Summary
Glenohumeral bone defects are a common finding in shoulder instability and they are strongly correlated with recurrence of dislocation and failure following arthroscopic Bankart repair. Most authors agree that open surgery should be considered in the presence of certain conditions: glenoid bone loss > 25%, a lesion involving > 30% of the humeral head, an engaging Hill-Sachs lesion, bipolar bone lesions even without engagement.
A careful imaging evaluation must therefore be performed in order to identify, quantify and characterize the bone defects. Even though magnetic resonance has important additional value in the assessment of the glenoid labrum and rotator cuff, computed tomography scan is the examination of choice for studying bone defects.
Several methods have been proposed to quantify the extent of the glenoid bone defect; the most accurate ones utilize two-dimensional computed tomography images with multiplanar reconstructions (PICO method) or more sophisticated three-dimensional reconstruction software. Conversely, the literature lacks studies that accurately quantify humeral bone defects and, above all, that demonstrate definitively the clinical and prognostic significance of the lesion location and size.
Keywords: bone defect, glenoid, Hill-Sachs, imaging, shoulder instability, PICO
Introduction
The glenohumeral joint is the joint most prone to traumatic dislocations (1). Static stabilizers (glenoid labrum, capsule and glenohumeral ligaments) and dynamic stabilizers (rotator cuff and long head of the biceps) work together to ensure the maintenance of the stability of this joint (2, 3). Disruption of this complex balance underlies the onset of recurrent shoulder instability. Glenohumeral bone defects are regarded as one of the main causes of recurrence of instability (4, 5). Numerous clinical (6, 7) and biomechanical (7–10) studies have highlighted the role of these defects in recurrent episodes of dislocation and instability after surgical treatment, showing how they cause changes in the contact forces on the joints and reduce their resistance to dislocation. Avulsion of the glenoid labrum and inferior glenohumeral ligament complex (Bankart lesion) is the lesion most commonly encountered after a first episode of anterior glenohumeral dislocation (11, 12). In between 5% and 55% of cases it is accompanied by detachment of a bone fragment (bony Bankart lesion) (13, 14). Isolated glenoid defects are found in 22% of patients with first-time anterior dislocation and in up to 73% of subjects with chronic instability (11, 16). Impaction fractures of the humeral head (Hill-Sachs lesions), caused by compressive forces that develop between the proximal humeral epiphysis and the anteroinferior margin of the glenoid in anterior dislocation, are a further factor predisposing to chronic instability. Up to 89% of patients with recurrent shoulder dislocation show glenohumeral bone loss (16, 17) and a clinically relevant correlation has been found between frequency of recurrence and the area of the missing glenoid (18). Sometimes the location of the lesions is more important than their size, as demonstrated in recent biomechanical studies (19).
The clinical workup and history, performed in order to identify general risk factors (age, sex, generalized ligamentous laxity, sporting activities, and work), and type of instability, must be followed by a careful imaging assessment. The literature shows that in addition to standard radiographic views, two-dimensional (2D) and three-dimensional (3D) computed tomography (CT) and magnetic resonance imaging (MRI) are fundamental for detecting the site and measuring the size of bone defects, both glenoid and humeral (17, 20). Recognizing and precisely evaluating the degree of bone loss in the pre-operative stage seems to be crucial in order to plan an appropriate treatment and reduce the risk of recurrence.
Glenoid bone defects
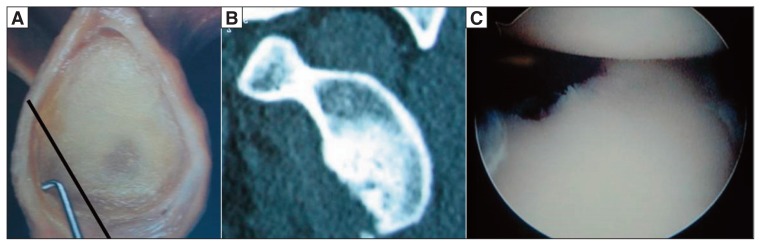
Many authors have highlighted the relationship between glenoid bone loss and recurrence of instability after surgical repair. Rowe (21) reported recurrence rates ranging from 6 to 62% in the presence of an anterior bone defect, while Burkhart and De Beer (4) found recurrence rates of 67% in patients with significant glenoid bone defects (“inverted pear” glenoid) versus 4% in those without such bone defects (Fig. 1). Tauber et al. (14) found bone loss in 57% of 41 patients re-operated on for recurrence of instability.
Figure 1.
A: “Inverted pear” glenoid bone defect. B: CT image of a 3 o’clock-to-6 o’clock defect (corresponding to 20–25% bone loss). C: Arthroscopic view of an “inverted pear” lesion.
It thus seems crucial to be able to identify the presence of glenoid bone loss, establish its location, study its morphology, and quantify its extent. Indeed, this is necessary because patients with glenoid bone loss in the range of 15% to 25% undergoing arthroscopic stabilization surgery show a high rate of recurrence at medium- and long-term follow-up. Given the demonstrated clinical importance of bone defects, all patients who are candidates for stabilization treatments should be carefully assessed for the presence of bone loss, particularly those presenting the most important risk factors, namely: many previous dislocations, a young age at first dislocation, and a long clinical history of instability (22).
Glenoid bone defects mainly involve the anterior rim of the glenoid (23, 24), and may be characterized morphologically by erosion (attritional bone loss) or fracture (8, 23, 25–27).
Bigliani et al. (28) coined the term bony Bankart lesion to indicate the presence of an anteroinferior bone fragment, and classified anterior rim bone lesions into three types:
type 1, displaced bone fragment with inferior glenohumeral ligament clearly recognizable;
type 2, poorly consolidated bone fragment and unrecognizable ligamentous connections;
type 3, bone defect corresponding to < 25% (type 3-A) or > 25% (type 3-B) of the total glenoid surface (indeed, they established 25% glenoid bone loss as the main risk factor for failure of Bankart repair).
Edwards et al. (29), studying 160 unstable shoulders, found osseous lesions in 90% and, after plain radiographs including anteroposterior (AP) and Bernageau views, classified the patterns of glenoid bone defects into three main groups:
those in which the bone fragment is still visible (bony Bankart lesion) (Fig. 2);
those with loss of the anteroinferior angle and with the bone fragment no longer visible (cliff sign);
those characterized by bone loss with compression and rounding of the glenoid rim (blunted angle).
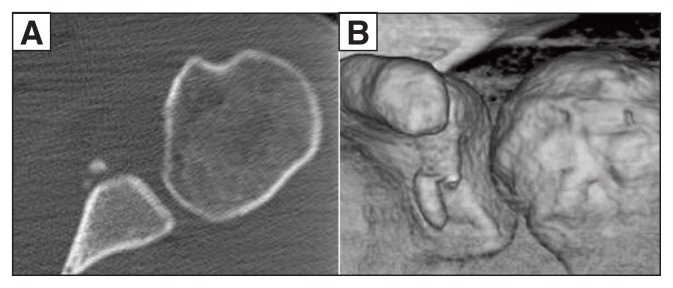
Figure 2.
Bony Bankart lesion. A: Axial 2D CT image. B: Coronal 3D CT image.
Sugaya et al. (27), in a series of 100 unstable shoulders, found a bony Bankart lesion in 50%, bone loss in 40% and normal glenoid morphology in just 10%.
There is no unanimous consensus on what method should be used to calculate a glenoid bone defect, or on what to consider the threshold value for defining a lesion as “severe”, or at risk of recurrence. Bigliani et al. (28) suggested that the Bankart procedure should not be performed in the presence of a glenoid defect width >25%. Itoi et al. (7), in a biomechanical study, found 21% to be a “critical” value, even though limitations of this study are the fact that the defect was artificially created and the authors considered only anteroinferior defects and not exclusively anterior ones. Many authors agree that, in terms of bone loss, the critical limit beyond which there is a clinically significant risk of recurrence after surgical repair (Bankart procedure) without bone grafting is between 20% and 25% of the total glenoid surface (4, 7, 9, 30, 31).
However, the real problem is not identifying the glenoid defect (this is now considered a relatively simple procedure, using true AP, Bernageau and West-Point views), but quantifying the bone loss or size of the fragment. The true AP and Bernageau views can show the lesion as a loss of continuity of the anteroinferior profile of the glenoid, with a sensitivity of 66% and a specificity of 100% (18); however, these views have a major limitation, in that they do not provide reliable indications on the real size of the bone defect. The West Point modified axillary view has shown a high correlation with CT in estimating glenoid bone loss (32).
Burkhart et al. (33) proposed an arthroscopic measurement based on the glenoid bare spot, i.e. the central area of the glenoid where the cartilage is absent or thinnest. The bare spot is considered to be the central point of the glenoid, from which using an arthroscopic probe, it is possible to measure the anterior or posterior glenoid radius. However, this method can be used only intra-operatively and therefore has no useful role to play in the preoperative planning stage; furthermore, anatomical studies have shown inter-individual variability in the position of the bare spot (34).
MRI is the gold standard method for examining the rotator cuff; conversely, it shows less sensitivity and specificity (in the ranges 44–100% and 66–95%, respectively) in detecting lesions of the glenoid labrum. MR arthrography, for the capsuloligamentous, labral and cartilaginous structures, showed sensitivity and specificity values of 86%–91% and 86%–98%, respectively (35). However, in order to quantify the glenoid defect on MR arthrography it is necessary to obtain a perfect sagittal image (en face view) of the glenoid. MR arthrography is, in any case, more sensitive and specific in the presence of attritional bone loss than Bankart bony lesions. Recent studies (20, 26) have demonstrated the validity of MR arthrography in estimating glenoid bone defects in patients with chronic instability of the shoulder, also in comparison with 2D and 3D CT. However, a series of factors limit the applicability of this technique: its high costs, relative invasiveness, general contraindications (presence of pacemakers, cochlear implants, ferromagnetic implants, etc.) and also the various complications that can arise (allergic reactions, infections, pain on injection of the contrast medium).
CT scanning is currently the method of choice for studying glenoid bone defects (9, 23–25, 27) (Fig. 3). Recurrent dislocations, a strongly positive apprehension test at minimum degrees of abduction, bone defects shown on plain radiographs, and the need for surgical stabilization are the main indications for performing a CT study (36). A study by De Wilde et al. was the first to confirm the pathological changes on whose basis various methods of measuring glenoid bone loss on CT or MRI studies were developed (37). In a cadaveric study, these authors, examining 98 scapulae, identified 11 reproducible anatomical points that can be used to trace a perfect circle including the inferior part of the glenoid. On this basis, Sugaya et al. (27) developed a method, based on the processing of 3D CT images of the glenoid (with the humeral head eliminated), for identifying and calculating glenoid bone loss, which they expressed as a percentage of the articular surface. CT scans of both the patient’s shoulders are acquired; a circle with a horizontal diameter (from 3’ o clock to 9 o’ clock) is drawn on the normal glenoid, following its lower edge; then, using dedicated CT software, the area of the inferior part of the normal glenoid is calculated. The procedure is then repeated on the unstable shoulder, tracing, on the glenoid, the circle of the normal (i.e. contralateral) glenoid and the area of bone loss is outlined. A limitation of this method is the need to have dedicated software (38).
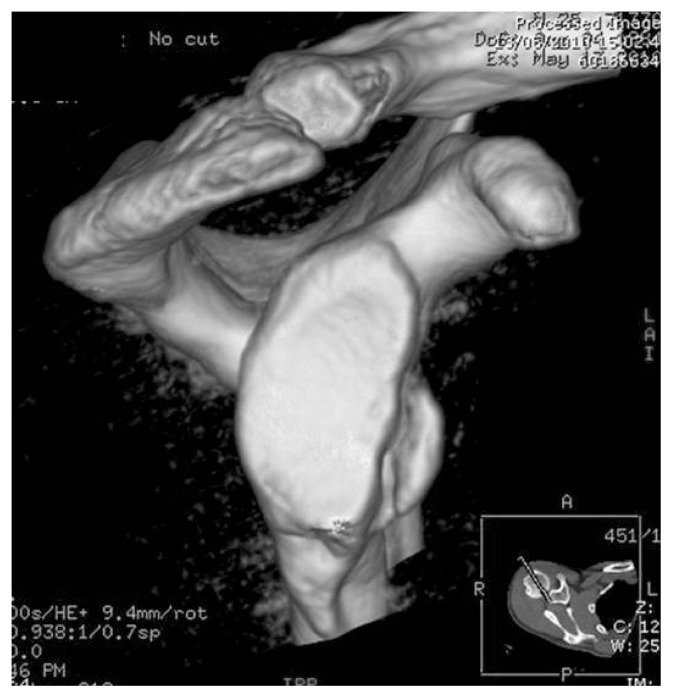
Figure 3.
Bony Bankart lesion. 3D CT image (en face view).
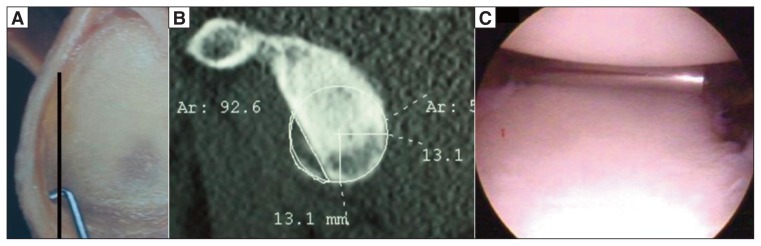
Griffith et al. (23) described a method for calculating glenoid defects, based on CT images with multiplanar reconstruction (MPR); they calculated the maximum width of the affected glenoid and compared it with that of the contralateral glenoid. Chuang et al. (9) developed a similar method using 3D CT images; these authors considered a glenoid index defined as the ratio between the diameter of the deficient glenoid and that of the healthy contralateral one. Instead, Barchilon et al. (39) proposed a method based on the tracing, on 3D CT en face images, a circle with a radius, “R”, centered on the lower two-thirds of the affected glenoid. A distance, “d”, is calculated between the center of the circle and the anterior edge of the bone lesion. The percentage of bone loss is identified using a function that takes into account the d/R ratio. Baudi et al. (40) developed a simple CT method for identifying and precisely evaluating the extent of the glenoid bone defects. The advantages of their technique, called “PICO”, derive from the fact that it combines simple 2D CT examination with subsequent MPR, and thus eliminates the need for 3D processing and dedicated software, yet can still be used to obtain 3D reconstructions. Standard images are processed in MPR in order to obtain an en face view of the glenoid. A circle with a horizontal diameter (from 3’ o clock to 9 o’ clock) is drawn on the inferior part of the healthy glenoid, and the circle area is measured in mm2 using commonly available software. The circle is then transferred to the contralateral glenoid and the area of bone loss outlined. The bone defect is quantified in mm2 and as a percentage (Fig. 4). A study performed using the PICO method on 115 patients with recurrent anterior shoulder dislocation showed glenoid loss in 65% of cases and a Hill-Sachs lesion in 80% (41). The glenoid bone lesions observed could be categorized into three main types. The first is the classic Bankart bony lesion. The second type, called the “straight pear” (Fig. 5) lesion, is determined by anterior glenoid bone loss, often as a result of compressive injuries, such as those caused by impaction of the humeral head against the anterior edge of the glenoid; lesions of this kind, found in 55%–65% of cases, are characterized by glenoid bone loss of between 5% and 15%, identifiable by drawing a straight line between 2 o’ clock and 5 o’ clock (25, 41). The third type of lesion, resulting from more severe injury, is bone loss >20%, identifiable by drawing an oblique line between 3 o’ clock and 6 o’ clock; this type of lesion is found in between 5% and 10% of cases (25, 41).
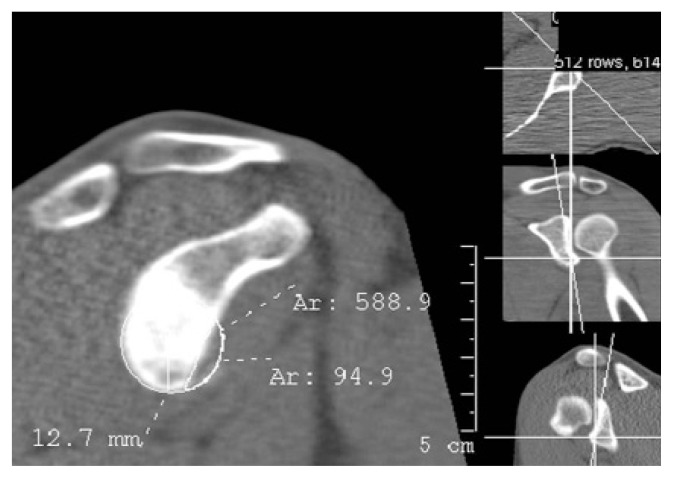
Figure 4.
Quantification of glenoid bone loss using the PICO method on en face 2D CT image of the glenoid with MPR.
Figure 5.
A: “Straight pear” glenoid bone defect. B: CT image of a 2 o’clock-to-5 o’clock defect (corresponding to 10–15% bone loss). C: Arthroscopic view of a “straight pear” lesion.
Various studies have documented the validity of this method (42). Magarelli et al. (43) applied it to 40 patients and confirmed its excellent reproducibility. In a recent study, the same authors (17) showed high agreement (97%) between 2D and 3D CT measurements for identifying the presence, size and type of glenoid bone defects in anterior glenohumeral instability, and claimed that the two measurements can be considered interchangeable. Finally, Bois et al. (16) studied the accuracy of the Glenoid Index (9), Ratio method (39) and PICO method (40) and concluded that 2D reconstruction is not recommended for these methods; on 3D CT, they found the PICO method to be the one that most accurately quantified bone loss, both anterior and anteroinferior, and thus recommended its wider use.
Humeral bone defects
The Hill-Sachs lesion, named after the authors who described it in 1940, is a fracture of the posterior-superior aspect of the humeral head, which is caused by impaction of the latter against the glenoid rim during anterior dislocation. In the rare event of posterior dislocation, a similar lesion is formed, in the same way, but on the anterior aspect of the head of the humerus (reverse Hill-Sachs lesion).
In the decision-making process in cases of recurrent anterior shoulder instability, humeral bone defects have always tended to be considered less important than glenoid ones, even though the authors who first described the Hill-Sachs lesion found it to be present in 74% of recurrent dislocations (44).
The prevalence of this lesion reported in the literature is highly variable, with values ranging from 38 to 93%, depending on the diagnostic instruments used and the sample observed. Bushnell et al. (45) reported a frequency of 70% in first-time dislocations and 93–100% in recurrences.
Hill-Sachs lesions can be identified using plain radiography, MRI, MR-arthrography and CT. The radiographic views that can highlight a Hill-Sachs lesion are: AP in maximum internal rotation and another more specific view called the Stryker notch view. Plain radiographs often underestimate humeral bone loss and are still considered inadequate for quantifying it (46). MRI is the technique that, thanks to the altered signal intensity of the injured (cancellous) bone compared with the intact humeral bone, detects Hill-Sachs lesions with the greatest reproducibility (47), and it shows high sensitivity (97%). However, MRI can underestimate bone loss (36). Furthermore, MRI can give false-positive results: erosions or small grooves can be mistaken for Hill-Sachs lesions, as can the bare area: this area is in fact visible as a small cartilage-free area on the posterolateral aspect of the humeral head and it is a normal finding; Hill-Sachs lesions, on the other hand, are located, on average, 0–24 mm from the top of the humeral head in a posterosuperior position (48).
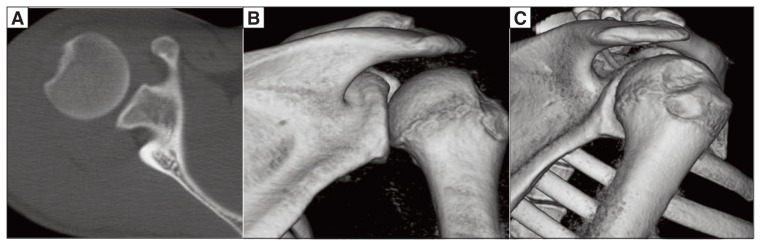
The gold standard for identifying and quantifying humeral bone defects is CT, especially with 3D reconstructions (46) (Fig. 6).
Figure 6.
Hill-Sachs lesion on the posterior-superior aspect of the humeral head. A: Axial 2D CT image. B: Coronal 3D CT image. C: Sagittal 3D CT image.
The first method for quantifying Hill-Sachs lesions dates back to 1959: the defect was calculated on plain radiographs as the percentage of lesion involvement in a 180° arc on the surface of the humeral head (49). Even though this method has its limits, given that it calculates the extent of the damage in only one plane and lacks prognostic value, is still widely used and can be quickly and easily applied to CT and MRI studies. It was used in 1984 by Rowe et al. (21), who also took into account the depth of the lesions. They calculated two dimensions (depth and length) of the lesions and correlated these results with clinical severity. This allowed them to grade the lesions as mild (<0.5cm deep and < 2cm long), moderate (0.5–1cm deep and 2–4cm long) and severe (>1cm deep and >4cm long). Flatow et al. (50) suggested quantifying the humeral bone loss as a percentage of involvement of the humeral head, classifying defects in three categories: <20%, 20–40% and >40%. Lesions >40% are clinically significant. Montgomery et al. (51) specified this method further: the lesion should be calculated on axial and coronal CT images with measurements expressed as a percentage of the total area of the humeral head.
Some authors have shown that not only the size, but also the location and orientation of Hill-Sachs lesions can have clinical and prognostic significance and introduced the concept of engagement of the glenoid and humeral head during movements at various degrees of abduction and external rotation (4, 46, 52, 53). Yamamoto et al. (19) used the term “glenoid track” to describe the zone of contact between the articular surfaces of the glenoid and humeral head during combined abduction and external rotation movements, and showed that there is a risk of engagement if the lesion extends beyond the medial margin of that zone. According to this theory, the depth and extent of the lesion, while contributing to the risk of recurrent shoulder instability, are less important than its location. The authors noted that the “medial margin of the glenoid track was located 18.4 ± 2.5 mm medial from the footprint” of the rotator cuff, a distance “equivalent to 84% ± 14% of the glenoid width” (19).
Engaging Hill-Sachs lesions are the ones with the worst prognosis, carrying a 100% risk of recurrence of instability (4). In addition to this type of lesion, there are a great amount of Hill-Sachs lesions, of different dimensions and locations, that do not give rise to engagement but are just as severe, in terms of prognosis, as the engaging type, so much so that many authors have sought to establish the existence of a critical lesion size, albeit they failed to document this. However, 25% bone loss has been recognized as a critical threshold beyond which surgery should be recommended, given that biomechanical studies and instrumental-clinical-arthroscopic correlations have shown this volume of humeral bone loss to lead to biomechanical alterations capable of inducing instability (36, 46, 54–56). Other authors have instead linked the risk of recurrent dislocations to the extent of bone loss calculated in relation to the glenoid, claiming that humeral bone loss greater than 21% of the superior-inferior length of the glenoid or 25% of its depth will result in instability (52).
It is important to point out that in the presence of a Hill-Sachs lesion it is essential to look for glenoid bone loss, given its remarkably high frequency in these cases (up to 100%). It is fundamentally important to diagnose this association, known as a bipolar injury (36, 57), because in the presence of glenoid bone loss the glenoid track area shrinks and the medial margin draws closer to the footprint, thus increasing the risk of engagement even for apparently innocuous lesions. Other authors have also studied the morphology of humeral bone loss, looking for correlations with clinical history. It has been shown that the volume of bone loss increases with increasing numbers of dislocations, but that its depth reduces, indicating more severe instability; conversely, the defect appears deeper in cases of acute dislocation following a high-energy trauma, repeated attempts at reduction of the dislocation under anesthesia, and in cases of chronic dislocation (53, 58). The mean depth of the lesion in cases of dislocation is 3.9 ± 0.9 mm, as opposed to 2.1 ± 1 mm in subluxation episodes (53).
Therefore, in recurrent shoulder instability, careful identification of the site and extent of a Hill-Sachs lesion is crucial in order to plan and to choose the appropriate treatment.
Surgical repair of a Hill-Sachs lesion may be indicated in the presence of a defect that is > 25% of the volume of the humeral head, or when there is engagement. It may also be indicated in young people who play collision sports and in cases of recurrence following a previous Bankart repair in patients with humeral bone loss. The following, on the other hand, are contraindications to the procedure: signs of osteoarthritis, avascular necrosis, advanced age (relative), rotator cuff tear associated with functional limitation (46).
An isolated Hill-Sachs lesion equal to or less than 25% of the humeral head should never be treated surgically if the Bankart lesion is well repaired. However, the presence of concomitant glenoid bone loss or rotator cuff damage needs to be carefully looked for in order to avoid failures (6, 46, 54).
For many years, the Hill-Sachs lesion was considered less important than glenoid bone loss, with the result that surgical repair techniques were performed simply to obtain joint stability, with little attention paid to the achievement of anatomical recovery and the restoration of joint congruity; this approach resulted in early onset of arthritic changes, decreased range of motion and a high number of failures. In 1984, Weber et al. (59) described, for the first time, a technique involving rotational humeral osteotomy of the proximal diaphyseal segment to prevent recurrent instability. In 1972, Connolly (60) introduced the remplissage technique, which was subsequently developed arthroscopically (61), in the form of a technique that involves transferring the infraspinatus and possibly the posterior capsule into the defect in such a way that the Hill-Sachs lesion becomes an extra-articular lesion. This solution guarantees full recovery of stability at the expense of a slight reduction in external rotation (55, 62). Various authors agree that treatment with remplissage is indicated when the defect involves more that 25% of the humeral head, and should be associated with anterior capsulolabral reconstruction (55, 63).
For an engaging Hill-Sachs lesion or a lesion involving > 30% of the humeral head associated with > 20% glenoid bone loss, authors recommend surgical autologous graft or allograft reconstruction, fixed with miniscrews (10, 36, 56).
In the presence of humeral head defects involving more than 40% of the articular surface, most authors recommend the implantation of shoulder resurfacing prostheses, albeit after very careful patient selection (46, 64).
References
- 1.Kazar B, Relovszky E. Prognosis of primary dislocation of the shoulder. Acta Orthop Scand. 1969;40:216–24. doi: 10.3109/17453676908989501. [DOI] [PubMed] [Google Scholar]
- 2.Chen AL, Hunt SA, Hawkins RJ, et al. Management of bone loss associated with recurrent anterior glenohumeral instability. Am J Sports Med. 2005;33:912–925. doi: 10.1177/0363546505277074. [DOI] [PubMed] [Google Scholar]
- 3.Rowe CR, Patel D, Southmayd WW. The Bankart procedure: a long-term end-result study. J Bone Joint Surg. 1978;60:1–16. [PubMed] [Google Scholar]
- 4.Burkhart SS, De Beer JF. Traumatic glenohumeral bone defect and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy. 2000;16:677–694. doi: 10.1053/jars.2000.17715. [DOI] [PubMed] [Google Scholar]
- 5.Greis PE, Scuderi MG, Mohr A, et al. Glenohumeral articolar contact areas and pressures following labral and osseus injury to the anteroinferior quadrant of the glenoid. J Shoulder Elbow Surg. 2002;11:442–451. doi: 10.1067/mse.2002.124526. [DOI] [PubMed] [Google Scholar]
- 6.Boileau P, Villalba M, Hery JY, et al. Risk factors for recurrence of shoulder instability after arthroscopic Bankart repair. J Bone Joint Surg. 2006;88A:1755–1763. doi: 10.2106/JBJS.E.00817. [DOI] [PubMed] [Google Scholar]
- 7.Itoi E, Lee SB, Berglund LJ, Berge LL, et al. The effect of a glenoid defect on anteroinferior stability of the shoulder after Bankart repair: a cadaveric study. J Bone Joint Surg. 2000;82A:35–46. doi: 10.2106/00004623-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Burkhart SS, Danaceau SM. Articular arc length mismatch as a cause of failed Bankart repair. Arthroscopy. 2000;16:740–744. doi: 10.1053/jars.2000.7794. [DOI] [PubMed] [Google Scholar]
- 9.Chuang TY, Adams CR, Burkhart SS. Use preoperative three-dimensional computer tomography to quantify glenoid bone loss in shoulder instability. Arthroscopy. 2008;24:376–382. doi: 10.1016/j.arthro.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Lynch JR, Clinton JM, Dewing CB, et al. Treatment of osseous defects associated with anterior shoulder instability. J Shoulder Elbow Surg. 2009;18:317–328. doi: 10.1016/j.jse.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Taylor DC, Arciero RA. Pathologic changes associated with shoulder dislocations. Arthroscopic and physical examination findings in first-time, traumatic anterior dislocations. Am J Sports Med. 1997;25:306–311. doi: 10.1177/036354659702500306. [DOI] [PubMed] [Google Scholar]
- 12.Thomas SC, Matsen FA., 3rd An approach to the repair of avulsion of the glenohumeral ligaments in the management of traumatic anterior glenohumeral instability. J Bone Joint Surg. 1989;71A:506–513. [PubMed] [Google Scholar]
- 13.Fujii Y, Yoneda M, Wakitani S, et al. Histologic analysis of Bony Bankart lesions in recurrent anterior instability of the shoulder. J Shoulder Elbow Surg. 2006;15:218–223. doi: 10.1016/j.jse.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Tauber M, Resch H, Forstner R, et al. Reasons for failure after surgical repair of anterior shoulder instability. J Shoulder Elbow Surg. 2004;13:279–285. doi: 10.1016/j.jse.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Calandra JJ, Baker CL, Uribe J. The incidence of Hill-Sachs lesions in initial anterior shoulder dislocations. Arthroscopy. 1989;5:254–257. doi: 10.1016/0749-8063(89)90138-2. [DOI] [PubMed] [Google Scholar]
- 16.Bois AJ, Fening SD, Polster J, et al. Quantifying glenoid bone loss in anterior shoulder instability: reliability and accuracy of 2-dimensional and 3-dimensional computed tomography measurement techniques. Am J Sports Med. 2012;40:2569–2577. doi: 10.1177/0363546512458247. [DOI] [PubMed] [Google Scholar]
- 17.Magarelli N, Milano G, Baudi P, et al. Comparison between 2D and 3D computed tomography evaluation of glenoid bone defect in unilateral anterior glenohumeral instability. Radiol Med. 2012;117:102–111. doi: 10.1007/s11547-011-0712-7. [DOI] [PubMed] [Google Scholar]
- 18.Gerber C, Nyffeler RW. Classification of glenohumeral joint instability. Clin Orthop Relat Res. 2002;400:65–76. doi: 10.1097/00003086-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto N, Itoi E, Abe H, et al. Contact between the glenoid and the humeral head in abduction, external rotation, and horizontal extension: a new concept of glenoid track. J Should Elbow Surg. 2007;16:649–656. doi: 10.1016/j.jse.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Owens BD, Burns TC, Campbell SE, et al. Simple method of glenoid bone loss calculation using ipsilateral magnetic resonance imaging. Am J Sports Med. 2013;41:622–624. doi: 10.1177/0363546512472325. [DOI] [PubMed] [Google Scholar]
- 21.Rowe CR, Zarins B, Ciullo JV. Recurrent anterior dislocation of the shoulder after surgical repair. Apparent causes of failure and treatment. 1984;66A:159–168. [PubMed] [Google Scholar]
- 22.Milano G, Grasso A, Russo A, et al. Analysis of risk factor for glenoid bone defect in anterior shoulder instability. Am J Sports Med. 2011;39:1870–1876. doi: 10.1177/0363546511411699. [DOI] [PubMed] [Google Scholar]
- 23.Griffith JF, Antonio GE, Tong CWC, et al. Anterior shoulder dislocation: quantification of glenoid bone loss with CT. AJR Am J Roentgenol. 2003;180:1423–1430. doi: 10.2214/ajr.180.5.1801423. [DOI] [PubMed] [Google Scholar]
- 24.Saito H, Itoi E, Sugaya H, et al. Location of the glenoid defect in shoulders with recurrent anterior dislocation. Am J Sports Med. 2005;33:889–893. doi: 10.1177/0363546504271521. [DOI] [PubMed] [Google Scholar]
- 25.Griffith JF, Antonio GE, Yung PS, et al. Prevalence, pattern, and spectrum of glenoid bone loss in anterior shoulder dislocation: CT analysis of 218 patients. AJR Am J Roentgenol. 2008;190:1247–1254. doi: 10.2214/AJR.07.3009. [DOI] [PubMed] [Google Scholar]
- 26.Huijsmans PE, Haen PS, Kidd M, et al. Quantification of a glenoid defect with three-dimensional computed tomography and magnetic resonance imaging: a cadaveric study. J Shoulder Elbow Surg. 2007;16:803–809. doi: 10.1016/j.jse.2007.02.115. [DOI] [PubMed] [Google Scholar]
- 27.Sugaya H, Moriishi J, Dohi M, et al. Glenoid rim morphology in recurrent anterior glenohumeral instability. J Bone Joint Surg. 2003;85A:878–884. doi: 10.2106/00004623-200305000-00016. [DOI] [PubMed] [Google Scholar]
- 28.Bigliani LU, Newton PM, Steinmann SP, et al. Glenoid rim lesions associated with recurrent anterior dislocation of the shoulder. Am J Sports Med. 1998;26:41–45. doi: 10.1177/03635465980260012301. [DOI] [PubMed] [Google Scholar]
- 29.Edwards TB, Boulahia A, Walch G. Radiographic analysis of bone defects in chronic anterior shoulder instability. Arthroscopy. 2003;19:732–739. doi: 10.1016/s0749-8063(03)00684-4. [DOI] [PubMed] [Google Scholar]
- 30.Lo IK, Parten PM, Burkhart SS. The inverted pear glenoid: an indicator of significant glenoid bone loss. Arthroscopy. 2004;20:169–74. doi: 10.1016/j.arthro.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto N, Muraki T, Sperling JW, et al. Stabilizing mechanism in bone-grafting of a large glenoid defect. J Bone Joint Surg. 2010;92A:2059–2066. doi: 10.2106/JBJS.I.00261. [DOI] [PubMed] [Google Scholar]
- 32.Itoi E, Lee SB, Amrami KK, et al. Quantitative assessment of classic anteroinferior bony Bankart lesions by radiography and computed tomography. Am J Sports Med. 2003;31:112–118. doi: 10.1177/03635465030310010301. [DOI] [PubMed] [Google Scholar]
- 33.Burkhart SS, De Beer JF, Tehrany AM, et al. Quantifying glenoid bone loss arthroscopically in shoulder instability. Arthroscopy. 2002;18:488–491. doi: 10.1053/jars.2002.32212. [DOI] [PubMed] [Google Scholar]
- 34.Aigner F, Longato S, Fritsch H, et al. Anatomical considerations regarding the “bare spot” of the glenoid cavity. Surg Radiol Anat. 2004;26:308–311. doi: 10.1007/s00276-003-0217-8. [DOI] [PubMed] [Google Scholar]
- 35.Steinbach LS. MRI of shoulder instability. Eur J Radiol. 2008;68:57–71. doi: 10.1016/j.ejrad.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 36.Bollier MJ, Arciero R. Management of glenoid and humeral bone loss. Sports Med Arthrosc Rev. 2010;18:140–148. doi: 10.1097/JSA.0b013e3181e88ef9. [DOI] [PubMed] [Google Scholar]
- 37.De Wilde LF, Berghs BM, Audenaert E, et al. About the variability of the shape of the glenoid cavity. Surg Radiolog Anat. 2004;26:54–59. doi: 10.1007/s00276-003-0167-1. [DOI] [PubMed] [Google Scholar]
- 38.Nofsinger C, Browning B, Burkhart SS, et al. Objective preoperative measurement of anterior glenoid bone loss: a pilot study of a computer-based method using unilateral 3-dimensional computed tomography. Arthroscopy. 2011;27:322–329. doi: 10.1016/j.arthro.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Barchilon VS, Kotz E, Barchilon Ben-Av M, et al. A simple method for quantitative evaluation of the missing area of the anterior glenoid in anterior instability of the glenohumeral joint. Skeletal Radiol. 2008;37:731–736. doi: 10.1007/s00256-008-0506-8. [DOI] [PubMed] [Google Scholar]
- 40.Baudi P, Righi P, Bolognesi D, et al. How to identify and calculate glenoid bone deficit. Chir Organi Mov. 2005;90:145–152. [PubMed] [Google Scholar]
- 41.Baudi P. How to manage bone defects in shoulder instability. Instructional Course Lecture, 13th ESSKA Meeting; 2008. [Google Scholar]
- 42.D’Elia G, Di Giacomo A. Quantification of traumatic anterior gleno-humeral bone loss by spiral CT. Radiol Med. 2008;113:496–505. doi: 10.1007/s11547-008-0274-5. [DOI] [PubMed] [Google Scholar]
- 43.Magarelli N, Milano G, Sergio P, et al. Intra-observer and interobserver reliability of the ‘Pico’ computed tomography method for quantification of glenoid bone defect in anterior shoulder instability. Skeletal Radiol. 2009;38:1071–1075. doi: 10.1007/s00256-009-0719-5. [DOI] [PubMed] [Google Scholar]
- 44.Hill HA, Sachs MD. The grooved defect of the humeral head. A frequently unrecognised complication of dislocation of the shoulder joint. Radiology. 1940;35:690–700. [Google Scholar]
- 45.Bushnell BD, Creighton RA, Herring MM. Bony instability of the shoulder. Arthroscopy. 2008;24:1061–1073. doi: 10.1016/j.arthro.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 46.Sekiya JK, Cutuk A. Humeral head defects - biomechanics, measurements, and treatments. In: Provencher MT, Romeo AA, editors. Shoulder instability: a comprehensive approach. Elsevier Saunders; Philadelphia: 2012. pp. 234–247. [Google Scholar]
- 47.Workman TL, Burkhard TK, Resnick D, et al. Hill-Sachs lesion: comparison of detection with MR imaging, radiography, and arthroscopy. Radiology. 1992;185:847–852. doi: 10.1148/radiology.185.3.1438774. [DOI] [PubMed] [Google Scholar]
- 48.Saito H, Itoi E, Minagawa H. Location of the Hill-Sachs lesion in shoulders with recurrent anterior dislocation. Arch Orthop Trauma Surg. 2009;129:1327–1334. doi: 10.1007/s00402-009-0854-4. [DOI] [PubMed] [Google Scholar]
- 49.Hall RH, Isaac F, Booth CR. Dislocations of the shoulder with special reference to accompanying small fractures. J Bone Joint Surg. 1959;41A:489–494. [PubMed] [Google Scholar]
- 50.Flatow EL, Miniaci A, Evans PJ, et al. Instability of the shoulder: complex problems and failed repairs: Part II. Failed repairs. AAOS Instr Course Lect. 1998;47:113–125. [PubMed] [Google Scholar]
- 51.Montgomery WH, Jr, Wahl M, Hettrich C, et al. Anteroinferior bone-grafting can restore stability in osseous glenoid defects. J Bone Joint Surg. 2005;87A:1972–1977. doi: 10.2106/JBJS.D.02573. [DOI] [PubMed] [Google Scholar]
- 52.Bhatia S, McGill K, Ghodarda NS, et al. Radiographic and arthroscopic evaluation of glenoid and humeral head bone loss. In: Provencher MT, Romeo AA, editors. Shoulder instability: a comprehensive approach. Elsevier Saunders; Philadelphia: 2012. pp. 178–183. [Google Scholar]
- 53.Ito H, Takayama A, Shirai Y. Radiographic evaluation of the Hill-Sachs lesion in patients with recurrent anterior shoulder instability. J Shoulder Elbow Surg. 2000;9:495–497. doi: 10.1067/mse.2000.106920. [DOI] [PubMed] [Google Scholar]
- 54.Burkhart SS, De Beer JF, Barth JR, et al. Results of modified Latarjet reconstruction in patients with anteroinferior instability and significant bone loss. Arthroscopy l. 2007;23:1033–1041. doi: 10.1016/j.arthro.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 55.Elkinson I, Giles JW, Faber KJ, et al. The Effect of the Remplissage Procedure on Shoulder Stability and Range of Motion. An in Vitro Biomechanical Assessment. J Bone Joint Surg. 2012;94A:1003–1012. doi: 10.2106/JBJS.J.01956. [DOI] [PubMed] [Google Scholar]
- 56.Miniaci A, Berlet G. Recurrent anterior instability following failed surgical repair: Allograft reconstruction of large humeral head defects. J Bone Joint Surg. 2001;83B(Suppl 1):19–20. [Google Scholar]
- 57.Widjaja AB, Tran A, Bailey M, et al. Correlation between Bankart and Hill-Sachs lesions in anterior shoulder dislocation. ANZ J Surg. 2006;76:436–438. doi: 10.1111/j.1445-2197.2006.03760.x. [DOI] [PubMed] [Google Scholar]
- 58.Cetik O, Uslu M, Ozsar BK. The relationship between Hill-Sachs lesion and recurrent anterior shoulder dislocation. Acta Orthop Belg. 2007;73:176–178. [PubMed] [Google Scholar]
- 59.Weber BG, Simpson LA, Hardegger F. Rotational humeral osteotomy for recurrent anterior dislocation of the shoulder associated with a large Hill-Sachs lesion. J Bone Joint Surg. 1984;66A:1443–1450. [PubMed] [Google Scholar]
- 60.Connolly J. Humeral head defects associated with shoulder dislocation: their diagnostic and surgical significance. AAOS Instr Course Lect. 1972;21:42–54. [Google Scholar]
- 61.Purchase RJ, Wolf EM, Hobgood ER, et al. Hill-Sachs “remplissage”: an arthroscopic solution for the engaging Hill-Sachs lesion. Arthroscopy. 2008;24:723–726. doi: 10.1016/j.arthro.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 62.Itoi E, Yamamoto N, Kurokawa D, et al. Bone loss in anterior instability. Curr Rev Musculoskelet Med. 2013;6:88–94. doi: 10.1007/s12178-012-9154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boileau P, O’Shea K, Vargas P, et al. Anatomical and Functional Results After Arthroscopic Hill-Sachs Remplissage. J Bone Joint Surg. 2012;94A:618–626. doi: 10.2106/JBJS.K.00101. [DOI] [PubMed] [Google Scholar]
- 64.Pritchett JW, Clark JM. Prosthetic replacement for chronic unreduced dislocations of the shoulder. Clin Orthop Relat Res. 1987;216:89–93. [PubMed] [Google Scholar]