simple hysterectomy with one or both ovaries retained (60–62). Most women with natural menopause in this sample reported having their last menstrual period before age 55, so using this cut-point for determining menopause status in women with simple hysterectomy is likely to have correctly classified the majority of older postmenopausal women. But this cut-point is insensitive for identifying younger postmenopausal women with simple hysterectomy, who could have been incorrectly included in the premenopausal category, especially if hysterectomy is associated with earlier ovarian failure (63). However, our findings appeared unchanged in sensitivity analyses reclassifying younger women with a simple hysterectomy from the premenopausal to postmenopausal category.
If there were birth cohort effects on ANA and estrogen use, changes in estrogen use and formulations could possibly create bias. To address changes in hormone therapy over the years of this study, we adjusted for collection cycle, but saw no confounding. We saw no confounding by smoking, but did not adjust for other environmental exposures (e.g., occupation, medications), and confounding by these factors is expected to be minimal given the lack of established associations and/or rarity of most of these factors. Little is known about environmental factors associated with ANA; plausible candidates include immune modifying factors such as vitamin D and endocrine disrupting chemicals that may also be related to reproductive health (64–66).
Conclusions
Our results suggest childbearing may play an important role in initial antigen stimulation or breaking tolerance to self-antigens, contributing to the development of ANA. Although we saw no evidence that exogenous estrogens were associated with ANA prevalence, our findings do not rule out estrogen as a potential modifier of autoimmunity. One recent study of breast cancer in postmenopausal women reported an elevation of tumor autoantibodies only in women who had not taken hormone replacement therapy (67). Given role of reproductive and hormonal factors in many cancers, these characteristics will be important to consider in future studies of autoimmunity and cancer.
Acknowledgments
Financial support: This study was supported by the Intramural Program of the NIH, National Institute of Environmental Health Sciences (Z01ES-044005 and Z01ES-101074), and NIH grant K12 ES019852 and P30 ES001247 (T.A. Jusko).
The authors are grateful for the input of many who have contributed to this project at various stages, including others members of the NIEHS NHANES Autoimmunity Study Group (Richard Cohn, Lindsey Ho, Dori Germolec, Irene Whitt), and for a critical reading by Donna Baird and Lauren Wilson.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Zaenker P, Ziman MR. Serologic autoantibodies as diagnostic cancer biomarkers--a review. Cancer Epidemiol Biomarkers Prev. 2013;22:2161–81. doi: 10.1158/1055-9965.EPI-13-0621. [DOI] [PubMed] [Google Scholar]
- 2.Tan EM, Zhang J. Autoantibodies to tumor-associated antigens: reporters from the immune system. Immunol Rev. 2008;222:328–40. doi: 10.1111/j.1600-065X.2008.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanash S. Harnessing the immune response for cancer detection. Cancer Epidemiol Biomarkers Prev. 2011;20:569–70. doi: 10.1158/1055-9965.EPI-11-0183. [DOI] [PubMed] [Google Scholar]
- 4.Hanash SM, Baik CS, Kallioniemi O. Emerging molecular biomarkers--blood-based strategies to detect and monitor cancer. Nat Rev Clin Oncol. 2011;8:142–50. doi: 10.1038/nrclinonc.2010.220. [DOI] [PubMed] [Google Scholar]
- 5.Vella LA, Yu M, Fuhrmann SR, El-Amine M, Epperson DE, Finn OJ. Healthy individuals have T-cell and antibody responses to the tumor antigen cyclin B1 that when elicited in mice protect from cancer. Proc Natl Acad Sci U S A. 2009;106:14010–5. doi: 10.1073/pnas.0903225106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nolen B, Winans M, Marrangoni A, Lokshin A. Aberrant tumor-associated antigen autoantibody profiles in healthy controls detected by multiplex bead-based immunoassay. J Immunol Methods. 2009;344:116–20. doi: 10.1016/j.jim.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinheiro SP, Hankinson SE, Tworoger SS, Rosner BA, McKolanis JR, Finn OJ, et al. Anti-MUC1 antibodies and ovarian cancer risk: prospective data from the Nurses’ Health Studies. Cancer Epidemiol Biomarkers Prev. 2010;19:1595–601. doi: 10.1158/1055-9965.EPI-10-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–33. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 9.Yadin O, Sarov B, Naggan L, Slor H, Shoenfeld Y. Natural autoantibodies in the serum of healthy women--a five-year follow-up. Clin Exp Immunol. 1989;75:402–6. [PMC free article] [PubMed] [Google Scholar]
- 10.Craig WY, Ledue TB, Johnson AM, Ritchie RF. The distribution of antinuclear antibody titers in “normal” children and adults. J Rheumatol. 1999;26:914–9. [PubMed] [Google Scholar]
- 11.Satoh M, Chan EK, Ho LA, Rose KM, Parks CG, Cohn RD, et al. Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheum. 2012;64:2319–27. doi: 10.1002/art.34380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hooper B, Whittingham S, Mathews JD, Mackay IR, Curnow DH. Autoimmunity in a rural community. Clin Exp Immunol. 1972;12:79–87. [PMC free article] [PubMed] [Google Scholar]
- 13.Njemini R, Meyers I, Demanet C, Smitz J, Sosso M, Mets T. The prevalence of autoantibodies in an elderly sub-Saharan African population. Clin Exp Immunol. 2002;127:99–106. doi: 10.1046/j.1365-2249.2002.01713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishikawa M, Konta T, Hao Z, Takasaki S, Abiko H, Takahashi T, et al. Relationship between antinuclear antibody and microalbuminuria in the general population: the Takahata study. Clin Exp Nephrol. 2008;12:200–6. doi: 10.1007/s10157-008-0030-0. [DOI] [PubMed] [Google Scholar]
- 15.Pennell LM, Galligan CL, Fish EN. Sex affects immunity. J Autoimmun. 2012;38:J282–91. doi: 10.1016/j.jaut.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Quintero OL, Amador-Patarroyo MJ, Montoya-Ortiz G, Rojas-Villarraga A, Anaya JM. Autoimmune disease and gender: plausible mechanisms for the female predominance of autoimmunity. J Autoimmun. 2012;38:J109–19. doi: 10.1016/j.jaut.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Chan EK, Fritzler MJ, Wiik A, Andrade LE, Reeves WH, Tincani A, et al. AutoAbSC.Org -- Autoantibody Standardization Committee in 2006. Autoimmun Rev. 2007;6:577–80. doi: 10.1016/j.autrev.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Korn EL, Graubard BI, editors. Analysis of Health Surveys. New York, NY: John Wiley & Sons; 1999. [Google Scholar]
- 19.Li WH, Zhao J, Li HY, Liu H, Li AL, Wang HX, et al. Proteomics-based identification of autoantibodies in the sera of healthy Chinese individuals from Beijing. Proteomics. 2006;6:4781–9. doi: 10.1002/pmic.200500909. [DOI] [PubMed] [Google Scholar]
- 20.Bracci PM, Zhou M, Young S, Wiemels J. Serum autoantibodies to pancreatic cancer antigens as biomarkers of pancreatic cancer in a San Francisco Bay Area case-control study. Cancer. 2012;118:5384–94. doi: 10.1002/cncr.27538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiemels JL, Bracci PM, Wrensch M, Schildkraut J, Bondy M, Pfefferle J, et al. Assessment of autoantibodies to meningioma in a population-based study. Am J Epidemiol. 2013;177:75–83. doi: 10.1093/aje/kws221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lleo A, Invernizzi P, Gao B, Podda M, Gershwin ME. Definition of human autoimmunity--autoantibodies versus autoimmune disease. Autoimmun Rev. 2010;9:A259–66. doi: 10.1016/j.autrev.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Eriksson C, Kokkonen H, Johansson M, Hallmans G, Wadell G, Rantapaa-Dahlqvist S. Autoantibodies predate the onset of systemic lupus erythematosus in northern Sweden. Arthritis Res Ther. 2011;13:R30. doi: 10.1186/ar3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olsen NJ, Li QZ, Quan J, Wang L, Mutwally A, Karp DR. Autoantibody profiling to follow evolution of lupus syndromes. Arthritis Res Ther. 2012;14:R174. doi: 10.1186/ar3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mariz HA, Sato EI, Barbosa SH, Rodrigues SH, Dellavance A, Andrade LE. Pattern on the antinuclear antibody-HEp-2 test is a critical parameter for discriminating antinuclear antibody-positive healthy individuals and patients with autoimmune rheumatic diseases. Arthritis Rheum. 2011;63:191–200. doi: 10.1002/art.30084. [DOI] [PubMed] [Google Scholar]
- 26.Carp HJ, Selmi C, Shoenfeld Y. The autoimmune bases of infertility and pregnancy loss. J Autoimmun. 2012;38:J266–74. doi: 10.1016/j.jaut.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 27.Jorgensen KT, Pedersen BV, Nielsen NM, Jacobsen S, Frisch M. Childbirths and risk of female predominant and other autoimmune diseases in a population-based Danish cohort. J Autoimmun. 2012;38:J81–7. doi: 10.1016/j.jaut.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Khashan AS, Kenny LC, Laursen TM, Mahmood U, Mortensen PB, Henriksen TB, et al. Pregnancy and the risk of autoimmune disease. PLoS One. 2011;6:e19658. doi: 10.1371/journal.pone.0019658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughes GC. Progesterone and autoimmune disease. Autoimmun Rev. 2012;11:A502–14. doi: 10.1016/j.autrev.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shelly S, Boaz M, Orbach H. Prolactin and autoimmunity. Autoimmun Rev. 2012;11:A465–70. doi: 10.1016/j.autrev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 31.Nelson JL. The otherness of self: microchimerism in health and disease. Trends Immunol. 2012;33:421–7. doi: 10.1016/j.it.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fugazzola L, Cirello V, Beck-Peccoz P. Fetal microchimerism as an explanation of disease. Nat Rev Endocrinol. 2011;7:89–97. doi: 10.1038/nrendo.2010.216. [DOI] [PubMed] [Google Scholar]
- 33.Gammill HS, Guthrie KA, Aydelotte TM, Adams Waldorf KM, Nelson JL. Effect of parity on fetal and maternal microchimerism: interaction of grafts within a host? Blood. 2010;116:2706–12. doi: 10.1182/blood-2010-02-270942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borchers AT, Naguwa SM, Keen CL, Gershwin ME. The implications of autoimmunity and pregnancy. J Autoimmun. 2010;34:J287–99. doi: 10.1016/j.jaut.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 35.Cutolo M, Capellino S, Straub RH. Oestrogens in rheumatic diseases: friend or foe? Rheumatology (Oxford) 2008;47(Suppl 3):iii2–5. doi: 10.1093/rheumatology/ken150. [DOI] [PubMed] [Google Scholar]
- 36.Ghosh M, Rodriguez-Garcia M, Wira CR. The immune system in menopause: pros and cons of hormone therapy. J Steroid Biochem Mol Biol. 2014;142:171–5. doi: 10.1016/j.jsbmb.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cunningham M, Gilkeson G. Estrogen receptors in immunity and autoimmunity. Clin Rev Allergy Immunol. 2011;40:66–73. doi: 10.1007/s12016-010-8203-5. [DOI] [PubMed] [Google Scholar]
- 38.Chighizola C, Meroni PL. The role of environmental estrogens and autoimmunity. Autoimmun Rev. 2012;11:A493–501. doi: 10.1016/j.autrev.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 39.Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–74. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 40.Sakiani S, Olsen NJ, Kovacs WJ. Gonadal steroids and humoral immunity. Nat Rev Endocrinol. 2013;9:56–62. doi: 10.1038/nrendo.2012.206. [DOI] [PubMed] [Google Scholar]
- 41.Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med. 2001;344:276–85. doi: 10.1056/NEJM200101253440407. [DOI] [PubMed] [Google Scholar]
- 42.Clancy KB, Klein LD, Ziomkiewicz A, Nenko I, Jasienska G, Bribiescas RG. Relationships between biomarkers of inflammation, ovarian steroids, and age at menarche in a rural polish sample. Am J Hum Biol. 2013;25:389–98. doi: 10.1002/ajhb.22386. [DOI] [PubMed] [Google Scholar]
- 43.D’Aloisio AA, DeRoo LA, Baird DD, Weinberg CR, Sandler DP. Prenatal and infant exposures and age at menarche. Epidemiology. 2013;24:277–84. doi: 10.1097/EDE.0b013e31828062b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morris DH, Jones ME, Schoemaker MJ, Ashworth A, Swerdlow AJ. Determinants of age at menarche in the UK: analyses from the Breakthrough Generations Study. Br J Cancer. 2010;103:1760–4. doi: 10.1038/sj.bjc.6605978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kwok MK, Leung GM, Lam TH, Schooling CM. Early life infections and onset of puberty: evidence from Hong Kong’s children of 1997 birth cohort. Am J Epidemiol. 2011;173:1440–52. doi: 10.1093/aje/kwr028. [DOI] [PubMed] [Google Scholar]
- 46.Boynton-Jarrett R, Harville EW. A prospective study of childhood social hardships and age at menarche. Ann Epidemiol. 2012;22:731–7. doi: 10.1016/j.annepidem.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bellinger DL, Lubahn C, Lorton D. Maternal and early life stress effects on immune function: relevance to immunotoxicology. J Immunotoxicol. 2008;5:419–44. doi: 10.1080/15476910802483415. [DOI] [PubMed] [Google Scholar]
- 48.Gogal RM, Jr, Holladay SD. Perinatal TCDD exposure and the adult onset of autoimmune disease. J Immunotoxicol. 2008;5:413–8. doi: 10.1080/10408360802483201. [DOI] [PubMed] [Google Scholar]
- 49.Simard JF, Karlson EW, Costenbader KH, Hernan MA, Stampfer MJ, Liang MH, et al. Perinatal factors and adult-onset lupus. Arthritis Rheum. 2008;59:1155–61. doi: 10.1002/art.23930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parks CG, D’Aloisio AA, DeRoo LA, Huiber K, Rider LG, Miller FW, et al. Childhood socioeconomic factors and perinatal characteristics influence development of rheumatoid arthritis in adulthood. Ann Rheum Dis. 2013;72:350–6. doi: 10.1136/annrheumdis-2011-201083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walitt BT, Constantinescu F, Katz JD, Weinstein A, Wang H, Hernandez RK, et al. Validation of self-report of rheumatoid arthritis and systemic lupus erythematosus: The Women’s Health Initiative. J Rheumatol. 2008;35:811–8. [PMC free article] [PubMed] [Google Scholar]
- 52.Rasch EK, Hirsch R, Paulose-Ram R, Hochberg MC. Prevalence of rheumatoid arthritis in persons 60 years of age and older in the United States: effect of different methods of case classification. Arthritis Rheum. 2003;48:917–26. doi: 10.1002/art.10897. [DOI] [PubMed] [Google Scholar]
- 53.Brix TH, Kyvik KO, Hegedus L. Validity of self-reported hyperthyroidism and hypothyroidism: comparison of self-reported questionnaire data with medical record review. Thyroid. 2001;11:769–73. doi: 10.1089/10507250152484619. [DOI] [PubMed] [Google Scholar]
- 54.Anonymous. [cited 2014 July 8]; Available from: http://www.cdc.gov/nchs/nhanes/response_rates_CPS.
- 55.Navarra SV, Ishimori MI, Uy EA, Hamijoyo L, Sama J, James JA, et al. Studies of Filipino patients with systemic lupus erythematosus: autoantibody profile of first-degree relatives. Lupus. 2011;20:537–43. doi: 10.1177/0961203310385164. [DOI] [PubMed] [Google Scholar]
- 56.Laustrup H, Heegaard NH, Voss A, Green A, Lillevang ST, Junker P. Autoantibodies and self-reported health complaints in relatives of systemic lupus erythematosus patients: a community based approach. Lupus. 2004;13:792–9. doi: 10.1191/0961203304lu2015oa. [DOI] [PubMed] [Google Scholar]
- 57.Ramos PS, Kelly JA, Gray-McGuire C, Bruner GR, Leiran AN, Meyer CM, et al. Familial aggregation and linkage analysis of autoantibody traits in pedigrees multiplex for systemic lupus erythematosus. Genes Immun. 2006;7:417–32. doi: 10.1038/sj.gene.6364316. [DOI] [PubMed] [Google Scholar]
- 58.Cooper GS, Dooley MA, Treadwell EL, St Clair EW, Gilkeson GS. Hormonal and reproductive risk factors for development of systemic lupus erythematosus: results of a population-based, case-control study. Arthritis Rheum. 2002;46:1830–9. doi: 10.1002/art.10365. [DOI] [PubMed] [Google Scholar]
- 59.Jorgensen KT, Nielsen NM, Pedersen BV, Jacobsen S, Frisch M. Hyperemesis, gestational hypertensive disorders, pregnancy losses and risk of autoimmune diseases in a Danish population-based cohort. J Autoimmun. 2012;38:J120–8. doi: 10.1016/j.jaut.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 60.Pike MC, Ross RK, Spicer DV. Problems involved in including women with simple hysterectomy in epidemiologic studies measuring the effects of hormone replacement therapy on breast cancer risk. Am J Epidemiol. 1998;147:718–21. doi: 10.1093/oxfordjournals.aje.a009515. [DOI] [PubMed] [Google Scholar]
- 61.Rosner B, Colditz GA. Age at menopause: imputing age at menopause for women with a hysterectomy with application to risk of postmenopausal breast cancer. Ann Epidemiol. 2011;21:450–60. doi: 10.1016/j.annepidem.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rockhill B, Colditz GA, Rosner B. Bias in breast cancer analyses due to error in age at menopause. Am J Epidemiol. 2000;151:404–8. doi: 10.1093/oxfordjournals.aje.a010220. [DOI] [PubMed] [Google Scholar]
- 63.Moorman PG, Myers ER, Schildkraut JM, Iversen ES, Wang F, Warren N. Effect of hysterectomy with ovarian preservation on ovarian function. Obstet Gynecol. 2011;118:1271–9. doi: 10.1097/AOG.0b013e318236fd12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lerchbaum E, Obermayer-Pietsch B. Vitamin D and fertility: a systematic review. Eur J Endocrinol. 2012;166:765–78. doi: 10.1530/EJE-11-0984. [DOI] [PubMed] [Google Scholar]
- 65.Mendola P, Messer LC, Rappazzo K. Science linking environmental contaminant exposures with fertility and reproductive health impacts in the adult female. Fertil Steril. 2008;89:e81–94. doi: 10.1016/j.fertnstert.2007.12.036. [DOI] [PubMed] [Google Scholar]
- 66.Cutolo M, Paolino S, Sulli A, Smith V, Pizzorni C, Seriolo B. Vitamin D, steroid hormones, and autoimmunity. Ann N Y Acad Sci. 2014;1317:39–46. doi: 10.1111/nyas.12432. [DOI] [PubMed] [Google Scholar]
- 67.Chao T, Ladd JJ, Qiu J, Johnson MM, Israel R, Chin A, et al. Proteomic profiling of the autoimmune response to breast cancer antigens uncovers a suppressive effect of hormone therapy. Proteomics Clin Appl. 2013;7:327–36. doi: 10.1002/prca.201200058. [DOI] [PMC free article] [PubMed] [Google Scholar]