Abstract
Background:
Peripheral nerve repair with sufficient functional recovery is an important issue in reconstructive surgery. Stem cells have attracted extensive research interest in recent years.
Objectives:
The purpose of this study was to compare the vein conduit technique, with and without the addition of mesenchymal stem cells in gap-less nerve injury repair in rats.
Materials and Methods:
In this study, 36 Wistar rats were randomly allocated to three groups: In the first group, nerve repair was performed with simple neurorrhaphy (control group), in the second group, nerve repair was done with vein conduit over site (vein conduit group) and in the third group, bone marrow stem cells were instilled into the vein conduit (stem cell group) after nerve repair with vein conduit over site. Six weeks after the intervention, the sciatic function index, electrophysiological study and histological examination were performed.
Results:
All animals tolerated the surgical procedures and survived well. The sciatic function index and latency were significantly improved in the vein conduit (P = 0.04 and 0.03, respectively) and stem cell group (P = 0.02 and 0.03, respectively) compared with the control group. No significant difference was observed in sciatic function and latency between the vein conduit and stem-cell groups. Moreover, histological analysis showed no significant difference in regenerative density between these two groups.
Conclusions:
The results of this study showed that the meticulous microsurgical nerve repair, which was performed using the vein tubulization induced significantly better sciatic nerve regeneration. However, the addition of bone marrow mesenchymal stem cell to vein conduit failed to promote any significant changes in regeneration outcome.
Keywords: Bone Marrow, Mesenchymal Stromal Cells, Regeneration, Trauma
1. Background
Repairing peripheral nerve injuries with sufficient functional recovery is an important issue in reconstructive surgery (1, 2). In recent decades, refinements in nerve repair and manipulation of the regeneration process with pluripotent stem cells or neurotrophic factors are the matter of extensive research (1, 2). Secure nerve repair is the most important key in successful nerve reconstruction (3). For this purpose, several techniques for enhancement of nerve recovery have been developed such as tubulization (nerve wrapping) and epineural sleeve technique (3, 4). Tubulization, which first introduced with decalcified bone, consists of the wrapping of nerve repair site with tubular structures that may or may not contain substances that promote axon regeneration (5, 6). Veins are well-studied for tubulization as they are easily available, inert, biodegradable, and not compressing (7-9). The Schwann cells play a crucial rule in cellular regeneration, by switching from a myelinating phenotype into a growth supportive one. They provide a trophic support for axons and also macrophage recruitment to degrade axon and myelin debris resulting from Wallerian degeneration (10). Therefore, the concept of improving the capacity of myelination and final quality of nerve function from supplementing the denervated distal environment with additional exogenous mature Schwann cells or their precursor cells was formed (10). Bone marrow mesenchymal stem cells have been used as alternatives to Schwann cells for treating peripheral neuropathies, showing great promise (11).
2. Objectives
The purpose of this study was to compare the vein tubulization technique, with and without mesenchymal stem cell, in gap-less nerve injury repair in rats.
3. Materials and Methods
3.1. Study Design and Animals
In this study, 36 Wistar rats (3-4 months old), weighting between 300 and 350 g were used. They were housed in cages, and maintained on a 12-hour light-dark cycle with free access to water and food. The rats were randomly allocated to three groups (n = 12, each group); in the first group nerve repair was performed with simple neurorrhaphy (group A: control group), in the second group nerve repair was done with vein conduit over neurorrhaphy (group B: vein conduit group) and in the third group after nerve repair with vein conduit over neurorrhaphy, marrow stem cells were instilled into the vein conduit (stem cell group). The animals were anesthetized with ketamine (90 mg/kg) and xylazine (9 mg/kg) and Ketamine was repeated if necessary during the procedure. Sciatic nerve function was evaluated for all groups at the beginning of the study; then, stem cell isolation and culture were performed in group C. Thereafter, an appropriate surgical intervention was done. Six weeks after the intervention, the sciatic function evaluation was repeated, electrophysiological study and histological examinations were also performed prior to euthanasia.
3.2. Bone Marrow Cell (Mesenchymal Stem Cells) Harvest
Under general anesthesia, 0.4 mL bone marrow were aspirated from left tibia of rat using 21 gauge needle, and mixed with 5 mL Dulbecco's modified eagle medium (DMEM) containing 10% fetal bovine serum (FBS), 100 IU/mL penicillin and 100 IU/ mL streptomycin. Bone marrow stem cells were washed by centrifugation for 3 minutes at 1200 rpm and followed by discarding the supernatant. The pellet was suspended in 1 mL DMEM and plated in 75 cm2 culture flasks at density of 105 cells per mL in a 15 mL DMEM containing 15% FBS and antibiotics. The cultures were incubated in an atmosphere of 5% CO2 at 37°C. Three days after culture initiation, the medium was changed to discard the nonadherent cells. The cultures were then allowed to achieve 70-80% confluency. At this time, they were tripsinized and subcultured at 1:3 ratio. Two additional passages were performed to obtain sufficient cells, which were used to conduct the following experiments and cells were used after 3 passage and mesenchymal stem cell confirmation (surface markers, bone, cartilage and adipose differentiation).
3.3. Surgical Procedures
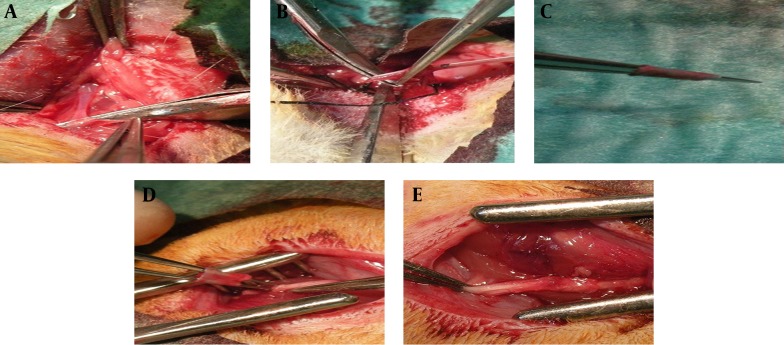
After the animals were anesthetized, the right sciatic nerve was exposed through an incision over the gluteal muscle incision and a 2-3 mm segment of the nerve was excised above the bifurcation area. The nerve specimen was sent for histological examination. In all groups, neurorrhaphy was performed under × 4.0 magnification loupe with two or three 11-0 nylon epineural sutures. For nerve conduit group a segment of right-sided jugular vein with an average length of 2 cm was harvested from right side and then proximal end of transected sciatic nerve was passed through the vein and after neurorrhaphy vein segment rolled to cover the repair site. For the stem cell group, repair was fashioned in the same way as the nerve conduit group, but after the completion of procedure 250 ×103 previously prepared cells suspended in 0.2 mL fibrin glue were injected into the vein. After completion of the procedure, skin was repaired with 4-0 nylon suture (Figure 1).
Figure 1. Surgical Procedure; (A-D): after exploration, a segment of vein is excised on to a tube; thereafter, vein segment is transferred on to a device to facilitate its use as a conduit; (E) final result after neurorrhaphy and its rolled vein segment to cover the repair site.
3.4. Functional Evaluation
Functional aspect of sciatic nerve was expressed by sciatic nerve function index (SFI) using the method described by Reynolds and Weiss (12). Rat’s hind feet were dipped in ink and the rats were allowed to walk across a tunnel so that the footprints could be recorded on white paper loaded onto the bottom of the tunnel. The footprints of normal feet (N) and experimental feet (E) were measured and evaluated with 3 indices: length of the footprint from third toe to heel (PL), width of toes from 1st to the 5th toe (TS) and width of middle toes from 2nd to 4th toe (ITS). The SFI was calculated according to the formula described by Varejao et al. (13): SFI=-38.3 [(EPL-NPL)/NPL] + 109.5 [(ETS-NTS)/NTS] + 13.3 [(EITS-NITS)/NITS]-8.8. SFI was calculated twice: once before an intervention for observing primary function of sciatic nerve and second, six weeks after operation for evaluation of regeneration quality. In general, SFI oscillates around 0 for normal nerve function, and around -100 for complete nerve dysfunction (14).
3.5. Electrophysiological and Histological Study
For electrophysiological and histological study the rats were anesthetized and sciatic nerves were re-exposed. Electrophysiological tests were performed by application of an electrical stimulation (duration of 0.1-0.2 milliseconds, frequency of 1 Hz, and intensity of 1-15 mm) to the proximal side of the injured sites, and a recording electrode in the extensor digitorum (14, 15). The distance between two electrodes was measured by a caliper. The onset latency was recorded. Then, nerve dissection was performed at and below the repair site. A block of nerve distal to the repair site was excised. After preparation of nerve specimens; evaluation with × 100 magnification (Olympus, PROVIS Ax 70, Japan) nerve axon count was done randomly for four separate fields and the average was reported as axon count.
3.6. Statistical Analysis
All data are presented as mean ± standard deviation (SD). Statistical analysis, after implementing Kolmogorov-Smirnov test for normality, was performed using one-way ANOVA and post-hoc tests with the SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA). P < 0.05 was considered statistically significant.
4. Results
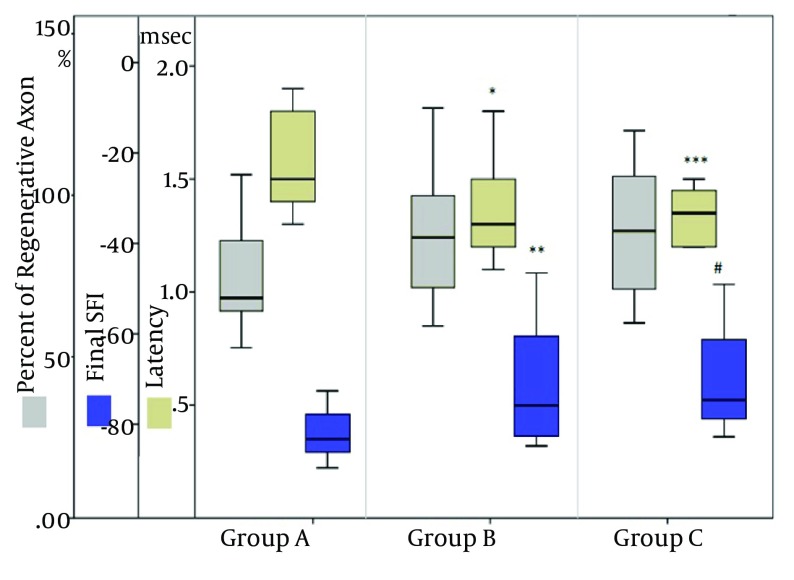
All animals tolerated surgical procedures and survived well without any serious surgical complication. Preoperative and postoperative results of sciatic function index, axon count and nerve conduction velocity (latency in milliseconds) are shown in Table 1 and Figure 2. After six weeks, gait analysis and electrophysiological study revealed better sciatic function index and latency in vein conduit and stem cell groups. The sciatic function was enhanced about 12% in the Vein conduit group (P = 0.04) and 14% in the stem-cell (P = 0.02) group compared to the control group. Also, latency was diminished about 13% in the vein conduit (P = 0.03) and 14% in the stem cell (P = 0.03) group compared with the control group.
Table 1. Data Analysis for Each Experimental Group a, b, c.
| Groups b | Axon Count /HPF | Postop Electrophysiology | Sciatic Function Index | ||
|---|---|---|---|---|---|
| Preop | Postop | Study: Latency mSec | Preop | Postop | |
| Group A (n = 12) | 176 ± 36 | 133 ± 34 | 1.55 ± 0.21 | -9.2 ± 3.99 | -81.8 ± 5.06 |
| Group B (n = 12) | 170 ± 31 | 149 ± 26 | 1.35 ± 0.21 | -10.1 ± 8.01 | -70.8 ± 12.96 |
| Group C (n = 12) | 163 ± 32 | 144 ± 29 | 1.34 ± 0.12 | -14.27 ± 9.09 | -69.9 ± 11.19 |
| Significance (P values) | None | none | A vs. B: 0.038; A vs. C: 0.030 | None | A vs. B: 0.046; A vs. C: 0.028 |
aAbbreviations: Preop, preoperative period; Postop: postoperative period
bGroup A: simple neurorrhaphy, group B: vein conduit over neurorrhaphy, group C: vein conduit containing bone marrow derived stem cells over neurorrhaphy.
c Data are presented as Mean ± SD.
Figure 2. Comparison Between Ultimate Sciatic Function Index, Latency and Percentage of Distal Axon Count With Normal Proximal Count in Three Different Groups; graph shows a significant improvement of sciatic function index and latency in vein conduit and stem cell group (Group B and C), compared to the control group (Group A) [* P =0.03; ** P =0.04; ***P =0.03; # P =0.02].
Histological analysis, including mean axon count density was performed in all groups and compared to the normal proximal sciatic nerve blocks. In comparison to proximal blocks, 73%, 85% and 87% successful axon regeneration into distal segment was observed in the control, vein conduit and stem-cell groups, respectively. Although better regenerative density was observed in the vein conduit and stem-cell groups (14% and 15%, respectively) compared to the control group, no statistically significant difference was observed (P = 0.30 and P = 0.39, respectively). Moreover, no significant regenerative density was observed between the vein conduit and stem cell groups (P =0.90).
5. Discussion
In the present study, rat sciatic nerve regeneration was significantly better when meticulous microsurgical nerve repair was performed with vein tubulization. However, the addition of bone marrow mesenchymal stem cells to the vein tubulization technique failed to promote any significant changes in regeneration outcome. It is suggested that due to the rich source of collagen and laminin in the adventitia and the medial layer of vein, tubulization with vein wrapping can facilitate nerve regeneration (16, 17). Other theoretical advantages of tubulization, which can explain enhanced outcome is providing an optimal biomechanical chamber at the repair site, which collect axoplasmic fluid from the transected nerve end and prevention of adherent scars and dispersion of fascicles and regenerating axons from the suture line (3, 7, 8, 18). The last advantage concerns the prevention of neuroma formation (7-9). Although, histological examinations revealed less epineural scarring, a thinner epineurium, more regenerated axons and fewer inflammatory cells when nerve repair is wrapped with vein (5, 18, 19). On the other hand, the efficacy of bone marrow stem cells to enhance nerve regeneration, optimal number of transplanted cells, in vivo survival of injected cells and true potential in differentiation into growth-supporting cell linage remain to be established (10); however, the present study did not support their benefit. Even with initial enthusiasm, limited capacity of these pluripotent cells toward a Schwann-cell-like linage (20, 21) and importantly, lack of definite evidence for their ability for in vivo production of myelin, decrease their significance for clinical use (10). In conclusion, the present study indicates that vein tubulization can be an effective adjunct to surgical nerve repair; however, bone marrow mesenchymal stem cell may not be a suitable cellular strategy to promote regeneration in acute nerve injury.
References
- 1.Keskin M, Akbas H, Uysal OA, Canan S, Ayyldz M, Agar E, et al. Enhancement of nerve regeneration and orientation across a gap with a nerve graft within a vein conduit graft: a functional, stereological, and electrophysiological study. Plast Reconstr Surg. 2004;113(5):1372–9. doi: 10.1097/01.prs.0000111596.61137.a1. [DOI] [PubMed] [Google Scholar]
- 2.Nie X, Zhang YJ, Tian WD, Jiang M, Dong R, Chen JW, et al. Improvement of peripheral nerve regeneration by a tissue-engineered nerve filled with ectomesenchymal stem cells. Int J Oral Maxillofac Surg. 2007;36(1):32–8. doi: 10.1016/j.ijom.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Lubiatowski P, Unsal FM, Nair D, Ozer K, Siemionow M. The epineural sleeve technique for nerve graft reconstruction enhances nerve recovery. Microsurgery. 2008;28(3):160–7. doi: 10.1002/micr.20472. [DOI] [PubMed] [Google Scholar]
- 4.Battiston B, Geuna S, Ferrero M, Tos P. Nerve repair by means of tubulization: literature review and personal clinical experience comparing biological and synthetic conduits for sensory nerve repair. Microsurgery. 2005;25(4):258–67. doi: 10.1002/micr.20127. [DOI] [PubMed] [Google Scholar]
- 5.Heijke GC, Klopper PJ, Dutrieux RP. Vein graft conduits versus conventional suturing in peripheral nerve reconstructions. Microsurgery. 1993;14(9):584–8. doi: 10.1002/micr.1920140908. [DOI] [PubMed] [Google Scholar]
- 6.Bastos Dos Santos E, Fernandes M, Gomes Dos Santos JB, Mattioli Leite V, Valente SG, Faloppa F. Study of tibial nerve regeneration in Wistar rats in primary neurorrhaphy with and without gap, wrapped in vein segments. Acta Ortop Bras. 2012;20(3):165–9. doi: 10.1590/S1413-78522012000300006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoo YM, Lee IJ, Lim H, Kim JH, Park MC. Vein wrapping technique for nerve reconstruction in patients with thyroid cancer invading the recurrent laryngeal nerve. Arch Plast Surg. 2012;39(1):71–5. doi: 10.5999/aps.2012.39.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leuzzi S, Armenio A, Leone L, De Santis V, Di Turi A, Annoscia P, et al. Repair of peripheral nerve with vein wrapping*. G Chir. 2014;35(3-4):101–6. [PMC free article] [PubMed] [Google Scholar]
- 9.Galeano M, Manasseri B, Risitano G, Geuna S, Di Scipio F, La Rosa P, et al. A free vein graft cap influences neuroma formation after nerve transection. Microsurgery. 2009;29(7):568–72. doi: 10.1002/micr.20652. [DOI] [PubMed] [Google Scholar]
- 10.Khuong HT, Midha R. Advances in nerve repair. Curr Neurol Neurosci Rep. 2013;13(1):322. doi: 10.1007/s11910-012-0322-3. [DOI] [PubMed] [Google Scholar]
- 11.Wang D, Liu XL, Zhu JK, Jiang L, Hu J, Zhang Y, et al. Bridging small-gap peripheral nerve defects using acellular nerve allograft implanted with autologous bone marrow stromal cells in primates. Brain Res. 2008;1188:44–53. doi: 10.1016/j.brainres.2007.09.098. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255(5052):1707–10. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 13.Varejao AS, Melo-Pinto P, Meek MF, Filipe VM, Bulas-Cruz J. Methods for the experimental functional assessment of rat sciatic nerve regeneration. Neurol Res. 2004;26(2):186–94. doi: 10.1179/016164104225013833. [DOI] [PubMed] [Google Scholar]
- 14.Zeng X, Zhang L, Sun L, Zhang D, Zhao H, Jia J, et al. Recovery from rat sciatic nerve injury in vivo through the use of differentiated MDSCs in vitro. Exp Ther Med. 2013;5(1):193–6. doi: 10.3892/etm.2012.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan HC, Yang DY, Chiu YT, Lai SZ, Wang YC, Chang MH, et al. Enhanced regeneration in injured sciatic nerve by human amniotic mesenchymal stem cell. J Clin Neurosci. 2006;13(5):570–5. doi: 10.1016/j.jocn.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Eren F, Yuksel F, Ulkur E, Cavdar S, Ercan F, Celikoz B. Nerve regeneration through a healthy nerve trunk: a new and hopeful conduit for bridging nerve defects. Plast Reconstr Surg. 2005;116(6):1697–705. doi: 10.1097/01.prs.0000186538.04622.7a. [DOI] [PubMed] [Google Scholar]
- 17.Wang KK, Costas PD, Bryan DJ, Eby PL, Seckel BR. Inside-out vein graft repair compared with nerve grafting for nerve regeneration in rats. Microsurgery. 1995;16(2):65–70. doi: 10.1002/micr.1920160205. [DOI] [PubMed] [Google Scholar]
- 18.Acar M, Karacalar A, Ayyildiz M, Unal B, Canan S, Agar E, et al. The effect of autogenous vein grafts on nerve repair with size discrepancy in rats: an electrophysiological and stereological analysis. Brain Res. 2008;1198:171–81. doi: 10.1016/j.brainres.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Valero-Cabre A, Tsironis K, Skouras E, Navarro X, Neiss WF. Peripheral and spinal motor reorganization after nerve injury and repair. J Neurotrauma. 2004;21(1):95–108. doi: 10.1089/089771504772695986. [DOI] [PubMed] [Google Scholar]
- 20.Dezawa M, Takahashi I, Esaki M, Takano M, Sawada H. Sciatic nerve regeneration in rats induced by transplantation of in vitro differentiated bone-marrow stromal cells. Eur J Neurosci. 2001;14(11):1771–6. doi: 10.1046/j.0953-816x.2001.01814.x. [DOI] [PubMed] [Google Scholar]
- 21.Tohill M, Mantovani C, Wiberg M, Terenghi G. Rat bone marrow mesenchymal stem cells express glial markers and stimulate nerve regeneration. Neurosci Lett. 2004;362(3):200–3. doi: 10.1016/j.neulet.2004.03.077. [DOI] [PubMed] [Google Scholar]