Abstract
Context:
Diabetes affects 9.2% of adults in India. About 8–16% of its population also suffer from depression. Both diseases pose a serious health challenge at individual and system level. The prevalence of depression in diabetes is much higher than in the general population. Undiagnosed and untreated depression puts people at higher morbidity and mortality risk.
Aim:
To study the prevalence of depression in diabetes and to identify associated risk factors.
Settings and Design:
Case control study carried out in an outpatient setting of a tertiary hospital in central India.
Materials and Methods:
One hundred and nine type 2 diabetes patients and 91 healthy controls formed the subjects of the study. Sociodemographic data were obtained on seven parameters. Comprehensive clinical data were obtained by means of standard procedures. Blood sugar levels and glycosylated hemoglobin levels were measured to assess glycemic control. Data of diabetic patients and controls as well as that of depressed and nondepressed diabetics were subjected to statistical analysis.
Results:
About 42.2% of diabetes patients and only 4.39% of controls had depression. About 19% of diabetics had peripheral neuropathy but had much higher neuropathic symptoms. Depression was not related to any sociodemographic or clinical factors but was strongly associated with poor glycemic control.
Conclusion:
Depression is highly prevalent in diabetes. Physical symptoms mask depression. Special attention needs to be paid to diagnose depression in diabetes and treat it appropriately along with effective glycemic control. Diabetes patients need to be treated collaboratively by physicians and psychiatrists.
Keywords: Centre for Epidemiological Studies Depression, clinical factors, depression, glycemic control, sociodemographic factors, type 2 diabetes
Diabetes mellitus comprises a group of common metabolic disorders that share the phenotype of hyperglycemia. The widespread metabolic dysregulation associated with diabetes impairs the functioning of multiple organ systems and imposes tremendous physical and psychological burden.[1] Both the ancient Egyptians and Indians described the condition as early as 1500 BCE. While the Egyptians described it as “too great emptying of urine” around the same time, or even earlier perhaps, the Indians went a step ahead to describe it as “Madhu meha” which when translated from Sanskrit literally means “honey urine.”[2] Perhaps depressive disorders are also of equal antiquity as descriptions of it figure quite prominently in the epics Ramayana and Mahabharata, as well as ancient medical monographs of India.[3] Today, the world is bracing against these two noncommunicable diseases.[4] India has the dubious distinction of being the possible capital of both the diseases.[5,6]
Currently, an estimated 382 million people are suffering from diabetes in the world. An additional 320 million people may be having impaired glucose tolerance and therefore at risk for diabetes. Indians are more susceptible for diabetes because of increased insulin resistance, abdominal obesity, decreased adinopectin and increased C-reactive protein levels. The prevalence of diabetes in India found to range from 2.7% to 15.1%.[5] As per the International Diabetic Federation 2013 9.1% of the adult population may be suffering from diabetes in India.[7] Globally, 4.3% of adult women and 3.2% of adult men suffer from depression.[8] Widely variable prevalence figures (1.7–74/1000) have been reported from India. In a recent study, from South India, 15.1% of the population were found to have depression.[9]
Depression and diabetes share a bidirectional relationship feeding positively back into each other. Diabetics are twice as likely to be depressed as those without diabetes.[10,11] The presence of depression significantly increases the likelihood of later developing type 2 diabetes mellitus.[12,13]
Depression and diabetes coexist in the world regardless of culture or country. An estimated 9% of diabetics are affected by depressive disorders while another 26% suffer from lesser forms of depression.[4] What makes the relationship unnerving is the fact that only a quarter, or even less, may be getting treatment[8] and that untreated depression is linked to adverse outcomes in the form of persisting hyperglycemia[14,15,16] poor adherence to medication and self-care micro and macrovascular complications;[17,18] all leading to poor quality of life, symptom burden[4] and even increased common mortality.[19,20]
In one of the earliest studies, Murrel et al.[21] investigated 175 diabetic patients and 227 healthy controls. They screened for depression using Center for Epidemiological Studies Depression (CES-D) with 20 as a cut-off score. Females constituted 66% of the sample. They found a prevalence of 21.7 in the sample while in the control it was 16%. More females had depression (25%) in comparison to males (15%). Lustman et al.[22] studied 114 type 1 and type 2 diabetes patients (females 66%) with a mean age of 40 years using diagnostic interview schedule (DIS) and found 14% had depression. Friis and Nanjundappa[23] compared 56 diabetic patients (mean age 60 years) with the same number of medically ill (mean age 63 years) for prevalence of depression using CES-D with 16 as the cut-off score. They found high rates of depression that is, 61% and 42% respectively. It is possible that the rather large depressives in their sample were due to a large number of females in their sample (71–73%).
Robinson et al.[24] investigated 130 mixed diabetic patients and an equal number of controls. Their sample was predominantly male (65%) and young (mean age 44 years). Using present status examination they found a prevalence of only 8.5% in both the study group and control. Popkin et al.[25] used DIS to study 75 type 1 cases and compared with 34 healthy 1st degree relatives and found a prevalence of 10.7 and 2.9% respectively. Mean age of their sample was 31. Using 50 as cut-off score on the Zung scale, Geringer et al.[26] found 18.8 prevalence in their sample of 64 type 2 female patients. Zhang et al.[27] compared 209 mixed diabetic patients with 1289 healthy controls using DIS and CES-D. They found a prevalence of 3.8 and 3.6% respectively in spite of having more females (58.4%) in the study group. They had no whites in their study group.
Songar et al.[28] used a Beck's Depression Inventory score of 14 as cut-off and studied 60 diabetic patients and 30 healthy controls. They found a prevalence of 43.3% and 3.3% respectively. Bourdel-Marchasson et al.[29] used a CES-D cut-off of 17 for males and 23 for females for 237 diabetics and found a prevalence 21.3% that was significantly high in comparison with controls (n = 2555). Only 12.7% of the controls had depression.
Peyrot and Rubin[30] investigated 634 diabetic patients. They used CES-D 16 as cut-off and found a prevalence of 41.3%. In a novel study, Pouwer et al.[31] investigated 52 diabetics without co-morbidity 162 with co-morbidity and 1184 healthy controls. They used the CES-D and set a cut-off of 16. Overall there was not much of difference when the diabetic subjects were combined and compared with controls (9.3% vs. 8.9%) but when diabetics with co-morbidity were compared with controls marked difference was evident (19.8% vs. 8.9% odds ratio [OR] 2.0)
In a recent fresh study, Pouwer et al.[32] took a random sample of 772 mixed cases of diabetes for study. They used composite international diagnostic interview and CES-D (cut-off 16) and found overall 9% major depression and 23% minor depression (total 32%) type 2 diabetics had relatively more depressed patients.
Raval et al.[33] from India examined 300 cases of diabetes of which males constituted 49% and the rest female. Medium duration was 8 years (4–13 years). They used the Patient Health Questionnaire (PHQ)-9 Hindi versions to ascertain depression and found in all 41% had depression (mild 18% moderate/severe 23%) mean age was 54.2 ± 10 years. They found strong association between depression and age (>54), neuropathy, nephropathy, body mass index (BMI) (>25), income >5000 INR/month, diabetic foot and pill burden. No association was found with hypertension, glycemic control, sex, duration of diabetes and cerebrovascular disease.
In another Indian study, Poongothai et al.[34] investigated 847 patients. They used PHQ-12 modified version and detected the depression in 198 patients (23.4%). Mean age in the depressives was significantly low (49.1 vs. 51.5). Mean BMI was nonsignificantly less (24.9 vs. 25.3). No difference in duration of diabetes, serum creatinine and glycosylated hemoglobin (HbA1c) was found. Patients having neuropathy, nephropathy, retinopathy, and peripheral vascular disease had significantly high depression.
Anderson et al.,[10] in one of the earliest meta-analysis, examined 42 studies with a combined sample size of 21,351 subjects. The selected studies included 20 controlled studies. In the controlled group, the odds of depression were twice that of the nondiabetic comparison group (OR = 2 95% confidence interval [CI]: 1.8–2.2). The prevalence was higher in diabetic women (28%) than in diabetic men (18%). Prevalence was high in un-controlled studies (30%) than in controlled studies (21%), in clinical (32%) than in community (20%) samples and when assessed by self-report questionnaires (31%) than by standard diagnostic interviews (11%).
Kruse et al.[35] in a cross-sectional study of 141 patients found that the psychiatric morbidity was same as that of nondiabetic individuals (26.6% vs. 26%). Das-Munshi et al.[36] in a study of 249 patients found no difference in morbidity between diabetics and nondiabetics.
Rustad et al.[37] in a comprehensive review enumerated the factors responsible for negative impact of depression over diabetes and these included worsening glycemic control, increased number and severity of complications, increased likelihood of cardiovascular risk factors, higher functional disability and nonadherence to diabetes self-care.
In a meta-analysis of 27 studies (n = 5374) de Groot et al.[38] found a significant association between retinopathy, nephropathy, neuropathy, macrovascular complications and sexual dysfunction. In another meta-analysis Lustman et al.[14] found depression was significantly associated with hyperglycaemia (P < 0.0001).
Lin et al.[39] followed-up 4623 patients over 5 years. They found 36% of patients with diabetes and depression are at high risk for microvascular complications while 25% are at risk for macrovascular complications. Vileikyte et al.[40] in a longitudinal study of 338 diabetic peripheral neuropathy (DPN) patients found that DPN severity was significantly correlated with depressive symptoms.
Richardson et al.[41] found depression was associated with persistently high HbA1c levels. Wagner et al.[15] also found that higher HbA1c levels and higher complications were associated with higher depressive symptoms. Van Tilburg et al.[16] found that depressive mood below the level of clinical depression was associated with a difference in glycemic control.
Both conditions are associated with impairment of brain morphology and function. Bilateral hippocampal volume reduction and abnormalities in amygdale found in depression were also found in diabetes. The highest insulin receptor densities that have been found in hippocampus, amygdale, septum and hypothalamus of diabetics were also important for the neurocognition and affect regulation.[42] Chronic hyperglycemia is associated with decreased cerebrospinal fluid insulin. Cerebral insulinopenia impacts neuronal growth and synaptogenesis. Decreased plasma brain derived factor (BDNF) levels were found in diabetes patients as well as in patients of depression.[37,42]
Over the last 50 years >4500 papers have been published all over the world on the topic of diabetes and depression.[4] Apart from two recent studies of Raval et al.[33] and Poongothai et al.[34] surprisingly not much of published material is available from India. The investigators believe that the present modest effort is one of the very few comprehensive efforts to understand depression in the context of diabetes and the first of its kind from Central India.
MATERIALS AND METHODS
The study was conducted at a large tertiary hospital, attached to a medical college, having multi-disciplinary reference facilities, which include inpatient and outpatient psychiatric services and regular endocrinology clinic. The study was conducted in a cross-sectional case control design after obtaining the approval of the Research and Ethical Committees of the institute.
Study group
The study group consisted of 109 type 2 diabetes mellitus patients. All patients were diagnosed as per the criteria laid down by the American Diabetes Association.[43] Fresh and old cases of diabetes were selected at random from the clinic.
Inclusion criteria
Patients of both sexes
Ages 18–65 years
Patients who have given consent to participate in the study.
Exclusion criteria
Individuals below 18 and above 65 years of age
Individuals with clinical evidence of current psychiatric disorder other than depression
Patients with impaired consciousness
Patients giving history suggestive of substance used disorders, patients who currently abusing or dependent on psychoactive substance.
Control group
All the inclusion and exclusion criteria as applicable to study group were applied to control the group also. Those who had undergone treatment for diabetes in preceding 1-year were not included. They were selected at random from healthy hospital staff and unrelated attendants of patients. However, those individuals whose blood sugar levels were high and satisfying the criteria for diabetes were taken off from the control group. Nine persons in the control group had high blood sugar levels and satisfied the criteria for diabetes hence they were included in the study group. In this way, control group got reduced to 91 individuals and the study group number increased to 109.
Methodology
Both the study group and control group persons were furnished with written information detailing the nature of the study and other relevant information. Informed consent was obtained from every person before their participation in the project.
Height was measured on a wall-mounted stadiometer with patient standing on bare feet placed closely to each other. Weight was measured for all on the same weighing machine in the morning with the patient on empty stomach. BMI was calculated with weight in kilograms as numerator and height in meter square as denominator.
Blood glucose and glycemic control
A volume of 5 ml blood was obtained under strict aseptic conditions. Fasting and postprandial plasma glucose was measured by glucose oxidase-peroxidase end point method at the central laboratory of the hospital. Glycemic control was assessed by measuring HbA1c by means of high-performance liquid chromatography.
Somatic and autonomic neuropathy symptom scales
Eleven items muscle weakness and sensory disturbance subscale and the 11 items autonomic symptoms subscale of the neurolgic symptom and impairment scale devised by Casellini and Vinik[44] was used for this purpose. The items consist of standard questions that are put to the subjects. Each subscale yields 11 points. Based on the score the symptoms are arbitrarily graded as mild, moderate and severe (0–3 mild, 4–7 moderate and 8–11 severe).
Semmes–Weinstein monofilament
Nylon filaments ranging from 4.17 to 6.10 calibers were devised by Josephine Semmes and Sydney–Weinstein in 1960 to objectively measure pressure sensation. The 5.07 caliber filament has been accepted as the standard for screening purposes. The buckling pressure required to just bend the filament is a force of 10 g. It was applied at 6 noncallous points of the feet: Great toe, over the heads of 1st, 3rd and 5th metatarsals, instep and heel-perpendicular to the skin with patients eyes closed. Absent sensation at 2 or above out of 12 points was taken as impaired sensation as per Kumar et al.[45]
Vibration perception threshold
A Biothesiometer supplied by Madras Diabetic Foot Care, India, was used for this purpose. Vibration perception threshold (VPT) was measured by placing the probe perpendicular to the plantar surface at 6 points of both feet. The voltage level at which the patient felt the first vibration sense was taken as the measure of threshold. Grading of VPT was done as per Saha et al.[46] that is, up to 15 mv normal, 16–25 mv grade 1 and >25 mv grade 2. The average of 6 points whichever side is higher is taken as VPT.
Centre for Epidemiologic Studies Depression Scale
The CES-D[47] used widely to screen for depression in diabetes.[19,23,27,29,31,32] It is a 20 items self-reported scale that asks individuals to rate how often over the past week they experienced symptoms associated with depression. The English version of CES-D was subjected to translation to suit local vernacular Hindi. After translation and back translation it was administered to 30 bilinguals in two sittings with half of the items given each time in each language. The correlation was good (r = 0.53 P ≤ 0.005). The translated version was used for the study.
Response options range from 0 to 3 for each item (0 = rarely or none of the time, 1 = some or little of the time, 2 = moderately or much of the time, 3 = most or almost all the time). Scores range from 0 to 60, with high scores indicating greater depressive symptoms. A score of 16 is taken as the cut-off point.[45]
Statistical analysis
Data generated were analyzed using various tests of significance and association. ORs and Pearson's Chi-square test was used for categorical data as appropriate. Student's t-test was used for continuous data. Simple binary logistic regression was used with depression as a dependent variable to ascertain, which of the variables predict depression in diabetes. SPSS 20 version (IBM Corp., Armonk, NY) was used for statistical analysis.
RESULTS
One hundred and nine patients of diabetes mellitus and 91 healthy controls were the subjects of this study. Table 1 shows that the diabetes and the control groups were matched for age (P = 0.88) gender (P = 0.67) education (P = 0.319) marital status (P = 0.413) monthly income (P = 0.422), place of living (P = 0.759) and religion (P = 0.28).
Table 1.
Demographic characterstics of study group and controls
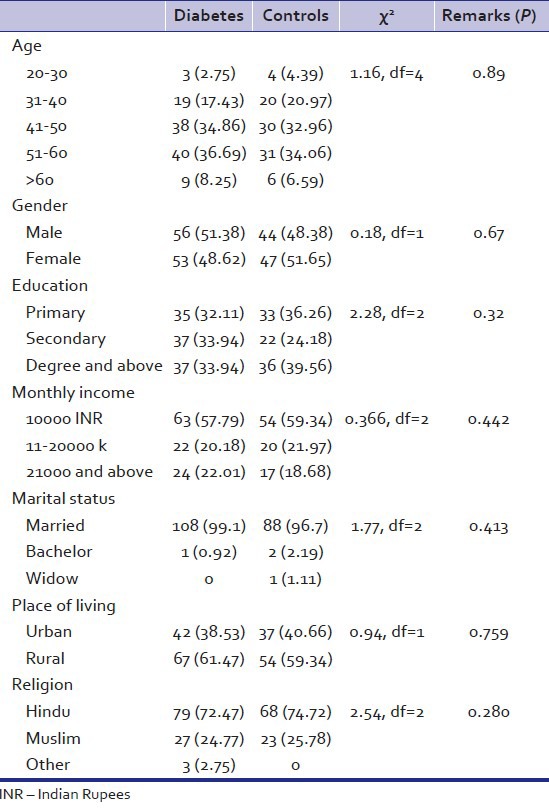
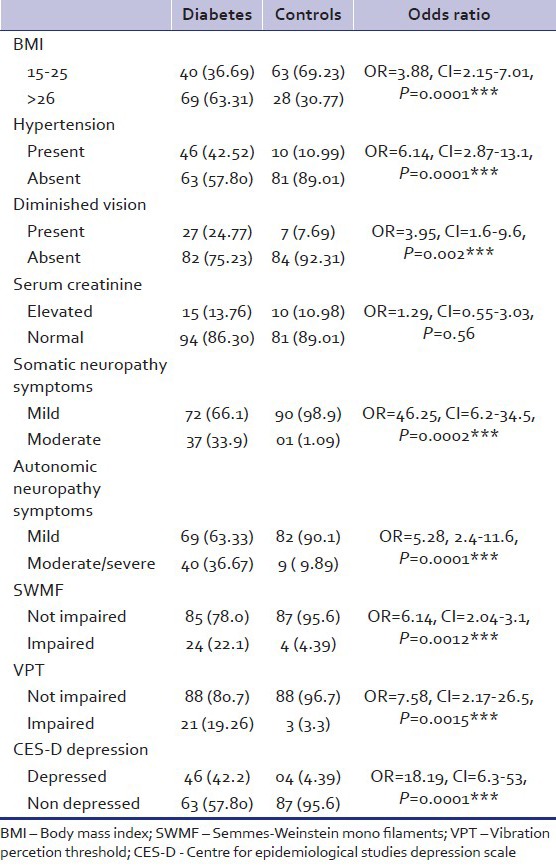
Table 2 describes the clinical characteristics of the study and the control groups. Study group differed in a highly significant way from the control group on the variables of hypertension (P - 0.0001) defective vision (P - 0.001) somatic neuropathy symptoms (P - 0.009) autonomic neuropathy symptoms (0.0001) Semmes–Weinstein monofilament (SWMF) impairment (P - 0.001) VPT impairment (P - 0.001). Serum creatinine levels were same in both groups (P - 0.56). 46 (42.2%) of the diabetic patients had depression as measured by CES-D while only 4 (4.39%) of the control group had depression (P - 0.0001).
Table 2.
Clinical characteristics and CES-D scores of diabetic patients and controls
The diabetic patients were dichotomized into depressed (n = 46) and nondepressed (n = 63) groups. Table 3 shows the comparison between the two groups on sociodemographic variables. Both groups did not differ on any of the parameters.
Table 3.
Socio-demographic characteristics of depressed and non-depressed diabetic patients
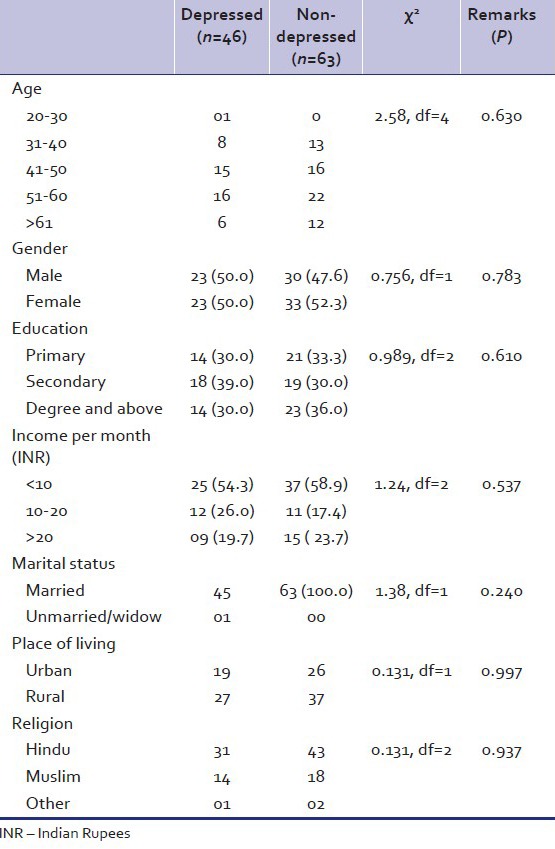
Table 4 describes the clinical profile of depressed and nondepressed diabetes patients. Depressed group had more moderate level somatic neuropathy symptoms (82.6% vs. 12.7%) while the nondepressed group had more mild (87.3% vs. 17.4%) symptoms. Depressed group did not differ from nondepressed group on all other variables, which included BMI, hypertension, defective vision, autonomic neuropathy symptoms, duration of diabetes and duration of treatment for diabetes.
Table 4.
Clinical profile of depressed and non-depressed diabetic patients
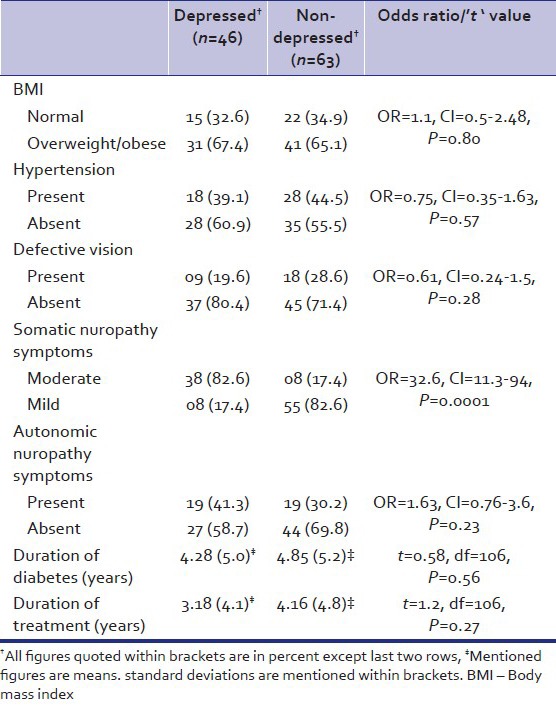
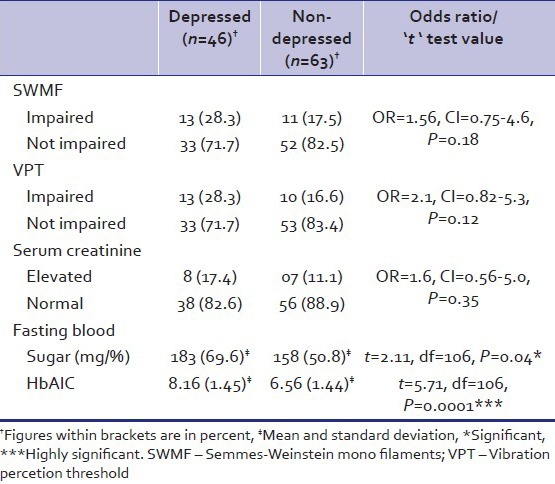
Table 5 depicts the objective evidence of peripheral neuropathy as measured by SWMF and VPT; glycemic control measured by blood glucose and HbA1c and nephropathy indicated by elevated creatinine levels. There was no difference in SWMF, VPT and serum creatinine levels of depressed and nondepressed groups. Fasting blood sugar (FBS) of the depressed group was noted to be significantly high (P - 0.04). HbA1C levels of the depressed group found to be elevated in a highly significant way (P - 0.0001).
Table 5.
Semmes-Weinstein mono filaments impairment, vibration percetion threshold, serum creatinine, fasting blood sugar and glycosylated haemoglobin levels in depressed and non-depressed diabetic patients
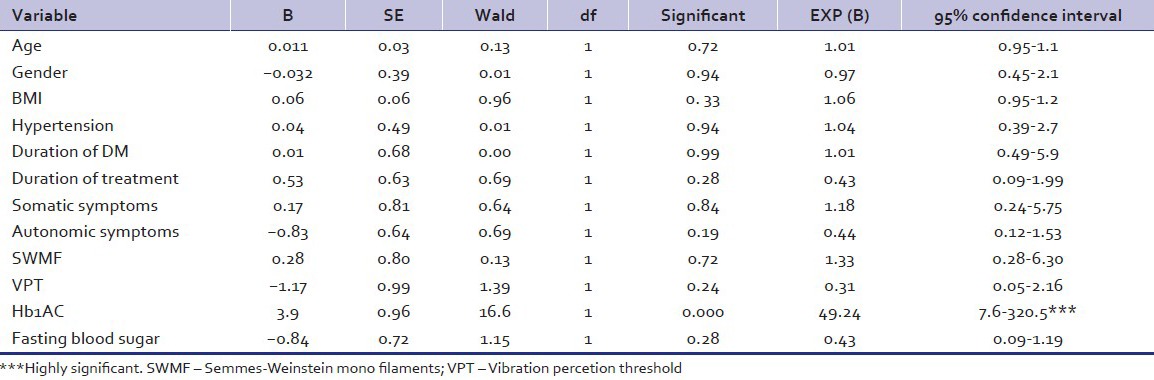
Binary logistic regression [Table 6] keeping CES-D cut-off score of 16 as dependent variable shows that only HbA1C levels predict depression in a significant way (OR: 49.24 95% CI: 7.6–320.5 P - 0.000).
Table 6.
Binary logistic regression
DISCUSSION
The present study was designed to ascertain the quantum of co-morbid depression in diabetes and to identify the variables that may be playing a part in their co-occurrence. The study itself is fairly rigorous and the sample size though small is comparable to that of many others.[22,23,24,25,28,30]
A glance at the clinical data makes the fact obvious that diabetes is a systemic disease. More diabetes patients are overweight or obese, hypertensive, visually impaired, have high levels of physical symptoms referable to somatic and autonomic nervous systems.
Both small and large fiber neuropathy was reported by investigators[31,36,42,48] but the issue here is whether the high symptom frequency (82.6%) of our study is due to peripheral neuropathy arising out of tissue glucopenia or they are due to central neurotransmitter dysregulation could be a common mechanism underpinning both depression and painful symptoms. The study group also had autonomic symptoms 6 times higher than controls. High levels of somatic symptoms ranging from 54% to 69% have been reported in both the WHO sponsored multinational study as well as the recent multicentric Indian study.[49,50] We found only 19.3% of diabetes patients have objective evidence of somatic neuropathy as evidenced by significantly impaired VPT that is in conformity with many other studies[39,46,51] but less than Jayaprakash et al.(34.9%)[52] and Raval et al.(65%).[33] Clearly neuropathic symptoms are disproportionate to objective evidence of peripheral neuropathy in our study indicating additional central mechanisms in operation. 46 (42.2%) of the diabetes patients had CES-D defined depression, which is 10 times higher than controls (4.39%). Since the prevalence in the controls conforms to that of the general population,[8] we concluded that the instrument tapped co-morbid depression accurately. This high prevalence of depression in diabetes is in conformity with that of other studies.[23,28,30,32] However, some investigators reported prevalence of depression in diabetics as same as controls.[24,27,35,36] In our country Raval et al.[33] reported a figure of 41% from the North of India while Poongathai et al.[34] reported only 23.4% from the South of India. Both groups used almost similar instruments, PHQ-9 and PHQ-12, to measure depression. It is, therefore, possible that some yet to be defined sociodemographic, clinical or biological factors are operating in causing depression in diabetes. Our findings are similar to the north Indian study.
We dichotomized the study group into depressed (n = 46) and nondepressed groups (n = 63) to compare both in terms of sociodemographic, clinical and objective variables to identify possible factors associated with depression. Both groups were found to be remarkably similar in all the seven sociodemographic parameters. Our findings are at variance with others who found low socioeconomic status[53] female gender[10] and young age[53] determinants of depression. Both sexes are equally represented in our study. Raval et al.[33] found higher age (>54) while Poongathai et al.[34] found lower age (49.1) was associated with depression. The mean age of our depressed group was 51 and did not differ from nondepressed group. Among the clinical variables Raval et al.[33] found high BMI was associated with depression while Poongathai et al.[34] found slightly lower BMI in their depressed group. In our study, no significant difference was found in the prevalence of high BMI, hypertension, defective vision, duration of diabetes and duration of treatment of diabetes.
Duration was not a factor in both the Indian studies[33,34] as it is in our study. Significantly high prevalence (P - 0.0001) of somatic symptoms was observed in the depressed group of our study. However, when objectively measured for neuropathy by Biothesiometer and SWMF no significant differences were observed between the depressed and nondepressed groups. Neuropathy was noted to be an important determinant of depression by some investigators.[33,34,38,40] Our findings are at variance and more in support of a central mechanism for the neuropathic pain. There are two reasons for this inference: Firstly, somatic neuropathy symptoms manifested in much higher frequency than actual neuropathy as assessed by SWMF and VPT; secondly there is no difference in the frequency of actual neuropathy between depressed and nondepressed groups. There is no indication of nephropathy as a determining factor for depression as creatinine levels were statistically same in both groups.
Mean FBS was significantly high in the depressed group. As raised FBS could be a short lasting phenomenon, it may not be considered relevant to a state of sustained mood impairment. HbA1C indicates glycemic control over a few weeks. We feel the robustly elevated HbA1C levels (P - 0.0001) in the depressed group is the most important finding of our study. Binary logistic regression analysis also revealed significant association of HbA1C levels with depression. Several investigators found poor glycemic control was a significant factor associated with depression.[14,15,16,37,41] Both the Indian studies however found no association of HbA1C levels with depression.[33,34] We are unable to explain the discrepancy.
Though fairly comprehensive, the study has certain limitations. Firstly it is a hospital-based study, and therefore findings may not reflect the situation in the community, secondly the sample size is relatively small. Even on a conservative estimate of 10% prevalence and with an allowable error of 20% the sample size would have been 225. We also should have addressed ourselves more for the assessment of autonomic, cardiovascular, sexual and cognitive functions but were thwarted by financial and other constraints.
To summarize, our study brings into bold relief the complexities involved in ascertaining relationships between depression and diabetes. That the prevalence of depression is high in diabetes is once again confirmed in this study. The second notable fact that has emerged out of our study is that somatic symptoms mask depression. Unless unmasked by deliberate screening, depression will remain undiagnosed and untreated putting patients at serious risk for further morbidity and even mortality. Finally, the strong association of poor glycemic control with depression drives home the point that diabetes demands sustained and meticulous management by a physician as well as a liaison psychiatrist. We feel the time for integrated treatment of diabetes has arrived and must be taken notice with due seriousness particularly in this part of the world where both diabetes and depression are looming large.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Pouwers A. Diabetes mellitus. In: Kasper DL, Braunwald E, Fauci A, Hauser C, Longo D, Jameson JL, editors. Harrison's Principles of Internal Medicine. New York: McGraw Hill; 2005. p. 2158. [Google Scholar]
- 2.Upadhyay OP, Upadhyay D. A few facts of historical interest relating to diabetes mellitus. Indian J Hist Sci. 1987;22:235–9. [PubMed] [Google Scholar]
- 3.Rao AV. Depressive illness in India. Indian J Psychiatry. 1984;26:301–11. [PMC free article] [PubMed] [Google Scholar]
- 4.Diabetes and Depression. Woodbridge, VA, USA: 2010. World Federation for Mental Health. [Google Scholar]
- 5.Mohan V, Sandeep S, Deepa R, Shah B, Varghese C. Epidemiology of type 2 diabetes: Indian scenario. Indian J Med Res. 2007;125:217–30. [PubMed] [Google Scholar]
- 6.Bromet E, Andrade LH, Hwang I, Sampson NA, Alonso J, de Girolamo G, et al. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011;9:90. doi: 10.1186/1741-7015-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diabetes Atlas. 6th Ed. Brussels: 2013. International Diabetes Federation. [PubMed] [Google Scholar]
- 8.Depression- A global crisis. Woodbridge, VA, USA: 2012. World Federation for Mental Health. [Google Scholar]
- 9.Grover S, Dutt A, Avasthi A. An overview of Indian research in depression. Indian J Psychiatry. 2010;52(Suppl 1):S178–88. doi: 10.4103/0019-5545.69231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: A meta-analysis. Diabetes Care. 2001;24:1069–78. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 11.Knol MJ, Twisk JW, Beekman AT, Heine RJ, Snoek FJ, Pouwer F. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia. 2006;49:837–45. doi: 10.1007/s00125-006-0159-x. [DOI] [PubMed] [Google Scholar]
- 12.Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: A meta-analysis. Diabetes Care. 2008;31:2383–90. doi: 10.2337/dc08-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carnethon MR, Kinder LS, Fair JM, Stafford RS, Fortmann SP. Symptoms of depression as a risk factor for incident diabetes: Findings from the national health and nutrition examination epidemiologic follow-up study, 1971-1992. Am J Epidemiol. 2003;158:416–23. doi: 10.1093/aje/kwg172. [DOI] [PubMed] [Google Scholar]
- 14.Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: A meta-analytic review of the literature. Diabetes Care. 2000;23:934–42. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- 15.Wagner JA, Abbott GL, Heapy A, Yong L. Depressive symptoms and diabetes control in African Americans. J Immigr Minor Health. 2009;11:66–70. doi: 10.1007/s10903-008-9147-1. [DOI] [PubMed] [Google Scholar]
- 16.Van Tilburg MA, McCaskill CC, Lane JD, Edwards CL, Bethel A, Feinglos MN, et al. Depressed mood is a factor in glycemic control in type 1 diabetes. Psychosom Med. 2001;63:551–5. doi: 10.1097/00006842-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez JS, Safren SA, Delahanty LM, Cagliero E, Wexler DJ, Meigs JB, et al. Symptoms of depression prospectively predict poorer self-care in patients with Type 2 diabetes. Diabet Med. 2008;25:1102–7. doi: 10.1111/j.1464-5491.2008.02535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez JS, Peyrot M, McCarl LA, Collins EM, Serpa L, Mimiaga MJ, et al. Depression and diabetes treatment nonadherence: A meta-analysis. Diabetes Care. 2008;31:2398–403. doi: 10.2337/dc08-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sullivan MD, O’Connor P, Feeney P, Hire D, Simmons DL, Raisch DW, et al. Depression predicts all-cause mortality: Epidemiological evaluation from the ACCORD HRQL substudy. Diabetes Care. 2012;35:1708–15. doi: 10.2337/dc11-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egede LE, Zheng D. Independent factors associated with major depressive disorder in a national sample of individuals with diabetes. Diabetes Care. 2003;26:104–11. doi: 10.2337/diacare.26.1.104. [DOI] [PubMed] [Google Scholar]
- 21.Murrell SA, Himmelfarb S, Wright K. Prevalence of depression and its correlates in older adults. Am J Epidemiol. 1983;117:173–85. doi: 10.1093/oxfordjournals.aje.a113528. [DOI] [PubMed] [Google Scholar]
- 22.Lustman PJ, Griffith LS, Clouse RE, Cryer PE. Psychiatric illness in diabetes mellitus. Relationship to symptoms and glucose control. J Nerv Ment Dis. 1986;174:736–42. doi: 10.1097/00005053-198612000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Friis R, Nanjundappa G. Diabetes, depression and employment status. Soc Sci Med. 1986;23:471–5. doi: 10.1016/0277-9536(86)90006-7. [DOI] [PubMed] [Google Scholar]
- 24.Robinson N, Fuller JH, Edmeades SP. Depression and diabetes. Diabet Med. 1988;5:268–74. doi: 10.1111/j.1464-5491.1988.tb00983.x. [DOI] [PubMed] [Google Scholar]
- 25.Popkin MK, Callies AL, Lentz RD, Colon EA, Sutherland DE. Prevalence of major depression, simple phobia, and other psychiatric disorders in patients with long-standing type I diabetes mellitus. Arch Gen Psychiatry. 1988;45:64–8. doi: 10.1001/archpsyc.1988.01800250078010. [DOI] [PubMed] [Google Scholar]
- 26.Geringer ES, Perlmuter LC, Stern TA, Nathan DM. Depression and diabetic neuropathy: A complex relationship. J Geriatr Psychiatry Neurol. 1988;1:11–5. doi: 10.1177/089198878800100103. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Markides KS, Lee DJ. Health status of diabetic Mexican Americans: Results from the Hispanic HANES. Ethn Dis. 1991;1:273–9. [PubMed] [Google Scholar]
- 28.Songar A, Kocabasoglu N, Balcioglu I, Karaca E, Kocabasoglu C, Haciosman M, et al. The relationship between diabetics metabolic control levels and psychiatric symptomatology. Integr Psychiatry. 1993;9:34–40. [Google Scholar]
- 29.Bourdel-Marchasson I, Dubroca B, Manciet G, Decamps A, Emeriau JP, Dartigues JF. Prevalence of diabetes and effect on quality of life in older French living in the community: The PAQUID Epidemiological Survey. J Am Geriatr Soc. 1997;45:295–301. doi: 10.1111/j.1532-5415.1997.tb00943.x. [DOI] [PubMed] [Google Scholar]
- 30.Peyrot M, Rubin RR. Levels and risks of depression and anxiety symptomatology among diabetic adults. Diabetes Care. 1997;20:585–90. doi: 10.2337/diacare.20.4.585. [DOI] [PubMed] [Google Scholar]
- 31.Pouwer F, Beekman AT, Nijpels G, Dekker JM, Snoek FJ, Kostense PJ, et al. Rates and risks for co-morbid depression in patients with type 2 diabetes mellitus: Results from a community-based study. Diabetologia. 2003;46:892–8. doi: 10.1007/s00125-003-1124-6. [DOI] [PubMed] [Google Scholar]
- 32.Pouwer F, Kupper N, Adriaanse MC. Does emotional stress cause type 2 diabetes mellitus? A review from the European Depression in Diabetes (EDID) Research Consortium. Discov Med. 2010;9:112–8. [PubMed] [Google Scholar]
- 33.Raval A, Dhanaraj E, Bhansali A, Grover S, Tiwari P. Prevalence and determinants of depression in type 2 diabetes patients in a tertiary care centre. Indian J Med Res. 2010;132:195–200. [PubMed] [Google Scholar]
- 34.Poongothai S, Anjana RM, Pradeepa R, Ganesan A, Unnikrishnan R, Rema M, et al. Association of depression with complications of type 2 diabetes – The Chennai Urban Rural Epidemiology Study (CURES- 102) J Assoc Physicians India. 2011;59:644–8. [PubMed] [Google Scholar]
- 35.Kruse J, Schmitz N, Thefeld W. German National Health Interview and Examination Survey. On the association between diabetes and mental disorders in a community sample: Results from the German National Health Interview and Examination Survey. Diabetes Care. 2003;26:1841–6. doi: 10.2337/diacare.26.6.1841. [DOI] [PubMed] [Google Scholar]
- 36.Das-Munshi J, Stewart R, Ismail K, Bebbington PE, Jenkins R, Prince MJ. Diabetes, common mental disorders, and disability: Findings from the UK National Psychiatric Morbidity Survey. Psychosom Med. 2007;69:543–50. doi: 10.1097/PSY.0b013e3180cc3062. [DOI] [PubMed] [Google Scholar]
- 37.Rustad JK, Musselman DL, Nemeroff CB. The relationship of depression and diabetes: Pathophysiological and treatment implications. Psychoneuroendocrinology. 2011;36:1276–86. doi: 10.1016/j.psyneuen.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 38.de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: A meta-analysis. Psychosom Med. 2001;63:619–30. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 39.Lin EH, Rutter CM, Katon W, Heckbert SR, Ciechanowski P, Oliver MM, et al. Depression and advanced complications of diabetes: A prospective cohort study. Diabetes Care. 2010;33:264–9. doi: 10.2337/dc09-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vileikyte L, Peyrot M, Gonzalez JS, Rubin RR, Garrow AP, Stickings D, et al. Predictors of depressive symptoms in persons with diabetic peripheral neuropathy: A longitudinal study. Diabetologia. 2009;52:1265–73. doi: 10.1007/s00125-009-1363-2. [DOI] [PubMed] [Google Scholar]
- 41.Richardson LK, Egede LE, Mueller M, Echols CL, Gebregziabher M. Longitudinal effects of depression on glycemic control in veterans with type 2 diabetes. Gen Hosp Psychiatry. 2008;30:509–14. doi: 10.1016/j.genhosppsych.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 42.McIntyre RS, Soczynska JK, Konarski JZ, Woldeyohannes HO, Law CW, Miranda A, et al. Should depressive syndromes be reclassified as “Metabolic Syndrome Type II”? Ann Clin Psychiatry. 2007;19:257–64. doi: 10.1080/10401230701653377. [DOI] [PubMed] [Google Scholar]
- 43.American Diabetes Association. Standards of medical care in diabetes--2010. Diabetes Care. 2010;33(Suppl 1):S11–61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Casellini CM, Vinik AI. Clinical manifestations and current treatment options for diabetic neuropathies. Endocr Pract. 2007;13:550–66. doi: 10.4158/EP.13.5.550. [DOI] [PubMed] [Google Scholar]
- 45.Kumar S, Fernando DJ, Veves A, Knowles EA, Young MJ, Boulton AJ. Semmes-Weinstein monofilaments: A simple, effective and inexpensive screening device for identifying diabetic patients at risk of foot ulceration. Diabetes Res Clin Pract. 1991;13:63–7. doi: 10.1016/0168-8227(91)90034-b. [DOI] [PubMed] [Google Scholar]
- 46.Saha D, Saha K, Dasgupta PK. Vibration sense impairment in diabetes mellitus. Indian J Physiol Pharmacol. 2011;55:381–3. [PubMed] [Google Scholar]
- 47.Radloff LS. The CES-D scale: A self report depression scale for research in the general population. Appl Physiol Meas. 1977;1:385–401. [Google Scholar]
- 48.Ashok S, Ramu M, Deepa R, Mohan V. Prevalence of neuropathy in type 2 diabetic patients attending a diabetes centre in South India. J Assoc Physicians India. 2002;50:546–50. [PubMed] [Google Scholar]
- 49.Simon GE, VonKorff M, Piccinelli M, Fullerton C, Ormel J. An international study of the relation between somatic symptoms and depression. N Engl J Med. 1999;341:1329–35. doi: 10.1056/NEJM199910283411801. [DOI] [PubMed] [Google Scholar]
- 50.Grover S, Avasthi A, Kalita K, Dalal PK, Rao GP, Chadda RK, et al. IPS multicentric study: Functional somatic symptoms in depression. Indian J Psychiatry. 2013;55:31–40. doi: 10.4103/0019-5545.105502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care. 2006;29:1518–22. doi: 10.2337/dc05-2228. [DOI] [PubMed] [Google Scholar]
- 52.Jayaprakash P, Bhansali A, Bhansali S, Dutta P, Anantharaman R, Shanmugasundar G, et al. Validation of bedside methods in evaluation of diabetic peripheral neuropathy. Indian J Med Res. 2011;133:645–9. [PMC free article] [PubMed] [Google Scholar]
- 53.Engum A, Mykletun A, Midthjell K, Holen A, Dahl AA. Depression and diabetes: A large population-based study of sociodemographic, lifestyle, and clinical factors associated with depression in type 1 and type 2 diabetes. Diabetes Care. 2005;28:1904–9. doi: 10.2337/diacare.28.8.1904. [DOI] [PubMed] [Google Scholar]


