Abstract
Background:
Night shift workers are more vulnerable to immune-related diseases. Immunoglobulin M (IgM) is a potent activator of complement, and complement has a crucial role in defense against bacterial infections. Circadian type is known as an effective agent on vulnerability and adaptation with shift work due to non-compliance with shift stress. The objective of this study was to investigate the correlation of circadian type and chronic fatigue with the serum concentration of IgM in a group of shift workers.
Materials and Methods:
This cross-sectional study was performed in an industrial organization in Isfahan, Iran. The study population consisted of 221 male employees working at night shifts who were selected by random cluster sampling. The following questionnaires were used: composite morningness (Torsvall and Akerstedt), circadian type (Folkard), and chronic fatigue (Barton and colleagues). The serum concentration of IgM was measured by the nephelometric method. The data were analyzed with the Pearson coefficient correlation and the path analysis for finding the pattern of the structural equations to evaluate the direct and indirect relationships between variables, using the SPSS 15 and LISREL 8.5 statistical software.
Results:
Significant correlation was documented between morningness, flexibility, languidness, and chronic fatigue with the serum concentration of IgM (P < 0.01).
Conclusion:
The results showed that the shift workers with morningness and languidness experienced more problems during the working hours due to more tiredness, and had decreased serum concentration of IgM. Correct management of shift work may attenuate fatigue in workers and also improve many health issues experienced by the shift workers.
Keywords: Circadian clocks, circadian rhythm, fatigue, immunoglobulin M, sleep disorders
INTRODUCTION
Shift working is one of the detrimental factors of ergonomics and the inevitable technology souvenir. It causes devastating effects on human life in different aspects.[1] From 2004, a fifth of the labor force works in shifts all around the world.[2] From a biological perspective, humans have a nature of rhythmic life and the circadian system is designed to accept the active awakening in daylight and having a restful sleep at night.[3] Humans are naturally remain awake during the day-time. On the other hand, they are day-direction, and by working at night, the biological rhythms go out of phase. This factor causes lower compliance with the requirements and is easily exposed by way of psychological and physiological disorders. Acquisition process for the circadian night is similar to the salmon moving in the opposite direction of water flow and jumping to the top of the waterfall.[4] Spurgeon et al. showed that only 10% of people enjoy working at night shifts and others handle it somehow.[5] The most important issues are the health and longevity of the shift workers, which are sacrificed by shift working.[6] Sleeping is essential for restoration of proteins in the brain and reducing the stress. Its deprivation is associated with an irregular circadian rhythm and the same factors may interfere with the functioning of the body and, in particular, the activity of the nervous system and the hypothalamic, pituitary, and adrenal functions.[7] The stress related to the shifts, which occurs due to sleeplessness, decreases the glucose levels,[8] reduces the maximal activity, limits the power of the individual due to the decrease of anaerobic power to sustain activity, and increases the heart's need for oxygen.[9] The continuation of this process would increase agitation and fatigue during shifts,[10] as well as the risk of heart and gastrointestinal diseases.[11] Studies of the Cancer International Association have shown that shift workers are among the high-risk group for cancer.[12] They are more prone to develop several infectious diseases.[13] Immune response parameters with a circadian organized regular rhythm help in the compliance of immune response by synthesis and release of large amounts of melatonin.[14] In the process of working at night shifts, the stress due to sleep deprivation increases the adrenocorticotropin secretion, and in response to environmental stress factors, increases the cortisol secretion, which is regulated by the brain and endocrine responses.[15,16] Following the positive effects of cortisol, it increases over the long period, and hence it acts as a mediator of stress.[17] If the process of sleep deprivation is applied continuously, the level of cortisol would increase up to the limit of pharmacological concentrations. In the short term, it keeps the individual safe; but in the long term, it would damage the brain due to the prompt breakdown of fats and proteins and an increase in the blood sugar, which can be dangerous.[18]
The increased secretion of cortisol also causes the atrophy of the lymphoid tissues and down-regulates the expression of interleukin (IL)-1 and IL-2 receptors on the T lymphocytes. These events eventually lead to decreased amounts of amino acids, which are essential for the B cell proliferation and IgM synthesis.[19,20] It also decreases the number of B lymphocytes and production of IgM. This immunoglobulin is an antigen receptor of naïve B lymphocytes, and binds to the specific antigens and plays an important role in the elimination of the bacteria entering the bloodstream through complement activation.[21]
By decreasing the serum concentration of IgM, the individual predisposes to several infectious diseases, allergies, cancers, and autoimmune disorders influenced by the psychological conditions.[22] Interferon production by lymphocytes increases during periods of insomnia and it reduces the capacity of multi-core white blood cells and natural killer cells (NKCs).[23] Individual differences as effective agents may be involved in predicting compatibility with the night work shifts and determine the degree of vulnerability of the individual.[24,25]
Because of the effective physiological changes in the circadian cycle, people are categorized into two groups, based on their own activity peak time in the morning and evening.[26] People in morningness group often wake up at sunrise and go to sleep early at night. In contrast, those in eveningness group are awake during the night and are most active late at night. Their performance is undesirable during the day.[27] Morningness type can evaluate three other factors of morning orientation, stable sleep pattern, and strength and power. People who are more flexible in stable sleep pattern and gain a high score of power have less damage caused by shift working than that experienced by others.[28] Personality traits are associated with chronic fatigue, such as flexibility, vitality, and eveningness, and predict the physical and psychological problems.[29] Therefore, night shift working affects the immune response components, and it seems that the circadian type of sleeping for working at the night shift may affect IgM change through fatigue by significant fluctuations in the biological rhythms of hormones. This study aimed to investigate the correlation of circadian type and chronic fatigue due to shift work with the serum concentration of IgM.
MATERIALS AND METHODS
Subjects
This cross-sectional study was conducted among male night shift workers in one of the industrial organizations of Isfahan, the second large city of Iran. They had to stay awake at night for work. Participants were selected by random cluster sampling; firstly, four industrial divisions were chosen among all the divisions, which were engaged at night shift work. Subsequently, 221 healthy workers were selected from these four divisions, based on the community size.[30] The sample size was calculated, based on the Krejcie and Morgan criteria, considering the permissible error of 5%, the confidence level of 95% and the standard deviation of the current variable in the community.
Written informed consent was obtained from all workers to contribute to this study and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected in a prior approval by the institution's human research committee. All workers were notified of their assay results. Workers were not included if they had chronic physical and mental illnesses according to the medical records of the Department of Industrial Medicine in the previous 6 months, or if they had used any medication that could have influenced the immune system (e.g. medications for hypersensitivity or rheumatologic disorders). In addition, smokers, those with hypertension, or those who participated in a working environment with more than 60 db continuous sound, or reported upper respiratory tract infection on the day of the visit, were not included either. Questionnaires were used in order to measure the variables related to circadian type in the two components of morningness and eveningness and chronic fatigue. The divisions were similar in terms of work rigidity, and workers worked in the technical division.
Composite morningness questionnaire
It consisted of 13 questions for the evaluating the individuals’ circadian type. This questionnaire was introduced by Torsvall and Akerstedt in 1980 to assess the circadian type. It assesses three aspects of sleep time (morningness, eveningness, and moderate). Scores of less than 22 indicate eveningness type, between 23 and 43 represent the moderate type, and scores above 44 indicate morningness type. The reliability coefficient obtained using Cronbach's alpha was equal to 0.91.
Circadian type inventory
The inventory consists of 30 questions and has two subscales of languidness/vigorousness and flexibility/rigidity. The reliability coefficients calculated using Cronbach's alpha were equal to 0.79 for the first factor and 0.83 for the second factor, respectively.
Chronic fatigue questionnaire
This questionnaire has 10 questions and it has been normalized for the shift workers by Barton and colleagues (1995). Response scale for this question is the 3-point Likert scale (never, sometimes, and always). Cronbach's alpha reliability coefficient of 0.84 was obtained for this questionnaire.[30]
Measurement of the serum IgM concentration
Blood samples were collected in vacutainer serum isolation test tubes at the end of the work shift. The samples were retained at room temperature for 30 min and then analyzed by the laboratory of the Industrial, Medicine Unit of the organization. Moreover, the workers were requested to refrain from smoking before the blood samples were obtained. Forty microliters of blood sample was added to 400 μl of diluents (Minineph IgM buffer, Minineph human IgM kit, product code: ZK012.R, the approximate measuring range is 0.25-4.00 g/l) in a sample dilution tube (1/11 dilution). Subsequently, 40 μl of Minineph human IgM antiserum was added to the mixture, which was finally placed in the instrument for IgM assay. Controls of same dilution (1/11) using the same diluents were also prepared for accuracy. The samples were analyzed by the nephelometry method (using the Behring nephelometer), based on light scattering after 180 s. Actual and quantitative concentrations of serum IgM are shown in Table 1. Data were analyzed using the Pearson correlation coefficient and path analysis for the structural equation modeling in Statistical Package for Social Sciences (SPSS) version 15.0 (SPSS Inc., Chicago, IL, USA) and LISREL 8.5 (analysis of linear structural relationships) software.
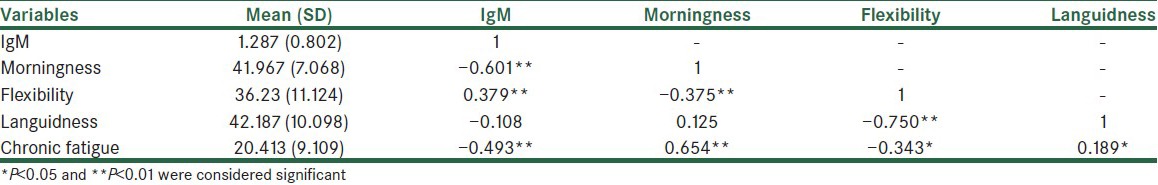
Table 1.
Descriptive indicators and correlations between the research variables
The proposed model provided for the path analysis of the variables is depicted in Figure 1. The obtained standardized coefficients are shown in this model.
Figure 1.
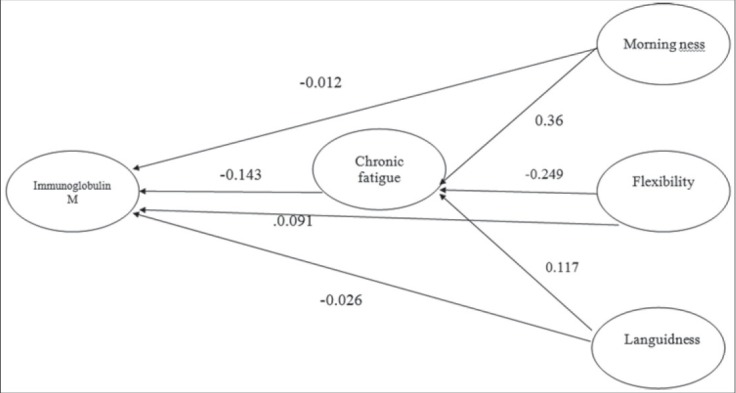
Theoritical model of the study
RESULTS
The mean and standard deviation (SD) of the age of the workers were 35.4 ± 2.49 (range 29-42) years.
Table 1 displays the descriptive indicators and correlations between the variables. It presents significant correlations of the serum IgM concentration with morningness (r = −0.601), flexibility (r = 0.379), and chronic fatigue (r = −0.493), respectively (P < 0.01).
Table 2 and Figure 1 display three model variables of morningness, languidness, and flexibility, which had indirect influence on the serum IgM concentration in three pathways through chronic fatigue. Among the three variables, morningness and flexibility had direct impact on the serum IgM concentration. The depicted model had the favorable conditions concerning fitting and fit indices. The Chi-square was equal to 1.56 and was significant (P < 0.05). On the other hand, Adjusted of fit good index =0.97, Goodness of fit Index =0.94, and Root mean squares error approximation =0.03. For the indexes related to AGFI and GFI, values higher than 0.9, and for the RMSEA, values lower than 0.06 have been reported favorable. Thus, by considering the values of model parameters, it could be pointed out that the final model was an appropriate fit with the research data.
Table 2.
Standardized coefficients of pathways of the revised and final pattern
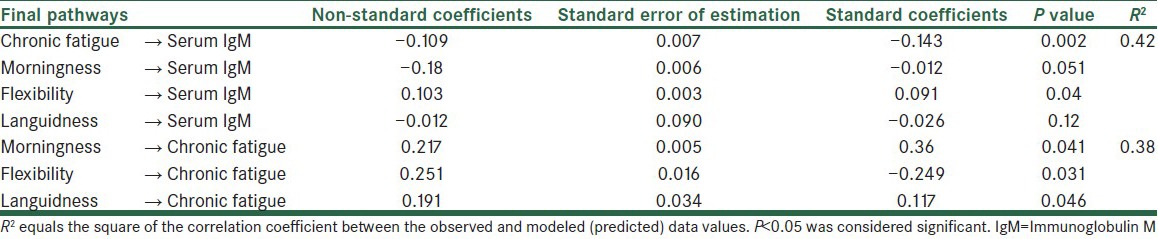
Table 2 shows that morningness, chronic fatigue, and flexibility provide 42% of the IgM variance. In addition, morningness, flexibility, and languidness show 38% of the chronic fatigue variance. However, the standard coefficients are significantly representative of all the indirect effects for the final model. The results of this study also show that the indirect effects of morningness, flexibility, and languidness on chronic fatigue are significant at a level of 0.01%.
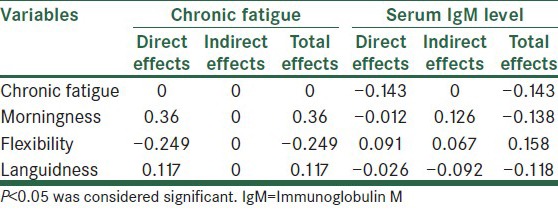
Table 3 shows that the standardized coefficients confirmed the statistical significance of all indirect effects for the final model. According to the survey data, most likely, the indirect effects of morningness, flexibility, and languidness through chronic fatigue were significant (P < 0.01).
Table 3.
Standardized direct and indirect effects of the fatigue model variables and IgM
DISCUSSION
This study aimed to investigate the correlation of circadian type and chronic fatigue due to shift work with the serum concentration of IgM. Sleep deprivation due to night shift causes irregular circadian rhythm and other sudden changes in the body. This deprivation also causes disturbances in the function of gastrointestinal tract, circulatory system, central nervous system (CNS), and brain. These disturbances lastly affect the immune system function badly, and thus break the resistance mechanisms against different diseases and ailments.[31] What makes this research distinct from others is presentation of a fundamental model, based on the theoretical interpretation, directed toward the purposes of this study.[32,33] The results of pattern-making of the structural equation and the prediction of suggested research pattern, confirmed that there was a correlation between the morningness, flexibility, languidness, and chronic fatigue with the serum concentration of IgM. We supposed that in the initial pattern, there was a correlation between chronic fatigue and the serum concentration of IgM. In addition, the effects of the three variables of morningness, flexibility, and languidness are mediated by chronic fatigue. The findings of this research model showed that there was a negative correlation between chronic fatigue and the serum concentration of IgM. To the best of our own knowledge, we did not find any similar study in literature for comparison of our findings. Nevertheless, the findings of this study agree with the assessment of other components of the immune system of the shift workers in other studies.[34,35,36] Morning types during shift work become more tired, and they find it difficult to stay awake at night or go to bed late. They are also more sensitive to the conditions of physical and social environments/deregulation of circadian type. Interestingly, their bio-circadian type is normally prone to prompt response to any stimulus. In other words, they act swiftly. Morning type is related to the duration of free dynamics of circadian type changes, so that duration in the morningness and eveningness groups is 24.3 and 25.5 hours, respectively. Because night shift demands delayed phase in the behavior and circadian type, the long period of free dynamics (25.5 hours) is more helpful. In addition, adaptation of morningness to the night shift is less and they become tired quickly and lose the defense mechanism against any disorder.[3]
Shift work deprives the body of sufficient sleep, and this causes detrimental effects on the endocrine glands, CNS, and activity of adrenal glands and, consequently, on the synthesis and release of corticosteroid hormones. These malfunctions cause more tiredness and also suppression of the immune system.[37,38] Morning and flexibility types are influenced by the genetic backgrounds, physiological conditions, changes in the nervous network function, and uncoordinated function of superior chiasmatic nucleus during shift work, which are sensitive to change due to shift work. Thus, these large numbers of factors prepare them to show more changes regarding the release of sleep-related hormones, melatonin and cortisol. Any uncoordinated and fluctuation in the synthesis and release of these hormones, which activate some components of the immune system, decline the production of IgM from the lymphoid tissues and organs.[39] Any change in the sleep time of morning type causes the hypothalamus nucleus to change the nervous network of consciousness and, hence, cannot create a good performance for the shift workers. Therefore, the first detrimental effect is rapid exhaustion. During this process, the cortisol level increases abruptly and then reduces quickly. This process, in turn, reduces the serum level of IgM. However, this process postpones the reduction of the serum concentration of IgM in the eveningness and flexibility types.[40,41,42,43]
CONCLUSIONS
This study suggests that the types of morningness, flexible, and languidness influence the serum concentration of IgM through the chronic fatigue. The morningness and languidness groups were facing more problems during night shifts due to failure of the immune system function. The model presented in this research highlights the importance of health promotion of the night shifts and proper selection of the shift workers. Thus, it is suggested to check other personality factors in order to provide the standard model for shift working.
ACKNOWLEDGEMENTS
We are very grateful to Mr. Ali Saffaei from the Pharmacy Students’ Research Committee, School of Pharmacy, Isfahan University of Medical Sciences, for his invaluable technical aids.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.British Medical Association. Shift Patterns Must Improve: Minimizing the Risks. 2010. [Last accessed on 2013 Jun 2]. Available from: http://www.bma.org.uk/images/shiftwork_tcm26-196305.pdf .
- 2.Pévet P. Melatonin and biological rhythms. Biol Signals Recept. 2000;9:203–12. doi: 10.1159/000014640. [DOI] [PubMed] [Google Scholar]
- 3.Folkard S. Do permanent night workers show circadian adjustment? A review based on the endogenous melatonin rhythm. Chronobiol Int. 2008;25:215–24. doi: 10.1080/07420520802106835. [DOI] [PubMed] [Google Scholar]
- 4.Reinberg A, Ashkenazi I. Internal desynchronization of circadian rhythms and tolerance to shift work. Chronobiol Int. 2008;25:625–43. doi: 10.1080/07420520802256101. [DOI] [PubMed] [Google Scholar]
- 5.Spurgeon A, Harrington JM, Cooper CL. Health and safety problems associated with long working hours: A review of the current position. Occup Environ Med. 1997;54:367–75. doi: 10.1136/oem.54.6.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knutsson A. Health disorders of shift workers. Occup Med (Lond) 2003;53:103–8. doi: 10.1093/occmed/kqg048. [DOI] [PubMed] [Google Scholar]
- 7.Swaab DF, Slob AK, Houtsmuller EJ, Brand T, Zhou JN. Increased number of vasopressin neurons in the suprachiasmatic nucleus (SCN) of ‘bisexual’ adult male rats following perinatal treatment with the aromatase blocker ATD. Brain Res Dev Brain Res. 1995;85:273–9. doi: 10.1016/0165-3806(94)00218-o. [DOI] [PubMed] [Google Scholar]
- 8.Jerjes WK, Cleare AJ, Wessely S, Wood PJ, Taylor NF. Diurnal patterns of salivary cortisol and cortisone output in chronic fatigue syndrome. J Affect Disord. 2005;87:299–304. doi: 10.1016/j.jad.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Prather AA, Marsland AL, Hall M, Neumann SA, Muldoon MF, Manuck SB. Normative variation in self-reported sleep quality and sleep debt is associated with stimulated pro-inflammatory cytokine production. Biol Psychol. 2009;82:12–7. doi: 10.1016/j.biopsycho.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi M, Tanigawa T, Tachibana N, Mutou K, Kage Y, Smith L, et al. Modifying effects of perceived adaptation to shift work on health, wellbeing, and alertness on the job among nuclear power plant operators. Ind Health. 2005;43:171–8. doi: 10.2486/indhealth.43.171. [DOI] [PubMed] [Google Scholar]
- 11.Copertaro A, Bracci M, Barbaresi M, Santarelli L. Assessment of cardiovascular risk in shift healthcare workers. Eur J Cardiovasc Prev Rehabil. 2008;15:224–9. doi: 10.1097/HJR.0b013e3282f364c0. [DOI] [PubMed] [Google Scholar]
- 12.Nagai M, Morikawa Y, Kitaoka K, Nakamura K, Sakurai M, Nishijo M, et al. Effects of fatigue on immune function in nurses performing shift work. J Occup Health. 2011;53:312–9. doi: 10.1539/joh.10-0072-oa. [DOI] [PubMed] [Google Scholar]
- 13.Godbout JP, Glaser R. Stress-induced immune dysregulation: Implications for wound healing, infectious disease and cancer. J Neuroimmune Pharmacol. 2006;1:421–7. doi: 10.1007/s11481-006-9036-0. [DOI] [PubMed] [Google Scholar]
- 14.Carrillo-Vico A, Guerrero JM, Lardone PJ, Reiter RJ. A review of the multiple actions of melatonin on the immune system. Endocrine. 2005;27:189–200. doi: 10.1385/ENDO:27:2:189. [DOI] [PubMed] [Google Scholar]
- 15.Ravetch J, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–90. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 16.Anderson BL. Cancer. In: Friedman HS, editor. Encyclopedia of Mental Health. San Diego: Academic Press; 1998. pp. 373–8. [Google Scholar]
- 17.Goodkin K, Visser AP. 1st ed. Washington, DC: American Psychiatric Publishing; 2000. Psychoneuroimmunology: Stress, Mental Disorders, and Health; pp. 211–216. [Google Scholar]
- 18.Barrett KE, Boitano S, Barman SM, Brooks HL. 24th ed. New York, NY: The McGraw-Hill Companies; 2012. Ganong's Review of Medical Physiology; pp. 146–169. [Google Scholar]
- 19.Guyton AC, Hall JE. 9th ed. Philadelphia: WB Saunders; 1996. Textbook of Medical Physiology; pp. 121–138. [Google Scholar]
- 20.Segerstrom SC, Miller GF. Psychological stress and the human immune system: A meta-analytic study of 30 years of inguriy. Psychol Bull. 2004;130:601–30. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciaccio C. B cell activation and antibody production. In: Abbas AK, Lichtman AH, Pillai S, editors. Cellular and Molecular Immunology. Philadelphia: WB Elsevier Saunders Company; 2012. pp. 243–68. [Google Scholar]
- 22.Casale G, Marinoni GL, d’Angelo R, de Nicola P. Circadian rhythm of immunoglobulins in aged persons. Age Ageing. 1983;12:81–5. doi: 10.1093/ageing/12.1.81. [DOI] [PubMed] [Google Scholar]
- 23.Copertaro A, Bracci M, Gesuita R, Carle F, Amati M, Baldassari M, et al. Influence of shift-work on selected immune variables in nurses. Ind Health. 2011;49:597–604. doi: 10.2486/indhealth.ms1210. [DOI] [PubMed] [Google Scholar]
- 24.Härmä M. Circadian adaptation to shift work. A review. In: Hornberger S, Knauth P, Costa G, Folkard S, editors. Shift Work in the 21st Century. Frankfurt: Peter Lang; 2000. pp. 125–30. [Google Scholar]
- 25.Davison GC, Neale J. 3rd ed. New York: John Wiley and Sons; 2001. Abnormal Psychology; p. 189. [Google Scholar]
- 26.Khodapanahi MK. Iran, Tehran: Publication of Samt; 2001. Physiological Psychology; p. 86. [Google Scholar]
- 27.Adan A, Fabbri M, Natale V, Prat B. Sleep beliefs scale (SBS) and circadian typology. J Sleep Res. 2006;15:125–32. doi: 10.1111/j.1365-2869.2006.00509.x. [DOI] [PubMed] [Google Scholar]
- 28.Folkard S, Monk TH, Lobban MC. Short and long-term adjustment of circadian rhythms in ‘permanent’ night nurses. Ergonomics. 1978;21:785–99. doi: 10.1080/00140137808931782. [DOI] [PubMed] [Google Scholar]
- 29.Smith CS, Folkard S, Schmieder RA, Parra LF, Spelten E, Almiral H, et al. Investigation of morning-evening orientation in six countries using the preferences scale. Pers Individ Dif. 2002;32:949–68. [Google Scholar]
- 30.Cohen L, Manion L, Morrison K. 5th ed. London: Routledge; 2000. Research Methods in Education; p. 211. [Google Scholar]
- 31.Barton J, Spelten E, Totterdell P, Smith LR, Folkard S, Costa G. The standard shiftwork index: A battery of questionnaires for assessing shift work-related problems. Work Stress. 1995;9:3–30. [Google Scholar]
- 32.Steinman L. Elaborate interactions between the immune and nervous systems. Nat Immunol. 2004;5:575–81. doi: 10.1038/ni1078. [DOI] [PubMed] [Google Scholar]
- 33.Díaz-Morales JF. Morning and evening-types: Exploring their personality styles. Pers Individ Dif. 2007;43:769–78. [Google Scholar]
- 34.Mitchell PJ, Radman JR. The relationship between morningness-eveningness, personality and habitual caffeine consumption. Pers Individ Dif. 1993;15:105–8. [Google Scholar]
- 35.Nagai M, Morikawa Y, Kitaoka K, Nakamura K, Sakurai M, Nishijo M, et al. Effects of fatigue on immune function in nurses performing shift work. J Occup Health. 2011;53:312–9. doi: 10.1539/joh.10-0072-oa. [DOI] [PubMed] [Google Scholar]
- 36.van Mark A, Weiler SW, Schröder M, Otto A, Jauch-Chara K, Groneberg DA, et al. The impact of shift work induced chronic circadian disruption on IL-6 and TNF-alpha immune responses. J Occup Med Toxicol. 2010;5:18. doi: 10.1186/1745-6673-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shakhar K, Valdimarsdottir HB, Guevarra JS, Bovbjerg DH. Sleep, fatigue, and NK cell activity in healthy volunteers: Significant relationships revealed by within subject analyses. Brain Behav Immun. 2007;21:180–4. doi: 10.1016/j.bbi.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Ader R, Cohen N, Felten D. Psychoneuroimmunology: Interactions between the nervous system and the immune system. Lancet. 1995;345:99–103. doi: 10.1016/s0140-6736(95)90066-7. [DOI] [PubMed] [Google Scholar]
- 39.Ziemssen T, Kern S. Psychoneuroimmunology--cross-talk between the immune and nervous systems. J Neurol. 2007;254(Suppl 2):II8–11. doi: 10.1007/s00415-007-2003-8. [DOI] [PubMed] [Google Scholar]
- 40.Matchock RL, Mordkoff JT. Chronotype and time-of-day influences on the alerting, orienting, and executive components of attention. Exp Brain Res. 2009;192:189–8. doi: 10.1007/s00221-008-1567-6. [DOI] [PubMed] [Google Scholar]
- 41.Richardson G, Tate B. Hormonal and pharmacological manipulation of circadian clock: Recent developments and future strategies. Sleep. 2000;23(Suppl 3):S77–85. [PubMed] [Google Scholar]
- 42.Arushanian EB, Baĭda OA, Mastiagin SS, Popov AV. Significance of chronotypic specificity of healthy individuals for the variability of cardiac rhythm. Fiziol Cheloveka. 2006;32:80–3. [PubMed] [Google Scholar]
- 43.Khaleghipour S, Masjedi M, Ahadi H, Enayate M, Pasha G, Nadery F, et al. Morning and nocturnal serum melatonin rhythm levels in patients with major depressive disorder: An analytical cross-sectional study. Sao Paulo Med J. 2012;130:167–72. doi: 10.1590/S1516-31802012000300006. [DOI] [PMC free article] [PubMed] [Google Scholar]


