Abstract
Background & Aims
Patient-reported outcomes (PRO) convey important aspects of health status, complementing physician-reported measures. The PRO Measurement Information System (PROMIS) provides valid, widely available measures applicable to patients with chronic illness and the general population. We sought to evaluate these measures in a large cohort of patients with inflammatory bowel disease (IBD).
Methods
Using data from the Crohn’s and Colitis Foundation Association Partners internet cohort, we performed cross-sectional and longitudinal analyses to evaluate associations between PROMIS measures and validated disease activity indices (Short Crohn’s Disease Activity Index and Simple Clinical Colitis Activity Index) and the Short IBD Questionnaire (SIBDQ) quality of life instrument.
Results
A total of 10,634 individuals (6689 with Crohn’s disease and 3945 with ulcerative colitis or indeterminate colitis) completed PRO testing. Compared with the general population (mean PROMIS score = 50), IBD patients in this cohort reported more depression (mean 54), anxiety (mean 52), fatigue (mean 56), sleep disturbance (mean 52), and pain interference (mean 53); they had less social satisfaction (mean 48). In each PROMIS domain, there was worse functioning with increasing levels of disease activity, and worsening SIBDQ scores (P<.001 for all). Longitudinal analyses demonstrated improved PROMIS scores with improved disease activity and worsening PROMIS scores with worsening disease (P<.001 for all comparisons).
Conclusions
In a cross-sectional and longitudinal study, we observed differences between patients with IBD and the general population in several important aspects of health. The improvement in diverse health outcome measures with improved disease control provides strong support for the construct validity of PROMIS measures in the IBD population. Their use should advance patient-centered outcomes research in IBD.
Keywords: CCFA, patients management, symptoms, patient reported outcomes
INTRODUCTION
Crohn’s disease (CD) and ulcerative colitis (UC), collectively known as inflammatory bowel disease (IBD), affect nearly 1.2 million Americans.1 To date, much of the evidence used to formulate treatment recommendations stems from placebo-controlled trials. However, real world, population-based clinical effectiveness and comparative effectiveness research are required to better understand the risks and benefits of IBD therapies, particularly in populations often excluded from clinical trials. Consequently, the Institute of Medicine has recently declared IBD as one of the top national priorities for comparative effectiveness research.2
Study outcomes of comparative effectiveness research often differ from endpoints of randomized trials. Practically speaking, in population-based research it is often not possible to complete assessments required to calculate disease activity scores and/or assess for endoscopic remission. Additionally, the generally accepted clinical trial endpoints do not necessarily reflect the well-being of patients with chronic illnesses, such as IBD. In contrast, patient reported outcomes (PROs) are direct responses from patients about how they feel or function in relation to a health condition and its therapy without interpretation by healthcare professionals or anyone else. PROs can evaluate symptoms, signs, functional status, perceptions, or other aspects such as convenience and tolerability. As such, PROs represent what is most important to patients about a condition or its treatment,3 and are important endpoints for clinical trials and comparative effectiveness studies.4
The Patient Reported Outcomes Measurement Information System (PROMIS®) initiative of the National Institutes of Health (NIH) was developed to advance the science and application of PROs among patients with chronic diseases for use in research and clinical practice.5 PROMIS instruments are general (not disease specific) measures that are valid and responsive, allow comparisons within and between conditions, and are grouped into item banks based on symptoms, function, well-being, and general health.6 PROMIS measures have not been comprehensively evaluated in patients with IBD. We sought to evaluate the performance of PROMIS measures in this patient population.
METHODS
Overall Study Design
Within a large internet cohort of adult patients with IBD, we performed a series of cross-sectional and longitudinal analyses to evaluate associations between PROMIS measures and disease activity indices, a disease-specific health related quality of life instrument, prednisone use, and Ileal Pouch Anal Anastamosis (IPAA) status.
Study Population
The Crohn’s and Colitis Foundation of America (CCFA) Partners study is a longitudinal internet-based cohort of patients with IBD. The development of the cohort has been described in detail previously.7 In brief, we recruited participants with a self-reported diagnosis of UC, CD, or indeterminate colitis (IC) who were older than 18 years of age through CCFA email rosters, the CCFA website, various social media outlets, and at educational and fundraising events. All participants completed a baseline survey including demographic information and questions about their IBD history, symptoms, and medication use. A random subset of patients completed an optional module regarding health related quality of life and various PROs. Follow-up questionnaires every 6 months ascertain changes in disease treatments, symptoms, and PROs.
The study population for the cross-sectional portion of this analysis includes all participants in the CCFA Partners cohort enrolled between June 2011 and October 2012 who completed PRO measures on at least one occasion. The study population for the longitudinal section of this analysis includes study participants who completed PRO measures on at least two occasions.
Patient reported outcome measures
Participants completed 4 items from each of 6 PROMIS item banks measuring individual dimensional constructs of health-related quality of life (HRQOL). Measured domains, selected based on prior literature, patient feedback, and input from gastroenterologists (MDK, MDL) and PROMIS methodologists (DAD), included Anxiety, Depression, Fatigue, Sleep Disturbance, Satisfaction with Social Role, and Pain Interference. Pain Interference items were included at a later date than the other items, and hence data are only available on a portion of the overall study population. Participants also completed a single question about general health. A complete list of all PROMIS items included in this study is included in Appendix 1. All PROMIS items have undergone rigorous development and validation based on qualitative research and item response theory in both general and chronically ill populations.8 Items are calibrated using a T-score metric with the mean of the US general population equal to 50 and standard deviation (SD) in the general population equal to 10. Minimal Important Differences (MIDs) refer to the score that is large enough to have implications for a patient’s treatment or care. As the PROMIS system is relatively new, MIDs are not well defined; however, research in cancer patients suggest that MIDs for many PROMIS domains are in the range of 2–6.9 Higher scores indicate more of the domain being measured. Hence, high scores for Anxiety, Depression, Fatigue, Sleep Disturbance, and Pain Interference indicate poorer health, whereas high scores for Satisfaction with Social Role indicate better health.
Other variables
The Short IBD Questionnaire (SIBDQ) was administered as a disease-specific measure of HRQOL.10 Disease activity was assessed using validated measures - the short Crohn’s Disease Activity Index (SCDAI) for CD11 and the Simple Clinical Colitis Activity Index (SCCAI) for UC and IC.12 A SCDAI < 150 or an SCCAI ≤ 4 indicated clinical remission for CD and UC respectively with values above this threshold indicating active disease.11, 12 Patient demographics, IBD medication use including oral 5-aminosalicylates, prednisone, immunomodulators, and biologic therapies (infliximab, adalimumab, certolizumab pegol, and natalizumab), and pouch and ostomy status were all measured by self-report.
Statistical Analysis
We first performed cross-sectional analyses using descriptive statistics and bivariate comparisons to assess the relationships between PROMIS T-scores and patient demographics, disease activity indices, the SIBDQ, current corticosteroid use, and other health measures. As disease activity indices and SIBDQ scores were not normally distributed, these values were categorized into quartiles. Mean PROMIS scores were compared across quartiles of disease activity and SIBDQ scores using a non-parametric test of trend for the ranks across ordered groups. We also used multinomial logistic regression to evaluate associations between PROMIS measures and disease activity, controlling for the effects of current corticosteroid use. As a secondary analysis, mean PROMIS scores were compared between patients in remission and with active disease.
We next performed longitudinal analyses by grouping participants into categories of stable disease, worsening disease, or improving disease based upon a threshold change between baseline and follow-up surveys of ≥ 100 points on the SCDAI (CD patients) or ≥ 2 points on the SCCAI (UC and IC patients). The mean change (and SD) in each PROMIS domain was calculated for each of these two groups.
All analyses were calculated for the entire cohort, and then stratified by patient sex and disease type (CD or UC/IC). For subjects who indicated a change in disease type between the baseline and follow-up survey, their disease type was categorized as that reported during the most recent survey. All statistical analyses were performed using SAS version 9.3. The study protocol was reviewed and approved by the Institutional Review Board of the University of North Carolina.
Results
Study Population
A total of 10,634 individuals with self-reported IBD joined CCFA Partners through October 22, 2012 and completed PRO testing. Of these, 6,689 reported having CD, and 3,945 reported UC or indeterminate colitis. Seventy-one percent of study participants were women. The mean age of the study population was 44 years, and the mean time from diagnosis to PRO testing was 14.9 years. Additional demographic details are provided in Table 1.
Table 1.
Baseline Characteristics of the Study Population
| Characteristic | Crohn’s disease n=6,689 Mean (SD) or percent |
Ulcerative Colitis or Indeterminate Colitis n=3,945 Mean (SD) or percent |
Overall IBD n=10,634 Mean (SD) or percent |
|
|---|---|---|---|---|
|
| ||||
| Demographics | Age, years | 44.0 (14.8) | 44.1 (14.7) | 44.0 (14.8) |
|
| ||||
| Female sex | 72.1% | 69.6% | 71.2% | |
|
| ||||
| Race/ethnicity | ||||
| White | 93.4% | 90.8% | 92.4% | |
| African American | 2.3% | 2.1% | 2.2% | |
| Asian | 0.6% | 1.9% | 1.1% | |
| Other | 3.7% | 5.2% | 4.2% | |
|
| ||||
| Hispanic | 2.3% | 4.6% | 3.2% | |
|
| ||||
| Education completed | ||||
| Less than 12th grade | 1.0% | 0.8% | 0.9% | |
| 12th grade | 8.3% | 6.8% | 7.7% | |
| Some college | 23.5% | 20.1% | 22.3% | |
| College graduate | 40.9% | 41.8% | 41.2% | |
| Graduate school | 26.3% | 30.5% | 27.9% | |
|
| ||||
| Current smoker | 13.8% | 6.3 | 11.1 | |
|
| ||||
| Disease characteristics | Years from IBD diagnosis | 16.3 (12.9) | 12.5 (11.1) | 14.9 (12.4) |
|
| ||||
| ≥1 hospitalizations in the past year | 16.4% | 10.4% | 14.1% | |
|
| ||||
| ≥1 bowel surgeries | 31.4% | 11.3% | 24.0% | |
|
| ||||
| Current Ileal or Koch pouch | 3% | 9.5% | 5.4% | |
|
| ||||
| Current ostomy | 9.1% | 4.6% | 7.4% | |
|
| ||||
| SCDAI or SCCAI | 149 (99) | 3.6 (2.9) | n/a | |
|
| ||||
| SIBDQ | 4.8 (1.2) | 4.9 (1.2) | 4.8 (1.2) | |
|
| ||||
| Current medication use | Aminosalicylates | 35.5% | 63.1% | 45.7% |
|
| ||||
| Prednisone | 10.4% | 12.1% | 11.0% | |
|
| ||||
| Immunomodulators (6-mercaptopurine, azathioprine, or methotrexate) | 29.5% | 21.3% | 26.4% | |
|
| ||||
| Biologic therapy (infliximab, adalimumab, certolizumab pegol, and natalizumab) | 39.8% | 17.3% | 31.4% | |
|
| ||||
| PROMIS# measures | Anxiety | 52 (10) | 52 (9) | 52 (10) |
|
| ||||
| Depression | 54 (10) | 54 (10) | 54 (10) | |
|
| ||||
| Fatigue | 56 (11) | 54 (11) | 56 (11) | |
|
| ||||
| Sleep Disturbance | 53 (9) | 52 (8) | 52 (9) | |
|
| ||||
| Satisfaction with Social Role | 48 (10) | 49 (10) | 48 (10) | |
|
| ||||
| Pain Interference | 53 (10) | 51 (10) | 53 (10) | |
Patient Reported Outcome Information Measurement System items are calibrated so that the mean of the US general population is 50 and the standard deviation is 10. Higher scores indicate more of the domain being measured.
PROMIS Testing
The mean PROMIS scores for Depression, Anxiety, Fatigue, Sleep Disturbance, Satisfaction with Social Role, and Pain Interference are shown in Table 1. For each of these domains, patients in this IBD population reported worse health as compared with the general population (T score in the general population = 50), and patients with CD reported marginally worse health than those with UC. The relationships between PROMIS scores and sex, age, race/ethnicity, educational status, and time from diagnosis are shown in Table 2. Across all measured domains, patients living with IBD for less than 1 year reported worse health outcomes than patients who have had IBD for longer periods of time. However, these differences were independent of disease activity only for anxiety and depression in CD patients and for anxiety and fatigue in UC patients. For most measures, older patients (age ≥ 60) reported better outcomes than younger ones (age 18–30), men reported better outcomes than women, and outcomes were better with increasing levels of education. Hispanics reported worse health than non-Hispanics. Other racial/ethnic differences in PROMIS measures were inconsistent.
Table 2.
Relationships between Patient Demographics and PROMIS# Scores
| Anxiety n=10,630 Mean (SD) |
Depression n=10,633 Mean (SD) |
Fatigue n=10,632 Mean (SD) |
Sleep Disturbance n=10,627 Mean (SD) |
Social Role Satisfaction n=10,633 Mean (SD) |
Pain Interference n=7,354 Mean (SD) |
|
|---|---|---|---|---|---|---|
|
| ||||||
| Age, years | ||||||
| 18–30 | 56 (10) | 53 (10) | 57 (11) | 52 (9) | 48 (10) | 53 (10) |
| 31–40 | 54 (10) | 52 (10) | 56 (11) | 53 (9) | 48 (10) | 52 (10) |
| 41–50 | 54 (10) | 52 (10) | 57 (11) | 53 (8) | 47 (10) | 54 (10) |
| 51–60 | 53 (9) | 52 (9) | 55 (11) | 52 (8) | 48 (10) | 53 (10) |
| >60 | 51 (9) | 50 (9) | 52 (11) | 51 (8) | 50 (10) | 51 (10) |
|
| ||||||
| Sex | ||||||
| Male | 52 (9) | 51 (9) | 53 (11) | 51 (8) | 49 (10) | 51 (10) |
| Female | 54 (10) | 52 (10) | 57 (11) | 53 (9) | 48 (10) | 53 (10) |
|
| ||||||
| Race/ethnicity | ||||||
| White | 53 (10) | 52 (10) | 56 (11) | 52 (9) | 48 (10) | 52 (10) |
| African American | 54 (11) | 52 (10) | 56 (12) | 54 (10) | 48 (11) | 55 (12) |
| Asian | 53 (9) | 51 (9) | 51 (10) | 51 (8) | 49 (10) | 52 (10) |
| Other | 57 (10) | 55 (11) | 58 (11) | 54 (9) | 46 (10) | 55 (11) |
|
| ||||||
| Hispanic | ||||||
| Yes | 56 (10) | 54 (11) | 57 (11) | 54 (9) | 47 (10) | 55 (11) |
| No | 54 (10) | 52 (10) | 56 (11) | 52 (9) | 48 (10) | 53 (10) |
|
| ||||||
| Education completed | ||||||
| Less than 12th grade | 62 (10) | 61 (12) | 63 (11) | 58 (10) | 40 (10) | 59 (12) |
| 12th grade | 55 (10) | 54 (10) | 58 (11) | 54 (10) | 46 (10) | 55 (10) |
| Some college | 55 (10) | 54 (10) | 58 (11) | 54 (9) | 46 (10) | 55 (10) |
| College graduate | 53 (10) | 51 (9) | 55 (11) | 52 (8) | 49 (10) | 52 (10) |
| Graduate school | 52 (9) | 50 (9) | 54 (11) | 51 (8) | 80 (9) | 51 (9) |
|
| ||||||
| Time since IBD diagnosis | ||||||
| <1 year | 58 (10) | 55 (10) | 59 (11) | 54 (9) | 45 (10) | 57 (10) |
| 1–5 years | 55 (10) | 53 (10) | 56 (11) | 52 (9) | 48 (10) | 53 (10) |
| 6–10 years | 54 (10) | 52 (10) | 56 (11) | 52 (9) | 48 (10) | 52 (10) |
| >10 years | 53 (10) | 51 (9) | 55 (11) | 52 (8) | 48 (10) | 52 (10) |
Patient Reported Outcome Information Measurement System items are calibrated so that the mean of the US general population is 50 and the standard deviation is 10. Higher scores indicate more of the domain being measured.
Associations with Disease Severity and the Short IBD Questionnaire
As expected, mean PROMIS scores for Depression, Anxiety, Fatigue, Sleep Disturbance, and Pain Interference all increased with increasing quartiles of disease activity, whereas mean scores for Social Satisfaction decreased (Table 3). These data indicate that, for each of the PROMIS domains, higher levels of IBD disease activity are associated with worsening health. Sex-stratified analyses indicated that the magnitude and strength of each of these associations was independent of patient sex. These relationships remained, after adjusting for current corticosteroid use (p<0.001 for all comparisons), indicating that PROMIS measures are associated with disease activity independent of corticosteroid use.
Table 3.
Relationships between Quartiles of Disease Activity Indices in Crohn’s Disease (n=5960) and Ulcerative/indeterminate colitis (n = 3394) and PROMIS Scores
| PROMIS# Domain | Quartile 1 SCDAI& ≤79 SCCAI* ≤1 |
Quartile 2 SCDAI& 80–128 SCCAI* 1–3 |
Quartile 3 SCDAI& 129–198 SCCAI* 4–5 |
Quartile 4 SCDAI& >199 SCCAI* >6 |
P^ | |
|---|---|---|---|---|---|---|
| Crohn’s disease | Anxiety Mean (SD) | 49 (8) | 52 (9) | 55 (9) | 59 (10) | <.0001 |
| Depression Mean (SD) | 47 (8) | 50 (9) | 53 (9) | 58 (10) | <.0001 | |
| Fatigue Mean (SD) | 49 (10) | 54 (10) | 59 (9) | 65 (9) | <.0001 | |
| Sleep Disturbance Mean (SD) | 48 (8) | 51 (8) | 54 (8) | 58 (8) | <.0001 | |
| Social Role Satisfaction Mean (SD) | 54 (9) | 50 (9) | 46 (8) | 41 (8) | <.0001 | |
| Pain Interference Mean (SD) | 46 (7) | 51 (8) | 56 (8) | 62 (8) | <.0001 | |
| Ulcerative colitis/Indeterminate colitis | Anxiety Mean (SD) | 48 (8) | 52 (9) | 56 (9) | 60 (9) | <.0001 |
| Depression Mean (SD) | 46 (7) | 51 (8) | 53 (9) | 58 (9) | <.0001 | |
| Fatigue Mean (SD) | 47 (9) | 53 (9) | 57 (10) | 62 (9) | <.0001 | |
| Sleep Disturbance Mean (SD) | 47 (8) | 51 (8) | 53 (8) | 56 (8) | <.0001 | |
| Social Role Satisfaction Mean (SD) | 55 (8) | 50 (9) | 47 (8) | 41 (9) | <.0001 | |
| Pain Interference Mean (SD) | 45 (6) | 50 (9) | 54 (8) | 59 (9) | <.0001 | |
| Total IBD | Anxiety Mean (SD) | 49 (8) | 52 (9) | 55 (9) | 59 (9) | <.0001 |
| Depression Mean (SD) | 47 (7) | 50 (8) | 53 (9) | 58 (10) | <.0001 | |
| Fatigue Mean (SD) | 48 (9) | 54 (9) | 58 (10) | 64 (9) | <.0001 | |
| Sleep Disturbance Mean (SD) | 48 (8) | 51 (8) | 54 (8) | 57 (8) | <.0001 | |
| Social Role Satisfaction Mean (SD) | 54 (9) | 50 (9) | 46 (8) | 41 (8) | <.0001 | |
| Pain Interference Mean (SD) | 45 (7) | 50 (9) | 55 (8) | 61 (9) | <.0001 |
Patient Reported Outcome Information Measurement System items are calibrated so that the mean of the US general population is 50 and the standard deviation is 10. Higher scores indicate more of the domain being measured.
Short Crohn’s Disease Activity Index. Scores are interpreted as follows: inactive disease (≤150), mild disease (151–199) and moderate to severe disease (≥200).
Simple Clinical Collitis Activity Index. A score of ≤2 is associated with remission (16) and a score of ≥5 defines a relapse of UC.
p values are from a non-parametric test of trend for the ranks of across ordered groups
PROMIS scores also differed between patients in remission and those with active disease (p<0.001 for all comparisons, Supplemental Table 1). Notably, among patients in remission, PROMIS scores were in the same range as members of the general U.S. population (T score = 50 in the general population).
Associations between PROMIS measures and Short IBD questionnaire scores demonstrate a similar relationship (Table 4). As expected, the direction of the effect is opposite that of the disease activity indices because higher scores on SIBDQ indicate improved health. We observed similar relationships within each of the 4 SIBDQ subdomains: Bowel, Emotional, Systemic, and Social (data not shown).
Table 4.
Relationships between Quartiles of the Short IBD Questionnaire in Crohn’s Disease (n=6689) and Ulcerative/indeterminate colitis (n = 3945) and PROMIS Scores
| PROMIS# Domain | Quartile 1 SIBDQ& ≤ 4 |
Quartile 2 SIBDQ& 4.1–5.0 |
Quartile 3 SIBDQ& 5.1–5.8 |
Quartile 4 SIBDQ& ≥ 5.9 |
P^ | |
|---|---|---|---|---|---|---|
| Crohn’s disease | Anxiety Mean (SD) | 61 (8) | 55 (8) | 51 (8) | 45 (7) | <.0001 |
| Depression Mean (SD) | 60 (9) | 53 (8) | 48 (7) | 44 (5) | <.0001 | |
| Fatigue Mean (SD) | 67 (7) | 59 (8) | 53 (8) | 45 (8) | <.0001 | |
| Sleep Disturbance Mean (SD) | 58 (8) | 54 (7) | 51 (7) | 46 (7) | <.0001 | |
| Social Role Satisfaction Mean (SD) | 39 (7) | 46 (7) | 51 (7) | 57 (8) | <.0001 | |
| Pain Interference Mean (SD) | 63 (7) | 55 (8) | 49 (8) | 44 (6) | <.0001 | |
| Ulcerative colitis/Indeterminate colitis | Anxiety Mean (SD) | 62 (8) | 56 (8) | 51 (8) | 46 (7) | <.0001 |
| Depression Mean (SD) | 60 (9) | 54 (8) | 49 (7) | 44 (5) | <.0001 | |
| Fatigue Mean (SD) | 65 (8) | 58 (8) | 52 (8) | 44 (8) | <.0001 | |
| Sleep Disturbance Mean (SD) | 57 (8) | 53 (7) | 50 (7) | 46 (7) | <.0001 | |
| Social Role Satisfaction Mean (SD) | 40 (7) | 46 (7) | 51 (8) | 57 (8) | <.0001 | |
| Pain Interference Mean (SD) | 62 (8) | 53 (8) | 48 (8) | 44 (6) | <.0001 | |
| Total IBD | Anxiety Mean (SD) | 62 (8) | 55 (8) | 51 (8) | 45 (7) | <.0001 |
| Depression Mean (SD) | 60 (9) | 53 (8) | 49 (7) | 44 (5) | <.0001 | |
| Fatigue Mean (SD) | 66 (8) | 58 (8) | 52 (8) | 44 (8) | <.0001 | |
| Sleep Disturbance Mean (SD) | 58 (8) | 53 (7) | 51 (7) | 46 (7) | <.0001 | |
| Social Role Satisfaction Mean (SD) | 40 (7) | 46 (7) | 51 (7) | 57 (8) | <.0001 | |
| Pain Interference Mean (SD) | 62 (7) | 54 (8) | 49 (8) | 44 (6) | <.0001 |
Patient Reported Outcome Information Measurement System items are calibrated so that the mean of the US general population is 50 and the standard deviation is 10. Higher scores indicate more of the domain being measured.
Short IBD questionnaire (SIBDQ) scores range from 10 to 70 with 10 associated with lower health-related quality of life.
p values are from a non-parametric test of trend for the ranks of across ordered groups
Additional Associations
All six PROMIS domains tested showed the expected correlation with the PROMIS measure of general health (p<0.001 for all comparisons). Prednisone use was associated with worsening patient reported functioning for all domains (Supplemental Table 2; p <0.001 for all comparisons). Notably, among UC patients, having a pouch was associated with higher functioning on all PROMIS domains, as compared with those in the highest quartile of disease activity (p< 0.001 for all domains, Supplemental Table 3). Conversely, having a pouch was associated with slightly worse functioning than patients in remission (p≤ 0.001 for all domains).
Longitudinal Evaluation of PROMIS Measures
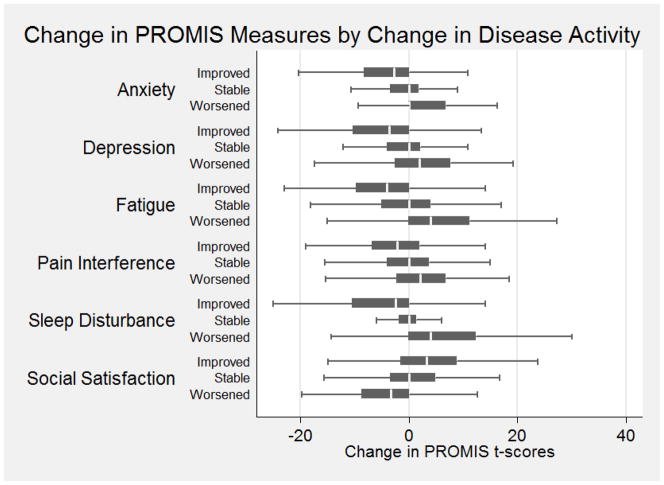
Data from 2,079 participants were available for longitudinal analyses. Of these, 229 had worsening disease activity, 1,633 had stable disease activity, and 217 had improved disease activity as measured by the SCDAI and SCCAI. The mean change in PROMIS measures for each of these groups is shown in Table 5 and Figure 1. As expected, patients with worsening disease activity had worse health outcomes for each of the PROMIS domains and those with improving disease had improved PROMIS outcome scores.
Table 5.
Relationships between Changes in Disease activity and Changes in PROMIS Scores
| PROMIS# Domain | Worsening Disease Activity& n=229 | Stable Disease Activity& n=1633 | Improving Disease Activity& n=217 | P^ | |
|---|---|---|---|---|---|
| Crohn’s disease (n=1303) | Anxiety Mean Change (SD) | 3 (9) | −1 (7) | −5 (7) | <.0001 |
| Depression Mean Change (SD) | 3 (7) | −0 (7) | −5 (7) | <.0001 | |
| Fatigue Mean Change (SD) | 6 (9) | −0 (7) | −7 (9) | <.0001 | |
| Sleep Disturbance Mean Change (SD) | 2 (9) | −0 (7) | −2 (7) | <.0001 | |
| Social Role Satisfaction Mean Change (SD) | −4 (8) | 0 (8) | 4 (8) | <.0001 | |
| Pain Interference Mean Change (SD) | 5 (9) | −0 (8) | −7 (10) | <.0001 | |
| Ulcerative colitis/Indeterminate colitis (n=776) | Anxiety Mean Change (SD) | 2 (8) | −1 (8) | −4 (10) | <.0001 |
| Depression Mean Change (SD) | 3 (7) | −1 (7) | −4 (8) | <.0001 | |
| Fatigue Mean Change (SD) | 5 (9) | −0 (8) | −4 (10) | <.0001 | |
| Sleep Disturbance Mean Change (SD) | 3 (8) | −0 (7) | −3 (8) | <.0001 | |
| Social Role Satisfaction Mean Change (SD) | −4 (9) | 1 (8) | 4 (10) | <.0001 | |
| Pain Interference Mean Change (SD) | 5 (10) | 0 (7) | −3 (10) | <.0001 | |
| Total IBD (n=2079) | Anxiety Mean Change (SD) | 3 (8) | −1 (7) | −4 (9) | <.0001 |
| Depression Mean Change (SD) | 3 (7) | −1 (7) | −4 (8) | <.0001 | |
| Fatigue Mean Change (SD) | 5 (9) | −0 (8) | −5 (10) | <.0001 | |
| Sleep Disturbance Mean Change (SD) | 2 (8) | −0 (7) | −3 (8) | <.0001 | |
| Social Role Satisfaction Mean Change (SD) | −4 (8) | 0 (8) | 4 (9) | <.0001 | |
| Pain Interference Mean Change (SD) | 5 (10) | −0 (7) | −4 (10) | <.0001 |
Patient Reported Outcome Information Measurement System items are calibrated so that the mean of the US general population is 50 and the standard deviation is 10. Higher scores indicate more of the domain being measured.
The thresholds used to indicate changes in disease activity were ≥ 100 points for the Short Crohn’s Disease Activity Index and o≥ 2 points for the Simple Clinical Colitis Index.
p values are from a non-parametric test of trend for the ranks of across ordered groups
Figure 1.
Mean Change in Patient Reported Outcome Information Measurement System (PROMIS) scores by Change in Disease Activity. PROMIS T-scores are calibrated so that the mean of the US general population is 50 and the standard deviation is 10. Higher scores indicate more of the domain being measured. The thresholds used to indicate changes in disease activity were ≥ 100 points for the Short Crohn’s Disease Activity Index and o≥ 2 points for the Simple Clinical Colitis Index.
Discussion
Patient-reported outcomes are an essential component of patient-centered research, including clinical trials and comparative effectiveness research. The Patient-Reported Outcomes Measurement Information System (PROMIS) provides measures that are efficient (minimizes item number without compromising reliability), flexible (enables optional use of interchangeable items), and precise (has minimal error in estimate).5, 6 PROMIS measures have been extensively evaluated in the general population and in individuals with chronic illness.8 Here, we report the first wide-scale cross-sectional and longitudinal evaluation of PROMIS measures in the IBD population. Health status and functioning measured by PROMIS are associated with self-reported validated disease activity indices and an IBD-specific HRQOL instrument, and changes in disease activity were associated with changes in PROMIS measures. These data demonstrate the construct validity of PROMIS PROs in the IBD population.
We found that IBD patients in this cohort had worse PROs as compared with the general population for each of the PROMIS domains tested and similar findings to those reported for other chronic diseases. For example, mean domain scores for Depression, Anxiety, Fatigue, and Social Satisfaction were 52, 52, 54, and 48 in an arthritis population and 53, 53, 55, and 48 in a COPD population.8 Among patients in remission, PRO’s were comparable to the general population.
Consistent with population-based data suggesting that healthcare utilization is highest in the year following IBD diagnosis13, we found that patients within 1 year of diagnosis reported worse health status in all measured domains. Generally speaking, these PROs trends were related to changes in disease activity. This may also be explained by the phenomenon of “response shift”-- a change in the meaning of one’s self-evaluation as a result of a re-calibration, a change in the importance of the outcome, or a re-definition of the outcome, which has been previously described among IBD patients.14 Notably, Fatigue was the PRO most affected among our IBD cohort and was strongly associated with quartiles of disease activity, consistent with recently published findings from a population-based study in Manitoba, Canada.15
The magnitude of differences in most PROMIS measures between IBD patients in this cohort and the general population were in the range of 2–6. Similarly, the magnitude of differences in PROMIS scores across quartiles of disease activity was also in this range. Hence, data from this cohort are consistent with emerging data suggesting that MIDs for PROMIS measures are in the range of 2–6.9
Another noteworthy finding was that UC patients who have undergone prior colectomy and ileal pouch anal anastomosis (IPAA) reported better health outcomes compared with UC patients in the highest quartile of disease activity, consistent with prior reports suggesting improvement in quality of life in UC patients following colectomy.16 In fact, patients following IPAA report only slightly worse outcomes than patients in remission. These data can be used to reassure UC patients contemplating surgery, and underscore another distinct advantage of non-disease specific measures such as PROMIS—the ability to compare disease populations with the general population. In this case, patients with UC following colectomy and IPAA report health outcomes within ½ of a standard deviation from the population norm. These findings are consistent with the results of a conjoint analysis demonstrating that UC patients are equally willing to accept colectomy and IPAA versus a partial response to medical therapy.17
There are several additional implications of these findings. First, PROMIS item banks appear to be very attractive as outcome measures for clinical and epidemiological research in IBD. They have excellent construct validity, are flexible and efficient, are easy to administer and interpret, and are publicly available. Additional PROMIS item banks not included in this study (i.e. Physical Function, Pain Intensity, etc.) are also available. Because the PROMIS instruments are designed to be applicable to a range of chronic illnesses, they offer some advantages over disease-targeted instruments, such as the Short IBD Questionnaire, by allowing for comparisons across a variety of chronic health conditions and studies. Given the recent policy support for comparative effectiveness research in IBD, including the American Recovery and Reinvestment Act, and more recently, the establishment of the Patient Centered Outcomes Research Institute, there will be abundant opportunities to utilize PROMIS measures in the near future. Secondly, the high burden of emotional distress (depression, anxiety) observed in this large cohort of IBD patients reinforces prior observations regarding the high level of psychological co-morbidity in this patient population,18 highlighting the need to include proper mental health screening and treatment in clinical practice particularly for patients with incompletely controlled disease. Finally, there may also be a role for PRO assessment in the context of clinical care, perhaps facilitated through computerized adaptive testing (CAT) and automated scoring. However, further research is needed to determine whether PRO assessment will influence treatment decisions and the impact of such decisions on clinical outcomes.
In this study, we used 4 item short forms for each PROMIS domain. This demonstrates remarkably low respondent burden with apparent little loss of precision in statistical comparisons in a large study. PROMIS provides short forms of varying length and CAT. Researchers can select the length of the short form or CAT that matches their research need. Specifically, longer forms and CAT provide more measurement precision. Studies with smaller sample size may choose longer forms to improve statistical power for group comparisons.
There are several strengths to this study, including the large and geographically diverse patient population, and the prospective nature of the cohort study which allowed both cross-sectional and longitudinal analyses of PROMIS instruments. Most of the prior evaluations of PROMIS instruments were based only on cross-sectional data.8 We acknowledge several limitations. First, CCFA Partners is a volunteer sample of patients. IBD patients enrolled in CCFA Partners differ from population-based IBD cohorts (i.e. higher percentage of women) limiting the ability to make broad generalizations about patient reported outcomes among the broader IBD. Nevertheless, the associations described here still have a high degree of internal validity. Indeed, after stratifying by sex, the direction, magnitude, and strength of most associations remained unaffected. Another limitation is that IBD status and disease type in this study were identified by self-report, rather than medical records. However, preliminary results from a validation study found that physicians confirmed IBD status in 96% and IBD subtype (CD or UC/IC) in 94% of cohort participants.19 Similarly, the use of symptom-based disease activity scores is also subject to limitations including influence by superimposed irritable bowel syndrome.
In conclusion, this cross sectional and longitudinal evaluation provides strong support for the construct validity of the PROMIS instruments in the IBD population. We anticipate that the use of these PROs will advance patient centered outcomes research in IBD.
Supplementary Material
Acknowledgments
Grant Support: This research was supported, in part, by a grant from the Crohn’s and Colitis Foundation of America and support from the National Institute for Diabetes and Digestive and Kidney Diseases Grant P30 DK034987 (RSS)
Footnotes
Disclosures: No authors have a conflict of interest to disclose
Author Contributions:
MDK: study concept and design; data collection; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content.
CM: study concept and design; data collection; acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; statistical analysis.
DAD: study design; data collection; analysis and interpretation of data; critical revision of the manuscript for important intellectual content MDL: study concept and design; data collection; analysis and interpretation of data; critical revision of the manuscript for important intellectual content
PMK: data collection; critical revision of the manuscript for important intellectual content.
WC: data collection; critical revision of the manuscript for important intellectual content.
JDL: study design; data collection; analysis and interpretation of data; critical revision of the manuscript for important intellectual content.
RSS: study design; data collection; analysis and interpretation of data; critical revision of the manuscript for important intellectual content
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kappelman MD, Moore KR, Allen JK, et al. Recent Trends in the Prevalence of Crohn’s Disease and Ulcerative Colitis in a Commercially Insured US Population. Dig Dis Sci. 2012 doi: 10.1007/s10620-012-2371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eden J, Wolman D, Greenfield S, et al. Initial National Priorities for Comparative Effectiveness Research. Institute of Medicine, National Academies Press; 2009. [Google Scholar]
- 3.Patrick D, Guyatt G, Acquadro C. Chapter 17: Patient-reported outcomes. In: Higgins JPTGS, editor. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.1. The Cochrane Collaboration; 2008. [Google Scholar]
- 4.US Department of Health and Human Services Food and Drug Administration. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims. p. 20009. [Google Scholar]
- 5.Adler D. Developing the Patient-Reported Outcomes Measurement Information System (PROMIS) Medical Care. 2007;45 (Suppl 1):S1–S2. [Google Scholar]
- 6.Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45:S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long MD, Kappelman MD, Martin CF, et al. Development of an internet-based cohort of patients with inflammatory bowel diseases (CCFA Partners): Methodology and initial results. Inflamm Bowel Dis. doi: 10.1002/ibd.22895. [DOI] [PubMed] [Google Scholar]
- 8.Rothrock NE, Hays RD, Spritzer K, et al. Relative to the general US population, chronic diseases are associated with poorer health-related quality of life as measured by the Patient-Reported Outcomes Measurement Information System (PROMIS) J Clin Epidemiol. 2010;63:1195–204. doi: 10.1016/j.jclinepi.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yost KJ, Eton DT, Garcia SF, et al. Minimally important differences were estimated for six Patient-Reported Outcomes Measurement Information System-Cancer scales in advanced-stage cancer patients. Journal of Clinical Epidemiology. 2011;64:507–516. doi: 10.1016/j.jclinepi.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irvine EJ, Zhou Q, Thompson AK. The Short Inflammatory Bowel Disease Questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohn’s Relapse Prevention Trial. Am J Gastroenterol. 1996;91:1571–8. [PubMed] [Google Scholar]
- 11.Thia K, Faubion WA, Jr, Loftus EV, Jr, et al. Short CDAI: development and validation of a shortened and simplified Crohn’s disease activity index. Inflamm Bowel Dis. 17:105–11. doi: 10.1002/ibd.21400. [DOI] [PubMed] [Google Scholar]
- 12.Jowett SL, Seal CJ, Phillips E, et al. Defining relapse of ulcerative colitis using a symptom-based activity index. Scand J Gastroenterol. 2003;38:164–71. doi: 10.1080/00365520310000654. [DOI] [PubMed] [Google Scholar]
- 13.Longobardi T, Bernstein CN. Utilization of health-care resources by patients with IBD in Manitoba: a profile of time since diagnosis. Am J Gastroenterol. 2007;102:1683–91. doi: 10.1111/j.1572-0241.2007.01232.x. [DOI] [PubMed] [Google Scholar]
- 14.Lix LM, Sajobi TT, Sawatzky R, et al. Relative importance measures for reprioritization response shift. Qual Life Res. 2013;22:695–703. doi: 10.1007/s11136-012-0198-3. [DOI] [PubMed] [Google Scholar]
- 15.Graff LA, Vincent N, Walker JR, et al. A population-based study of fatigue and sleep difficulties in inflammatory bowel disease. Inflammatory Bowel Diseases. 17:1882–1889. doi: 10.1002/ibd.21580. [DOI] [PubMed] [Google Scholar]
- 16.Heikens JT, de Vries J, van Laarhoven CJ. Quality of life, health-related quality of life and health status in patients having restorative proctocolectomy with ileal pouch-anal anastomosis for ulcerative colitis: a systematic review. Colorectal Dis. 2012;14:536–44. doi: 10.1111/j.1463-1318.2010.02538.x. [DOI] [PubMed] [Google Scholar]
- 17.Bewtra M, Kilambi V, Siegel CA, et al. Patient Preferences for Surgical and Pharmaceutical Treatment of Ulcerative Colitis: When is Surgery Better Than Drugs? Gastroenterology. 142:S-68–S-69. [Google Scholar]
- 18.Graff LA, Walker JR, Bernstein CN. Depression and anxiety in inflammatory bowel disease: a review of comorbidity and management. Inflamm Bowel Dis. 2009;15:1105–18. doi: 10.1002/ibd.20873. [DOI] [PubMed] [Google Scholar]
- 19.Randell RL, Cook S, Wrennall CE, et al. 982 Validation of an Internet-Based Cohort of Patients With Inflammatory Bowel Diseases (CCFA Partners) Gastroenterology. 2013;144:S–180. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.