Abstract
Background:
Wastewater contains a variety of pathogens and bio -aerosols generated during the wastewater treatment process, which could be a potential health risk for exposed individuals. This study was carried out to detect Legionella spp. in the bio -aerosols generated from different processes of a wastewater treatment plant (WWTP) in Isfahan, Iran, and the downwind distances.
Materials and Methods:
A total of 54 air samples were collected and analyzed for the presence of Legionella spp. by a nested- polymerase chain reaction (PCR) assay. A liquid impingement biosampler was used to capture bio -aerosols. The weather conditions were also recorded.
Results:
Legionella were detected in 6% of the samples, including air samples above the aeration tank (1/9), belt filter press (1/9), and 250 m downwind (1/9).
Conclusion:
The result of this study revealed the presence of Legionella spp. in air samples of a WWTP and downwind distance, which consequently represent a potential health risk to the exposed individuals.
Keywords: Air, Bio –aerosol, Legionella spp., nested- PCR, wastewater treatment plant
INTRODUCTION
Legionella species are gram negative and non -spore bacilli, which are ubiquitous in natural and man -made aquatic environments, such as, lakes, rivers, reservoirs, cooling towers, and whirlpools.[1,2,3,4] Legionella can survive in extreme ranges of environmental conditions, with a variety of different physiochemical factors. This is related to the fact that some protozoa and blue green algae support the growth of the Legionella spp. This association increases the resistance of Legionella to extreme ranges of environmental conditions, such as, high temperature, low pH, and biocides.[3,4,5]
Several species of Legionella, especially L. pneumophila, are human pathogens and have been associated with Legionnaires’ disease, a type of acute pneumonia, with a relatively high fatality rate, or Pontiac fever, a milder non -fatal form of Legionella infection.[3,5,6,7]
Legionella is transmitted mainly by aerosolization of contaminated water. Inhalation of aerosols from contaminated water sources leads to Legionella infections or legionellosis.[2,4,8]
Several studies have reported the detection of Legionella spp. in the wastewater of various units of WWTPs.[1,2,3,9,10] Therefore, Legionella aerosols can be produced whenever aeration or mechanical agitation is used in wastewater treatment processes. There is evidence that the presence of Legionella in wastewater has been linked with increased levels of antibodies among wastewater irrigation workers.[11] Kusnetsov et al., (2010) have reported two cases of severe pneumonia in employees working at two separate industrial WWTPs in Finland.[12]
A few studies have reported the emission of Legionella spp. in bio -aerosols generated from WWTPs.[2,7,9,13] However, the presence of Legionella in bio -aerosols of WWTPs could be influenced by several parameters, including, the concentration of these bacteria in the wastewater, characteristics of WWTP units, and the aerosolization at the different steps of treatment and weather conditions.[11,14] This study was carried out to detect Legionella spp. in the bio -aerosols generated from different processes of an activated sludge WWTP and downwind distances, using a nested -PCR method. The aim of this study was to improve our understanding about health hazards due to inhalation of bio -aerosols generated from a wastewater treatment plant in our region.
MATERIALS AND METHODS
Site description and sampling procedure
The study was carried out in a municipal WWTP of Isfahan, Iran. The WWTP has 48-hectare area, receiving a maximum wastewater flow rate in the final design of 130,000 m3/day produced by 800,000 inhabitants. The plant is operated by using the activated sludge biological system, with horizontal surface aeration.
Air samples for detection of Legionella bio -aerosols were collected from three points in the WWTP, 100 m upwind, as a background sample, and 100 and 250 m downwind from the treatment plant. A total of 54 air samples were collected during nine months, from September 2012 to July 2013. A liquid impingement biosampler (SKC Biosampler) was used to capture the airborne Legionella. The biosampler was calibrated for a flow rate of 12 l/minute at a height of 1.5 m above ground level, to simulate the breathing zone. The location of the sampling points and the average volume of air samples are presented in Table 1. The samples were transferred to the laboratory in an insulated box with cooling packs.
Table 1.
Sampling sites and air samples volumes tested in this study

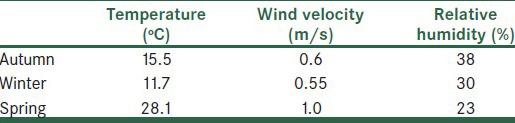
The weather conditions including temperature (°C), relative humidity (%), and wind speed (m/s) were also monitored and recorded by use of a portable weather station (Kimo), at each sampling location during collection of the Legionella bio –aerosol samples.
Detection of Legionella spp. in air samples by polymerase chain reaction assay
The impingement solution was concentrated by centrifugation. To extract the DNA, pellets were resuspended in distilled water and transferred to FTA -cards (Whatman, USA). DNA was then extracted from the FTA cards and used in the PCR. In the first PCR step, a ~ 1,420 base pair (bp) fragment of the bacterial 16S rRNA gene region was amplified using the primer set Eubac 27F (5’ -AGAGTTTGATCCTGGCTCA <G>) and 1492R (5’ -TACGGYTACCTTGTTACGACT <T>)[15] to verify the nucleic acid extraction as well as check the probable presence of inhibitors. For detection of the Legionella species, a nested -PCR technique was applied as described previously by Baghal –Asghari et al. (2013).[16] Briefly, PCR amplification was conducted in a final volume of 25 μl consisting of 2.5 μl of 10 × PCR buffer, 0.2 μM of LEG 448 and JRP primers, 0.2 mM of each dNTP, two units of Taq DNA polymerase, and 1 μl of DNA. All PCR assays contained a positive (DNA of L. pneumophila) [L. pneumophila NCTC 12821, FEPTU, HPA center for infections, United Kingdom, London] and a negative control (molecular grade water without DNA). The PCR was performed with an initial denaturation for five minutes at 95°C, followed by 30 cycles at 94°C for 45 seconds, at 55˚C for 1 minute, at 72°C for 1.30 minutes, and a final extension at 72°C for 5 minutes. The PCR products were analyzed by agarose gel electrophoresis. The gels were viewed on a ultraviolet (UV) transilluminator (UV Tech, France), and the DNA fragment sizes were compared with the 50 and 100 bp ladder DNA.
RESULTS
A total of 54 bioaerosol samples were taken at three locations in a WWTP (pump station, aeration tank, and sludge dewatering process), upwind, and downwind distances, and examined for the presence of Legionella spp. The nested -PCR assay revealed that about 6% (three out of 54 samples) of the air samples were positive for Legionella. Samples of air above the aeration tank (1/9), the belt filter press (1/9), and 250 m downwind distance (1/9), were positive for Legionella. However, all the samples were found to be positive with the PCR assay with the Eubac 27F -1492R primer set and PCR inhibition was seen in none of the 54 samples tested. Table 2 shows the results of Legionella spp. detection at the sampling points. The weather conditions are also presented in Table 3. The ambient temperature ranged from 11.7 to 28.1°C and the relative humidity from 23 to 38%. The temperature, wind speed, and humidity values during air sampling showed no relationship with the presence of positive or negative results.
Table 2.
The number of positive samples for detection of airborne Legionella spp. in WWTPs with regard to seasons and points of sampling
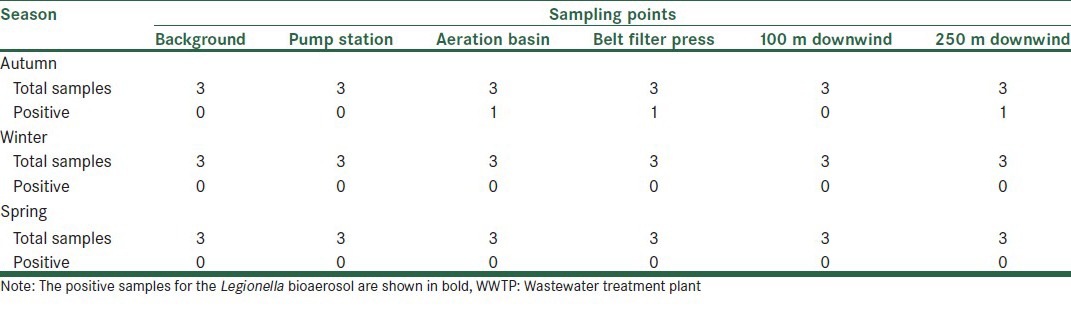
Table 3.
Meteorological parameters in the different seasons
DISCUSSION
Wastewater contains a variety of pathogenic microorganisms and WWTP workers may be exposed to these pathogens from various routes. Some of wastewater treatment processes, such as activated sludge, lead to the production of bio –aerosols, which could result in workers exposure to pathogens. Exposure to the bio -aerosols is associated with a wide range of adverse health effects and is a concern from a public-health point of view. In the present study the occurrence of Legionella bio -aerosols in the air of an activated sludge treatment plant and downwind distances was investigated.
As shown in Table 2 Legionella spp. were detected in air samples of an activated sludge and a belt filter press, simultaneously. Aeration of wastewater or application of mechanical devices could assist in the aerosolization of wastewater. As wastewater and sludge provide a suitable environment for the presence and growth of Legionella spp., aerosolization of wastewater could lead to the emission of Legionella spp. into the environment. Medemu et al., (2004), detected Legionella spp. in air samples of 10 m3 at three of the five WWTPs tested by PCR. Similar to our study, they found Legionella in the air samples of aeration tanks (2/2) and the belt filter press (1/1).[14] In the study of Pascual et al., (2001), in a WWTP; three samples from the pretreatment point (30%) and one from the biological treatment (11%) were positive for Legionella.[9] However, both studies found Legionella in air samples with only PCR, but not with a culture. Although, culture on a selective medium has commonly been used for detection of Legionella spp. in environmental samples, but the method has low sensitivity, and overgrowth by other bacteria, especially in environmental samples, could hamper the identification of Legionella.[16,17] Besides, the presence of Legionella in a viable, but non -culturable (VBNC) state, can be caused by aerosolization of airborne microorganisms, which cannot be detected by the culture method. To overcome these shortages, molecular methods such as polymerase chain reaction (PCR) amplification of DNA sequences, specific for Legionella spp., were used as sensitive and rapid methods for detection of the bacteria in the environmental samples.[18,19]
We used a PCR assay with the LEG448 -JRP primer set, with high sensitivity and specificity for detection of the Legionella spp.[16] The nested -PCR assay was also applied to further increase the sensitivity. However, the nested -PCR assay is a qualitative assay that only recognizes the presence or absence of Legionella spp., and quantitative techniques such as real- time PCR should be taken into use for the analysis of health risks posed by the Legionella bio -aerosols in the WWTPs.
The results of the study also showed the presence of Legionella in an air sample at a 250 m downwind distance from the WWTP. Some studies have reported that a downwind distance of 250 m or higher from the microbial emission sources is a safe distance from the viewpoint of safety.[20] However, bio -aerosol dispersion is affected by a number of factors such as individual bio -aerosol properties and meteorological conditions.[9,20] The presence of Legionella at a 250 m downwind distance could be a potential risk to neighbors of WWTP, however, more studies in this field are required.
As shown in Table 2 all the positive samples were related to autumn. Comparison of meteorological parameters in different seasons of sampling show higher values of relative humidity for positive samples than for negative samples [Table 3]. Bacteria generally survive better at higher relative humidity.[13] This relationship is also comparable with the results of Pascual et al., (2001), which showed that the humidity values of the Legionella positive samples at a WWTP were significantly higher than for negative samples.[9]
CONCLUSION
The results of this study revealed the presence of Legionella spp. in air samples of a WWTP and downwind distances, which consequently represent a potential health risk for exposed individuals. Further studies, however, are needed for risk assessment of inhalation of Legionella bio –aerosols from WWTPs.
ACKNOWLEDGMENT
This research was conducted with funding from the Vice Chancellery for Research at the Isfahan University of Medical Sciences (Grant No. 391081), as a part of a M.Sc dissertation.
Footnotes
Source of Support: This research was conducted with funding from the Vice Chancellery for Research at the Isfahan University of Medical Sciences (Grant No. 391081), as a part of a M.Sc dissertation
Conflict of Interest: None declared.
REFERENCES
- 1.Catalan V, Garcia F, Moreno C, Vila MJ, Apraiz D. Detection of Legionella pneumophila in wastewater by nested polymerase chain reaction. ResMicrobiol. 1997;148:71–8. doi: 10.1016/S0923-2508(97)81902-X. [DOI] [PubMed] [Google Scholar]
- 2.Palmer CJ, Bonilla GF, Roll B, Paszko-Kolva C, Sangermano LR, Fujioka RS. Detection of Legionella species in reclaimed water and air with the EnviroAmp Legionella PCR kit and direct fluorescent antibody staining. Appl Environ Microbiol. 1995;61:407–12. doi: 10.1128/aem.61.2.407-412.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmer CJ, Tsai Y, Paszko-Kolva C, Mayer C, Sangermano LR. Detection of Legionella species in sewage and ocean water by polymerase chain reaction, direct fluorescent-antibody, and plate culture methods. Appl Environ Microbiol. 1993;59:3618–24. doi: 10.1128/aem.59.11.3618-3624.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devos L, Boon N, Verstraete W. Legionella pneumophila in the environment: The occurrence of a fastidious bacterium in oligotrophic conditions. Rev Environ SciBiotechnol. 2005;4:61–74. [Google Scholar]
- 5.Atlas RM. Legionella: From environmental habitats to disease pathology, detection and control. Environ Microbiol. 1999;1:283–93. doi: 10.1046/j.1462-2920.1999.00046.x. [DOI] [PubMed] [Google Scholar]
- 6.Hilbi H, Jarraud S, Hartland E, Buchrieser C. Update on Legionnaires’ disease: Pathogenesis, epidemiology, detection and control. MolMicrobiol. 2010;76:1–11. doi: 10.1111/j.1365-2958.2010.07086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roll BM, Fujioka RS. Detection of Legionella bacteria in sewage by polymerase chain reaction and standard culture method. Water SciTechnol. 1995;31:409–16. [Google Scholar]
- 8.Stout JE, Yu VL. Legionellosis. N Engl J Med. 1997;337:682–7. doi: 10.1056/NEJM199709043371006. [DOI] [PubMed] [Google Scholar]
- 9.Pascual L, Pérez-Luz S, Amo A, Moreno C, Apraiz D, Catalán V. Detection of Legionella pneumophila in bioaerosols by polymerase chain reaction. Can J Microbiol. 2001;47:341–7. [PubMed] [Google Scholar]
- 10.Huang SW, Hsu BM, Ma PH, Chien KT. Legionella prevalence in wastewater treatment plants of Taiwan. Water SciTechnol. 2009;60:1303–10. doi: 10.2166/wst.2009.410. [DOI] [PubMed] [Google Scholar]
- 11.Bitton G. Fourth edition. Wiley -Blackwell; 2011. Wastewater Microbiology; pp. 437–463. [Google Scholar]
- 12.Kusnetsov J, Neuvonen LK, Korpio T, Uldum SA, Mentula S, Putus T, et al. Two Legionnaires’ disease cases associated with industrial waste water treatment plants: A case report. BMC Infect Dis. 2010;10:343. doi: 10.1186/1471-2334-10-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stetzenbach LD, Alvarez AJ, Buttner MP. Rapid detection of targeted airborne micro-organisms using polymerase chain reaction. Biotechnology Risk Assessment. 1999 [Google Scholar]
- 14.Medema G, Wullings B, Roeleveld P, van der Kooij D. Risk assessment of Legionella and enteric pathogens in sewage treatment works. Water SciTechnol. 2004;4:125–32. [Google Scholar]
- 15.Lane D. 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic Acid Techniques in Bacterial Systematics. New York: Wiley; 1991. pp. 15–117. [Google Scholar]
- 16.Asghari FB, Nikaeen M, Hatamzadeh M, Hassanzadeh A. Surveillance of Legionella species in hospital water systems: The significance of detection method for environmental surveillance data. J Water Health. 2013;11:713–9. doi: 10.2166/wh.2013.064. [DOI] [PubMed] [Google Scholar]
- 17.Devos L, Clymans K, Boon N, Verstraete W. Evaluation of nested PCR assays for the detection of Legionella pneumophila in a wide range of aquatic samples. J ApplMicrobiol. 2005;99:916–25. doi: 10.1111/j.1365-2672.2005.02668.x. [DOI] [PubMed] [Google Scholar]
- 18.Alvarez AJ, Buttner MP, Stetzenbach LD. PCR for bioaerosol monitoring: Sensitivity and environmental interference. Appl Environ Microbiol. 1995;61:3639–44. doi: 10.1128/aem.61.10.3639-3644.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toze S. PCR and the detection of microbial pathogens in water and wastewater. Water Res. 1999;33:3545–56. [Google Scholar]
- 20.Stellacci P, Liberti L, Notarnicola M, Haas CN. Hygienic sustainability of site location of wastewater treatment plants: A case study. II. Estimating airborne biological hazard. Desalination. 2010;253:106–11. [Google Scholar]


