Abstract
As endoscopic ultrasound (EUS) is the most sensitive imaging modality for diagnosing pancreatic disorders, it can demonstrate subtle alterations in the pancreatic parenchymal and ductal structure even before traditional imaging and functional testing demonstrate any abnormality. In spite of this fact and abundant literature, the exact role of EUS in the diagnosis of chronic pancreatitis (CP) is still not established. The EUS features to diagnose CP have evolved over a period from a pure qualitative approach to more advanced and complicated scoring systems incorporating multiple parenchymal and ductal EUS features. The rosemont criteria have attempted to define precisely each EUS criterion and thus have good inter-observer agreement. However, initial studies have failed to demonstrate any significant improvement in the inter-observer variability and further validation studies are needed to define the exact role of these criteria. The measurement of strain ratio using quantitative EUS elastography and thus allowing quantification of pancreatic fibrosis seems to be a promising new technique.
Keywords: Chronic pancreatitis, endosonography, pancreatic duct
INTRODUCTION
Chronic pancreatitis (CP) is characterized by irreversible damage to the pancreas that may lead on to the development of abdominal pain as well as varying degrees of endocrine and exocrine insufficiency.[1] The ideal diagnostic standard for any disease is histopathological classification and diagnostic system. However, pancreatic biopsy is usually not performed in routine clinical settings because obtaining a histological diagnosis of CP is risky and carries a high rate of potentially severe complications. The diagnosis of CP is thus based upon the demonstration of morphological and/or functional changes in the pancreas that occurs because of irreversible damage to the pancreatic parenchyma.[1] The morphological changes in the pancreas can be appreciated by various imaging methods such as abdominal X-ray, transabdominal ultrasound (ultrasonography), computed tomography (CT), magnetic resonance imaging and endoscopic retrograde cholangio pancreatography. However, these imaging modalities can pick up advanced morphological changes only, and the diagnostic ability of these modalities to diagnose early and minimal CP is limited.[1,2] The pancreatic function tests have been shown to improve the diagnostic sensitivity and specificity but because of associated technical challenges, patient discomfort and limited availability, these functional tests are infrequently performed.[3]
Due to the ability to place the transducer in close proximity to the pancreas, endoscopic ultrasound (EUS) is considered to be the most sensitive imaging modality for diagnosing pancreatic disorders.[2,4] EUS has been shown to demonstrate subtle alterations in the pancreatic parenchymal and ductal structure even before traditional imaging and functional testing demonstrate any abnormality.[3,4,5,6] The EUS diagnosis of CP is based upon the demonstration of ductal and parenchymal features, and a number of diagnostic criteria incorporating these EUS features have been proposed to diagnose CP. Improvement in EUS equipment and availability of newer and sophisticated EUS applications like color Doppler, contrast EUS and elastography, have further improved the diagnostic ability of EUS.
ENDOSCOPIC ULTRASOUND FEATURES OF CHRONIC PANCREATITIS
The EUS features to diagnose CP [Figures 1–9] have evolved over a period from a pure qualitative approach to more advanced and complicated scoring systems incorporating multiple parenchymal and ductal EUS features.[7,8] Increasing the number of these EUS features required for diagnosis of CP increases the specificity and lowering the threshold number of criteria increases the sensitivity, but decreases the already poor specificity of EUS.[7,8] The rosemont criteria developed by leading experts has attempted to surmount the limitations of the current diagnostic criteria, define precisely each EUS criterion and thus develop criteria that have good inter-observer agreement.[9] The various EUS features that have to be demonstrated for the diagnosis of CP are:
Figure 1.
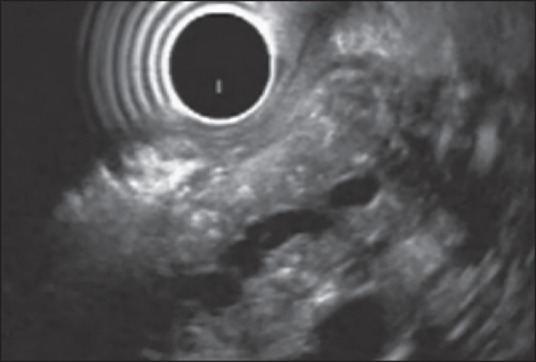
Examination of pancreatic body from stomach using radial echoendoscope. The main pancreatic duct is dilated with an irregular contour and hyper echoic wall. The pancreatic parenchyma shows hyper echoic foci with as well as without shadowing. Thus, this patient has one Major A and 4 Minor endoscopic ultrasound features and is consistent with chronic pancreatitis
Figure 9.
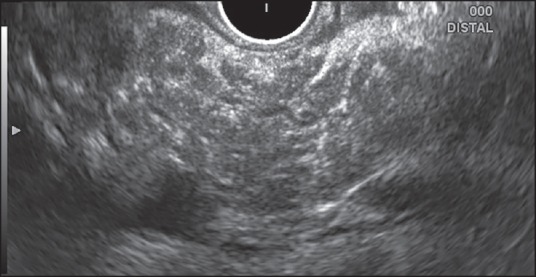
Examination of pancreatic body from stomach using radial echoendoscope. The main pancreatic duct is of normal diameter with hyperechoic wall. The pancreatic parenchyma shows hyperechoic foci with shadowing, stranding and lobularity with honeycombing. Thus, this patient has Major A and B criteria along with 1 Minor criteria and is consistent with chronic pancreatitis
Figure 2.
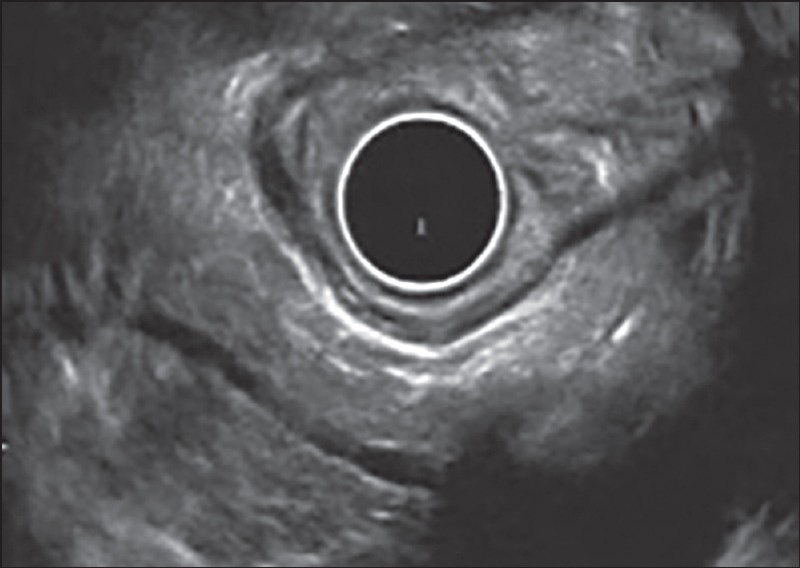
Examination of pancreatic body from stomach using radial echoendoscope. The main pancreatic duct is dilated with hyper echoic wall. The duct is seen communicating with cyst. The pancreatic parenchyma shows stranding. Thus, this patient has only 4 Minor endoscopic ultrasound features and is indeterminate for chronic pancreatitis
Figure 3.
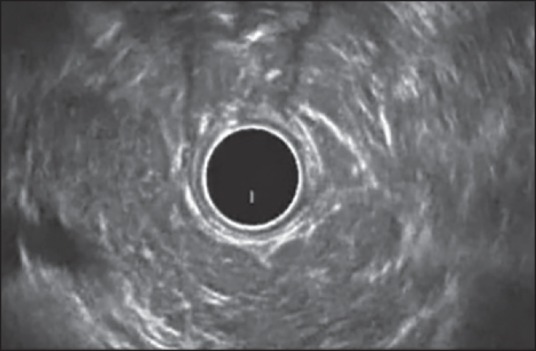
Examination of pancreatic body from stomach using radial echoendoscope. The main pancreatic duct is of normal diameter with hyperechoic wall. The pancreatic parenchyma shows hyperechoic foci without shadowing, stranding and lobularity without honeycombing. Thus, this patient has only 4 Minor endoscopic ultrasound features and is indeterminate for chronic pancreatitis
Figure 4.
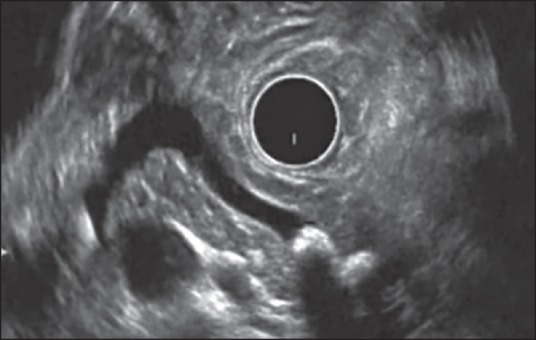
Examination of pancreatic body from stomach using radial echoendoscope. The main pancreatic duct is dilated and has multiple echogenic structures. The pancreatic parenchyma shows hyperechoic foci without shadowing. Thus, this patient has one Major A and 2 Minor endoscopic ultrasound features and is suggestive of chronic pancreatitis
Figure 5.
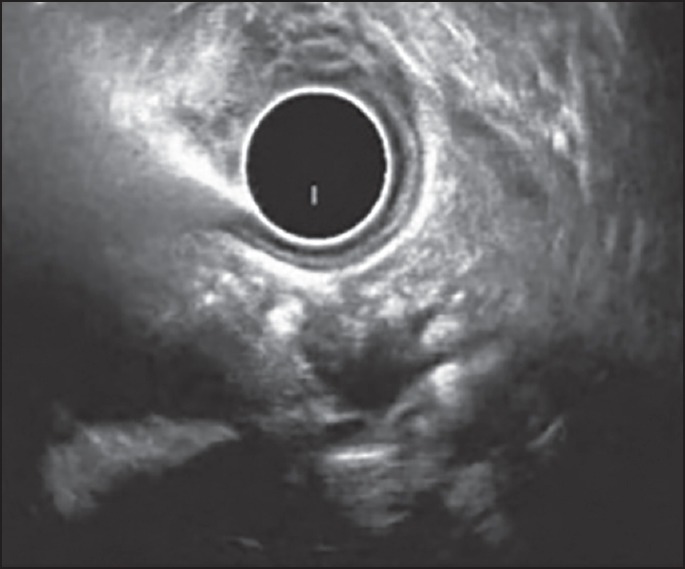
Examination of pancreatic body from stomach using radial echoendoscope. The main pancreatic duct is dilated with an irregular contour and has echogenic structures suggestive of calculi. The pancreatic parenchyma shows hyper echoic foci with shadowing. Thus, this patient has two major a endoscopic ultrasound features and is consistent with chronic pancreatitis
Figure 6.
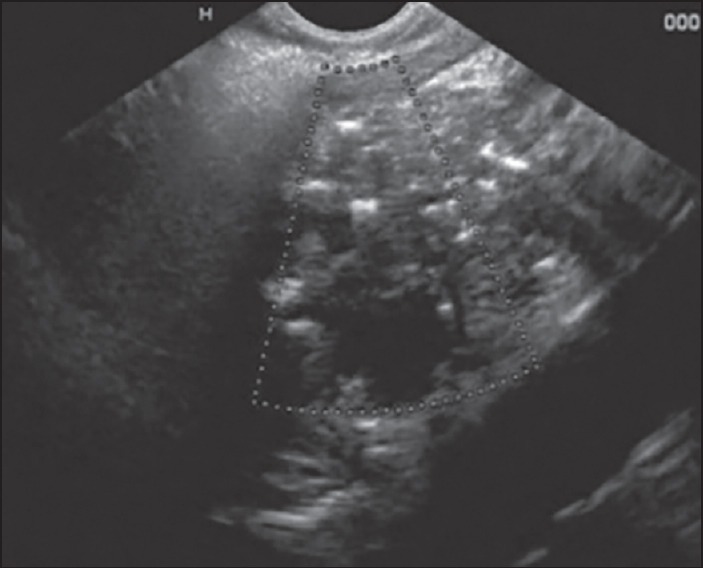
Examination of pancreatic body from stomach using linear echoendoscope. The main pancreatic duct is dilated. The pancreatic parenchyma shows hyper echoic foci with as well as without shadowing. Thus, this patient has one Major A and 2 Minor endoscopic ultrasound features and is suggestive of chronic pancreatitis
Parenchymal features
Hyperechoic foci with shadowing (Major A): They have been defined as echogenic structures ≥2 mm in length and width that shadow. They histologically correlate with pancreatic parenchymal calcification.
Lobularity with honeycombing (Major B): Well-circumscribed, ≥5 mm structures with rims that are hyperechoic relative to the echogenicity of its central areas are defined as lobules. At least three contiguous lobules that are present in the body or tail of the pancreas are labeled as “honeycombing” lobularity. The histological correlate of lobularity is not known.
Lobularity without honeycombing (Minor): Lobules as defined above that are non-contiguous and present in body and tail.
Hyperechoic foci without shadowing (Minor): Defined as above but without shadowing.
Cysts (Minor): Anechoic, rounded/elliptic structures that should measure ≥2 mm in short axis.
Stranding (Minor): Hyperechoic lines ≥3 mm in length seen in at least two different directions with respect to the imaged plane. At least three strands are considered necessary.
Ductal features
Except for ductal calculi all other features should be looked in the body and tail of pancreas only
Main pancreatic duct (MPD) calculi (Major A): Echogenic structures within MPD with acoustic shadowing located anywhere in the head, body and tail.
Irregular MPD contour (Minor): MPD that is uneven or irregular in outline and ectatic in its course. These features should be demonstrated in the body and tail of pancreas only.
Dilated side branches (Minor): presence of ≥3 tubular anechoic structures each measuring ≥1 mm in width and communicating with MPD. These should be demonstrated in the body and tail of pancreas only.
Main pancreatic duct dilatation (Minor): MPD diameter ≥3.5 mm in the pancreatic body or ≥1.5 mm in the pancreatic tail.
Hyper echoic duct margin (Minor): Relatively hyperechoic duct wall found in >50% of the entire MPD of the body and tail.
Endoscopic ultrasound diagnosis of chronic pancreatitis based upon Rosemont criteria
Consistent with CP: One Major A feature with ≥3 Minor features or 1 Major A feature and Major B feature or 2 Major A features.
Suggestive of CP: One Major A feature with <3 Minor features or 1 Major B feature with ≥3 Minor features or ≥5 Minor features.
Indeterminate for CP: 3 or 4 Minor features with no major features or Major B feature alone or with <3 Minor features.
Normal: <3 Minor features, no major features.
Although promising, there are several issues associated with EUS features of CP. A number of conditions such as aging, smoking, obesity, and chronic alcohol consumption may cause similar EUS changes in the pancreas.[7] Another important issue is of high interobserver variability. Although, rosemont criteria has refined the past criteria with more objectivity initial studies have failed to demonstrate any significant improvement in kappa scores.[10] To decrease interobserver variability adjunctive imaging technologies like digital image analysis and elastography have been used for diagnosis of CP with encouraging results.[10] However, further studies with larger sample size are needed to determine the exact diagnostic role of these ancillary EUS techniques.
TECHNICAL ISSUES IN THE ENDOSCOPIC PROCEDURE
The EUS procedure for looking for changes of CP is similar to the standard EUS examination of the pancreas. The complete EUS examination of the pancreas is possible from gastric and duodenal stations with uncinate process being visualized from the second part of the duodenum. Both radial and linear EUS have been shown to be equally accurate for the diagnosis of CP.[11] Radial EUS, however, provides cross sectional imaging similar to other cross sectional imaging modalities like CT and this makes the imaging of MPD easier as it is seen as a long linear structure in contrast to a dot like appearance on linear EUS. In order to correctly identify the changes of CP, the endoscopist needs to know the normal appearances of the pancreas as well as the normal variations seen in a nonpathological pancreas.
The important things to keep in mind are:
The normal pancreas has a diffusely speckled “salt and pepper” pattern of the body and tail with a barely visualized single, smooth and anechoic MPD.
With the current advanced EUS imaging systems side branches of MPD can also be seen in normal individuals especially the elderly and only if side branches are ≥1 mm, they are considered to be abnormal.
Even in normal individuals the MPD can be mildly tortuous and therefore the MPD should be carefully evaluated for a gradual decrease in its diameter from the head to tail, which is an important feature of the normal duct.
The duct of alternating sizes or beading is abnormal.
The dorsal and ventral pancreas can have different echogenicity with the dorsal anlage of the pancreas being more echogenic than the ventral pancreas.
As mentioned above most of these EUS features of CP are visualized in the body and tail and currently it is not clear how best to diagnose focal CP in the head as the isolated EUS findings like lobularity in the head with normal body and tail of pancreas are generally reported as normal.
ROLE OF ENDOSCOPIC ULTRASOUND ELASTOGRAPHY
Endoscopic ultrasound elastography has also been shown to be an accurate tool for the diagnosis of CP and strain ratio obtained using quantitative EUS elastography has been shown to vary significantly in various rosemont classification groups thus allowing quantification of pancreatic fibrosis.[12] In quantitative elastography, the tissue stiffness is measured in the targeted area (region of interest [ROI] A) and outside the targeted area in a region representing normal tissue (ROI B). Thereafter, the strain ratio value is calculated as the quotient B/A, which seems to be better and more objective than visual color changes of qualitative elastography [Figures 7 and 8]. In a study on EUS elastography in CP, region B selected was the normal surrounding gastroduodenal wall and strain ratio cut-off of 2.25 yielded an accuracy of 91.1% for diagnosis of CP.[12] The results appear promising, but further studies with larger sample size are needed to confirm these interesting results.
Figure 7.
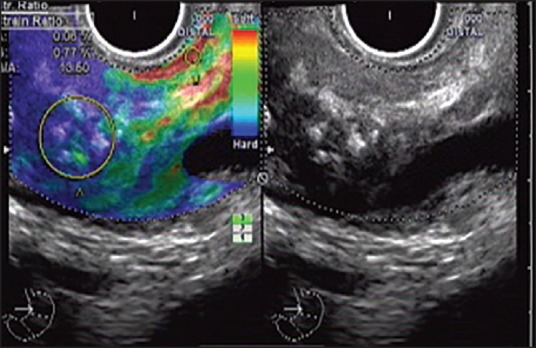
Quantitative endoscopic ultrasound (EUS) elastography and strain ratio measurement with radial echoendoscope at the body of pancreas: The EUS shows echogenic foci with shadowing (Right side). A stable EUS image for at least 5 s is obtained for EUS quantitative analysis. The region of interest for the elastographic evaluation is manually selected to include the targeted area of the pancreas (region A) and soft (red) reference area corresponding to normal gastric wall (region B). A strain ratio of 13.6 has been obtained
Figure 8.
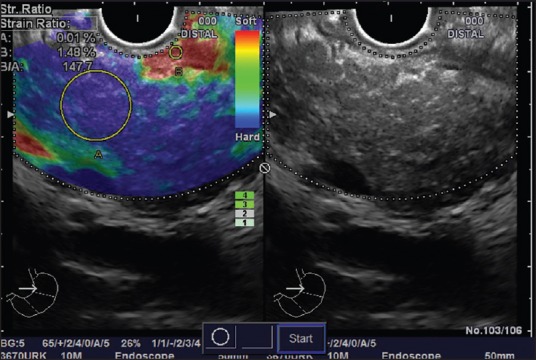
Quantitative endoscopic ultrasound (EUS) elastography and strain ratio measurement with radial echoendoscope at the body of pancreas: The EUS shows stranding with echogenic foci and lobularity (Right side). A stable EUS image for at least 5 s is obtained for EUS quantitative analysis. The region of interest for the elastographic evaluation is manually selected to include the targeted area of the pancreas (region A) and soft (red) reference area corresponding to normal gastric wall (region B). A strain ratio of 147.7 has been obtained
IMPORTANT FACTS ABOUT ENDOSCOPIC ULTRASOUND IN CHRONIC PANCREATITIS
Although EUS can demonstrate subtle alterations in the pancreatic parenchymal and ductal structure, the exact role of EUS in the diagnosis of CP is still not established.
The EUS features to diagnose CP have evolved over a period of time from a pure qualitative approach to more advanced and complicated scoring systems incorporating multiple parenchymal and ductal EUS features.
The rosemont criteria have attempted to define precisely each EUS criterion and thus have good inter-observer agreement. But, initial studies have failed to demonstrate any significant improvement in the inter-observer variability.
A number of conditions such as aging, smoking, obesity, and chronic alcohol consumption may cause EUS changes similar to CP. Therefore, EUS findings should be interpreted in appropriate clinical context.
Most of the EUS features of CP except ductal calculi and cysts should be located in the body and tail only.
In order to correctly identify the changes of CP, the endoscopist needs to know the normal appearances of the pancreas as well as the normal variations seen in nonpathological pancreas.
Quantitative EUS elastography appear promising technique for diagnosis of CP.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Braganza JM, Lee SH, McCloy RF, et al. Chronic pancreatitis. Lancet. 2011;377:1184–97. doi: 10.1016/S0140-6736(10)61852-1. [DOI] [PubMed] [Google Scholar]
- 2.Gardner TB, Levy MJ. EUS diagnosis of chronic pancreatitis. Gastrointest Endosc. 2010;71:1280–9. doi: 10.1016/j.gie.2010.02.038. [DOI] [PubMed] [Google Scholar]
- 3.Raimondo M, Wallace MB. Diagnosis of early chronic pancreatitis by endoscopic ultrasound. Are we there yet? JOP. 2004;5:1–7. [PubMed] [Google Scholar]
- 4.Morris-Stiff G, Webster P, Frost B, et al. Endoscopic ultrasound reliably identifies chronic pancreatitis when other imaging modalities have been non-diagnostic. JOP. 2009;10:280–3. [PubMed] [Google Scholar]
- 5.Gleeson FC, Topazian M. Endoscopic retrograde cholangiopancreatography and endoscopic ultrasound for diagnosis of chronic pancreatitis. Curr Gastroenterol Rep. 2007;9:123–9. doi: 10.1007/s11894-007-0006-3. [DOI] [PubMed] [Google Scholar]
- 6.Bhutani MS. Endoscopic ultrasound in pancreatic diseases. Indications, limitations, and the future. Gastroenterol Clin North Am. 1999;28:747–70. doi: 10.1016/s0889-8553(05)70085-6. xi. [DOI] [PubMed] [Google Scholar]
- 7.Stevens T. Update on the role of endoscopic ultrasound in chronic pancreatitis. Curr Gastroenterol Rep. 2011;13:117–22. doi: 10.1007/s11894-010-0167-3. [DOI] [PubMed] [Google Scholar]
- 8.Catalano MF, Sahai A, Levy M, et al. EUS-based criteria for the diagnosis of chronic pancreatitis: The Rosemont classification. Gastrointest Endosc. 2009;69:1251–61. doi: 10.1016/j.gie.2008.07.043. [DOI] [PubMed] [Google Scholar]
- 9.Stevens T, Lopez R, Adler DG, et al. Multicenter comparison of the interobserver agreement of standard EUS scoring and Rosemont classification scoring for diagnosis of chronic pancreatitis. Gastrointest Endosc. 2010;71:519–26. doi: 10.1016/j.gie.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 10.Stevens T. Role of endoscopic ultrasonography in the diagnosis of acute and chronic pancreatitis. Gastrointest Endosc Clin N Am. 2013;23:735–47. doi: 10.1016/j.giec.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Stevens T, Zuccaro G, Jr, Dumot JA, et al. Prospective comparison of radial and linear endoscopic ultrasound for diagnosis of chronic pancreatitis. Endoscopy. 2009;41:836–41. doi: 10.1055/s-0029-1215061. [DOI] [PubMed] [Google Scholar]
- 12.Iglesias-Garcia J, Domínguez-Muñoz JE, Castiñeira-Alvariño M, et al. Quantitative elastography associated with endoscopic ultrasound for the diagnosis of chronic pancreatitis. Endoscopy. 2013;45:781–8. doi: 10.1055/s-0033-1344614. [DOI] [PubMed] [Google Scholar]


