Abstract
Background:
Uncontrolled hemorrhage is a well-recognized cause of mortality in trauma victims and the control of active hemorrhage is among the initial steps in resuscitation.
Objectives:
The purpose of this study was to assess the role of a hemostatic agent “celox” in the management of civilian stab-wound trauma.
Patients and Methods:
In this clinical trial study, 160 patients with penetrating limb trauma were randomly allocated to either the control or intervention group (n = 80, each group). Controls were treated with the simple pressure dressing, while the celox-coated gauze was used in the intervention group. The time for achievement of hemostasis and the amount of bleeding were recorded. Data were analyzed using SPSS Version 21 and Stata 13. A P value of less than 0.05 was considered statistically significant.
Results:
The mean age of participants was 30.5 and the majority of patients were male (90.6%). The forearm and distal leg were the most sites of injury. Hemostasis was achieved within 5 minutes in 32.5% of the control group and 51.3% of the intervention group. Using the celox-coated gauze significantly reduced the time to hemostasis (P = 0.01). Moreover, the blood loss was significantly lower in the celox group compared to the controls (P < 0.05).
Conclusions:
Using the celox-coated gauze is able to achieve hemostasis in penetrating limb trauma faster than the conventional pressure bandage. Further research is required to clarify the subset of patients who will benefit the most from this effect in the emergency department.
Keywords: Stab Wound, Hemostasis, Occlusive Dressing
1. Background
Uncontrolled bleeding is frequently named as the leading cause of preventable death in military combat, and the second cause of death in victims of civilian trauma (1, 2). For this reason, meticulous attention to control of bleeding as soon as possible is highlighted in advanced trauma life support guidelines (3). Methods currently in use for hemorrhage control are prioritized differently in the battle field and civilian circumstances; however, they generally include applying direct pressure to the wound, tourniquets, ligation of the bleeding vessel, and pressure bandage. If these measures fail to control the bleeding, the patient faces the dire consequence of hemorrhagic shock and increased mortality (4, 5). With the aim of early hemostasis and prevention of this fatal complication, different materials have been introduced as hemostatic agents (6-9). Through variable mechanisms, these agents promote clot formation and hemorrhage control at the site of bleeding. A good hemostatic agent should be available in the emergency setting, easy to apply, and have the ability to control bleeding from great vessels (10-12).Celox is a granular chitosan available in powder, gauze, and nasal tampon forms as a hemostatic agent. Upon contact with blood, it interacts with red blood cells and platelets to form a barrier against ongoing hemorrhage. This mechanism promotes hemostasis independent of the coagulation system (7). Moreover, Aktop et al. recently demonstrated initiates the coagulation cascade by activating the tissue factor, thus further promoting hemostasis through a thrombin burst (13). The commercially prepared chitosan-coated gauze has the advantage of easy applicability and not interfering with other treatment modalities, such as wound exploration. It is applied directly on the wound where, after coming in contact with blood, forms a complicated web, entraps red blood cells, and stops the bleeding (11). Until now, the interest for celox and similar material was limited to combat and scene of hospital patient care.
2. Objectives
Since most of the available literature regarding celox is based on animal, lab, or military reports, and its role in civilian hospitals remains undefined (7-9, 14-16), this trial aimed to evaluate the role of celox in the management of civilian penetrating trauma.
3. Patients and Methods
This pragmatic superiority, randomized clinical trial was performed on 160 patients suffering from stab wounds with knives, glass, motor vehicle collisions, and other mechanisms between March 2014 and August 2014. Due to the emergent nature of treatment provided, obtaining informed consent was not feasible, but the study protocol was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences (SBMU). Patients aged 18 - 50 years were included if they had suffered a stab injury to a limb, had a minimal wound length of 3 cm, and bleeding was a concern regardless of the source. The patients were excluded from the study if a foreign body was retained in the wound, had a history of anticoagulation, required blood products for resuscitation, or other hemostatic products had been used for the control of bleeding in the prehospital setting. The study was set in the Emergency Department (ED) of Emam Hossein Hospital, a first level urban trauma Center in Tehran and registered in Iranian Registry of Clinical Trial (IRCT) with IRCT201104206238N1. Patients who met the inclusion criteria were recognized and enrolled in the study by the physician upon arrival at the trauma room. Based on similar studies, initial pilot study conducted on 20 patients. Successful hemostasis achieved in the intervention and control groups were 82% and 62%, respectively in the pilot study. With a type one error of 5%, study power of 80%, and a significant appreciable difference of 20% between the two groups, the minimum number of patients to be allocated to each group was determined to be 76. The patients were allocated to either the intervention or the control group. A randomized block sampling system was implemented in blocks of 2 to 8. The control group was treated with pressure bandage using a regular 10 × 10 cm gauze, while a celox-coated gauze was used in the intervention group. Demographic information and vital signs on presentation were gathered in both groups. The role of celox-coated gauze in management of civilian stab wounds was addressed by analysis of two specific outcomes: first its effect on time till achieving hemostasis, and second the amount of blood loss through the wound after the initiation of treatment. The time until hemostasis was determined by checking the wound for hemorrhage every five minutes by the treating physician. The amount of bleeding was calculated by counting the number of blood-soaked gauze again by the treating physician. Because this study was a pragmatic study and outcomes were measured objectively, it was carried out as an open-label trial. The patients were followed-up until the end of their stay in the ED. If during this time it was shown that the patients met any of the exclusion criteria (foreign body, coagulation studies outside of normal reference levels declared by the laboratory), their data were excluded from the study. Data were analyzed using SPSS Inc., USA 21. Variables in the two groups were analyzed using Mann-Whitney and chi-square tests. Time to cessation of bleeding was compared in the two groups by chi2 -trend analysis using Stata 13 software. A P value of < 0.05 was considered statistically significant.
4. Results
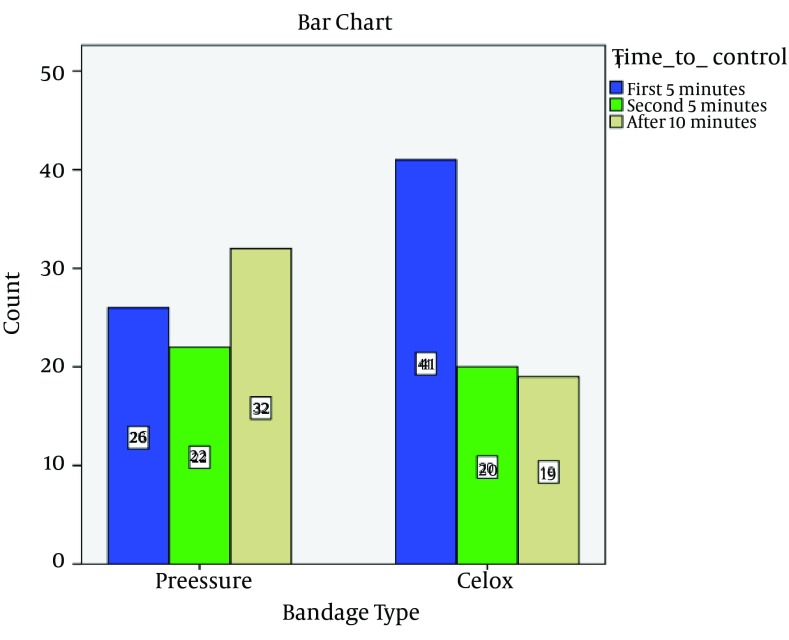
A total of 160 patients were included in the study, 80 in each group. There were no lost cases because the follow-up period was limited to the initial ED stay. No missing data were recorded for the patients included in the study. The mean age of patients was 30.5 (age range, 18 to 50). The majority of patients were male (90.6%) and most of the wounds were < 10 cm long (81.3%). The forearm and distal leg were the most common sites of injury (31.9%), followed by the hand (24.4%), foot (18.8%), arm (10.6%), and thigh (6.3%). Seventy-five patients had received pressure dressing prior to arrival at the hospital, 39 in the control and 36 in the intervention group. Most of these dressings had been applied by emergency medical services (82.6%). No statistically significant difference was found between the two groups regarding pretest variables (Table 1). As a measure of total bleeding after the initiation of therapy, the mean number of blood-soaked 10 × 10 cm gauzes was 3.06 and 2.63 in the control and celox groups, respectively. The difference was found to be statistically significant by Mann-Whitney test (P = 0.049). Furthermore, the chi-squared trend analysis shows dressings using the celox-coated gauze achieved a greater success rate faster than the group receiving a pressure bandage alone (P = 0.01) (Table 2, Figure 1). Based on the results achieved at five minutes, considering the sample size of 80 in each group, and accepting α = 5, the power of the study is determined to be 0.77, which is considered acceptable. Subgroup analyses of celox-coated gauze in relation to hemostasis by categories of wound depth (dermis, facia, and muscle) and wound Length (< 10 cm and > 10 cm) suggested a stronger association among dermal wounds and among wounds with size over 10 cm (P values were 0.01 and 0.04, respectively). There was no significant association among fascial, muscular, and smaller (< 10 cm) wounds (data not shown). The role of celox in the management of civilian stab wounds in foot seems more efficient (P value = 0.001).
Table 1. Pretest Comparison of Control and Intervention Groups a, b.
| Variables | Controls | Intervention | P Value | Test |
|---|---|---|---|---|
| Gender | 0.786 | Chi2 | ||
| Male | 72 (90) | 73 (91.25) | ||
| Female | 8 (10) | 7 (8.75) | ||
| Wound Length | 0.685 | Chi2 | ||
| > 10 cm | 16 (20) | 14 (17.5) | ||
| < 10 cm | 64 (80) | 66 (83.5) | ||
| Wound Depth | 0.095 | Chi2 | ||
| Dermis | 33 (41.25) | 27 (33.75) | ||
| Facia | 25 (31.25) | 18 (22.5) | ||
| Muscle | 22 (27.5) | 35 (43.75) | ||
| Wound Location | 0.856 | Chi2 | ||
| Hand | 22 (27.5) | 17 (21.25) | ||
| Forearm and leg | 26 (32.5) | 25 (31.25) | ||
| Elbow | 4 (5) | 3 (3.75) | ||
| Arm | 6 (7.5) | 11 (13.75) | ||
| Shoulder | 0 (0) | 1 (1.25) | ||
| Foot | 15 (18.75) | 15 (18.75) | ||
| Knee | 2 (2.5) | 1 (1.25) | ||
| Thigh | 4 (5) | 6 (7.5) | ||
| Buttock | 1 (1.25) | 1 (1.25) | ||
| Prehospital pressure dressing | 0.28 | Chi2 | ||
| By Medics | 34 (42.5) | 28 (35) | ||
| By Patient | 5 (6.25) | 8 (10) | ||
| None | 41 (51.25) | 44 (55) | ||
| Systolic BP, Mean ± SD | 11.44 ± 1.32 | 11.64 ± 1.13 | 0.30 | Mann-Whitney |
| Diastolic BP, Mean ± SD | 7.35 ± 0.64 | 7.46 ± 0.55 | 0.23 | Mann-Whitney |
| Age, Mean ± SD | 31.01 ± 10.16 | 29.99 ± 9.68 | 0.52 | Mann-Whitney |
a Data presented as No (%).
bValues are presented as Mean ± SD.
Table 2. Distribution of Patients Based on Time of Achieving Hemostasis in Both Groups a.
| Bandage Type | Test | ||
|---|---|---|---|
| Pressure | Celox | ||
| Time to control | Nonparametric Chi2 Trend, z = - 2.59, Prob > |z| = 0.010 | ||
| Less than 5 minutes | 26 (38.81) | 41 (61.19) | |
| 5 to 10 minutes | 22 (52.38) | 20 (47.62) | |
| More than 10 minutes | 32 (62.75) | 19 (37.25) | |
aData are presented as No. (%).
Figure 1. Comparison of Times to Cessation of Bleeding Between Two Types of Bandage.
5. Discussion
The optimum treatment of isolated open limb injuries requires a well-coordinated multidisciplinary approach. The sooner primary measures are done, the faster definite treatment can be initiated, and better results can be expected (17). This study shows that the celox-coated gauze may play a role in reducing this time window. As previously mentioned, most of the available literature on celox is derived from animal models and human trials are lacking. In two separate studies Kozen et al. in 2008 and Littlejohn et al. in 2011, compared the effects of celox on controlling bleeding due to femoral artery injury in swine models with other commercially available hemostatic agents. Both studies found that celox was superior in reducing blood loss, bleeding recurrence, and mortality (7, 9). Based on our results it appears that celox has similar effects in humans, reducing the amount of blood loss and the time needed to achieve hemostasis. Available human reports are mostly derived from the military experience. Pozza et al. reported 21 cases of hemorrhagic wounds in American soldiers serving in Afghanistan (18). In 18 out of these cases, celox was able to achieve hemostasis after a single application. In the remaining 3 cases, repeated attempts achieved hemostasis. With the success celox has enjoyed in tactical circumstances, recent reports are emerging regarding its use in civilian medicine as well. Two such examples report utilization of this hemostatic agent for traumatic pelvic injury (14), and thoracic surgery (19). In our study celox gauze was used in a different setting (civilian ED) and for a different type of trauma, therefore, comparison is difficult if not impossible. However, it seems that celox enjoys the same advantages in this new setting. In this study, we aimed to evaluate the effects of celox on the management of civilian stab wounds. To the best our knowledge, this is the first clinical trial evaluating the effects of this hemostatic agent in civilian trauma. Our findings showed that the celox-coated gauze is able to control hemorrhagic wounds sooner than the traditional pressure dressing. It also significantly reduced the amount of blood loss after the initiation of treatment. These effects appear to be more significant in dermal wounds as well as wounds affecting the foot. Furthermore, wounds that were more than 10 cm long benefited more from the use of celox than those below 10 cm. These findings might be an early guide to the target population that may benefit the most from this hemostatic agent. Active external bleeding is a life-threatening event. In a study by Bostrom et al. 15% of deaths resulting from stab wounds were caused by stabs to the extremities (20). Furthermore, a recent study reported that among stab victims 19% suffered from multiple injuries (21). In such settings the control of hemorrhage can be difficult and time consuming. The results of our study showed that using celox in the civilian EDs may be helpful in such situations. This is especially true in a selected group of patients, including those with larger wounds. The results showed that hemostasis can be achieved sooner and with less blood loss using the celox gauze. This means that probably fewer wounds will require more advanced and invasive attempts at hemostasis, such as emergency clamping or ligation. Moreover, earlier control of active bleeding allows the physician to focus on other possible life-threatening conditions sooner. This was designed as a pragmatic study and efforts were made not to control the study to an extent that its results would not be applicable in practice. For instance, we did not attempt to clarify the source of bleeding or the amount of bleeding prior to the ED admission. The reason was that in real life situations such information can rarely be gained with certainty. These limitations may influence the results and their effects need to be clarified the larger studies. Moreover, the authors believe that because the patients were selected from the ED population then the results will be applicable to ED stab victims. Other limitations include lack of blinding and assessing the patients for possible side effects. Although blinding is not impossible in a pragmatic trial design, the authors believed that since the entire outcome measures objectively gathered, the blinding process would add undue complexity to the management of the patients. Also, this study was not designed to record any acute side-effects and the short follow-up period (during the initial ED stay) does not allow us to comment on issues such as the role of dressing on wound healing and other later complications. Delving into such questions will require more meticulous study designs. Furthermore, our study was not designed to compare the total cost of care, which may be an important factor in the ultimate implementation of this treatment. To our knowledge, this study is the first human trial assessing the effects of celox-coated gauze in civilian trauma. The results showed that the use of celox-coated gauze reduces the time needed to achieve hemostasis and the amount of blood loss after initiation of the treatment. The challenge is to select patients who gain the most benefit from it. This is the core question that will define the role of such agents in the EDs and has to be answered in future trials.
Acknowledgments
The authors would like to gratefully thank the ED nurses of the Emam Hossein Hospital, without their help this work could not be done. We would also like to thank the Emergency Medicine Faculties and residents of Shahid Beheshti University of Medical Sciences at the aforementioned hospital for their participation and useful hints that made this research a fruitful experience. We also would like to thank the Celox Company (Tehran, Iran) for providing the celox-coated gauze for this study.
Footnotes
Authors’ Contributions:Hamid Reza Hatamabadi: critical revision of the manuscript for important intellectual content and study supervision; Fatemeh Asayesh Zarchi: participated in data gathering and acquisition of data; Hamid Kariman and Ali Arhami Dolatabadi: drafting of the manuscript; Ali Tabatabaey: analysis and interpretation of data; Afshin Amini: study concept and design and administrative, technical, and material support. All authors approved the final version of the manuscript.
References
- 1.Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, et al. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38(2):185–93. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Mabry R, McManus JG. Prehospital advances in the management of severe penetrating trauma. Crit Care Med. 2008;36(7 Suppl):S258–66. doi: 10.1097/CCM.0b013e31817da674. [DOI] [PubMed] [Google Scholar]
- 3.Atls Subcommittee, American College of Surgeons' Committee on T, International AWG. Advanced trauma life support (ATLS(R)): the ninth edition. J Trauma Acute Care Surg. 2013;74(5):1363–6. doi: 10.1097/TA.0b013e31828b82f5. [DOI] [PubMed] [Google Scholar]
- 4.Alam HB, Burris D, DaCorta JA, Rhee P. Hemorrhage control in the battlefield: role of new hemostatic agents. Mil Med. 2005;170(1):63–9. doi: 10.7205/milmed.170.1.63. [DOI] [PubMed] [Google Scholar]
- 5.Pusateri AE, Holcomb JB, Kheirabadi BS, Alam HB, Wade CE, Ryan KL. Making sense of the preclinical literature on advanced hemostatic products. J Trauma. 2006;60(3):674–82. doi: 10.1097/01.ta.0000196672.47783.fd. [DOI] [PubMed] [Google Scholar]
- 6.Acheson EM, Kheirabadi BS, Deguzman R, Dick EJ, Holcomb JB. Comparison of hemorrhage control agents applied to lethal extremity arterial hemorrhages in swine. J Trauma. 2005;59(4):865–74. doi: 10.1097/01.ta.0000187655.63698.9f. discussion 874-5. [DOI] [PubMed] [Google Scholar]
- 7.Kozen BG, Kircher SJ, Henao J, Godinez FS, Johnson AS. An alternative hemostatic dressing: comparison of CELOX, HemCon, and QuikClot. Acad Emerg Med. 2008;15(1):74–81. doi: 10.1111/j.1553-2712.2007.00009.x. [DOI] [PubMed] [Google Scholar]
- 8.Devlin JJ, Kircher S, Kozen BG, Littlejohn LF, Johnson AS. Comparison of ChitoFlex(R), CELOX, and QuikClot(R) in control of hemorrhage. J Emerg Med. 2011;41(3):237–45. doi: 10.1016/j.jemermed.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 9.Littlejohn LF, Devlin JJ, Kircher SS, Lueken R, Melia MR, Johnson AS. Comparison of Celox-A, ChitoFlex, WoundStat, and combat gauze hemostatic agents versus standard gauze dressing in control of hemorrhage in a swine model of penetrating trauma. Acad Emerg Med. 2011;18(4):340–50. doi: 10.1111/j.1553-2712.2011.01036.x. [DOI] [PubMed] [Google Scholar]
- 10.Sondeen JL, Pusateri AE, Coppes VG, Gaddy CE, Holcomb JB. Comparison of 10 different hemostatic dressings in an aortic injury. J Trauma. 2003;54(2):280–5. doi: 10.1097/01.TA.0000037431.19185.B4. [DOI] [PubMed] [Google Scholar]
- 11.Koksal O, Ozdemir F, Cam Etoz B, Isbil Buyukcoskun N, Sigirli D. Hemostatic effect of a chitosan linear polymer (Celox(R) in a severe femoral artery bleeding rat model under hypothermia or warfarin therapy. Ulus Travma Acil Cerrahi Derg. 2011;17(3):199–204. [PubMed] [Google Scholar]
- 12.Wedmore I, McManus JG, Pusateri AE, Holcomb JB. A special report on the chitosan-based hemostatic dressing: experience in current combat operations. J Trauma. 2006;60(3):655–8. doi: 10.1097/01.ta.0000199392.91772.44. [DOI] [PubMed] [Google Scholar]
- 13.Aktop S, Emekli-Alturfan E, Ozer C, Gonul O, Garip H, Yarat A, et al. Effects of Ankaferd Blood Stopper and Celox on the tissue factor activities of warfarin-treated rats. Clin Appl Thromb Hemost. 2014;20(1):16–21. doi: 10.1177/1076029613490254. [DOI] [PubMed] [Google Scholar]
- 14.Arul GS, Bowley DM, DiRusso S. The use of Celox gauze as an adjunct to pelvic Packing in otherwise uncontrollable pelvic haemorrhage secondary to penetrating trauma. J R Army Med Corps. 2012;158(4):331–3. doi: 10.1136/jramc-158-04-12. [DOI] [PubMed] [Google Scholar]
- 15.Burgert J, Gegel B, Neal AR, Kammer KE, Paul ME, Schwartz DJ, et al. The effects of arterial blood pressure on rebleeding when BleedArrest, Celox and TraumaDex are used in a porcine model of lethal femoral injury. Mil Med. 2012;177(3):340–4. doi: 10.7205/milmed-d-11-00310. [DOI] [PubMed] [Google Scholar]
- 16.Burgert JM, Gegel BT, Austin R3, Davila A, Deeds J, Hodges L, et al. Effects of arterial blood pressure on rebleeding using Celox and TraumaDEX in a porcine model of lethal femoral injury. AANA J. 2010;78(3):230–6. [PubMed] [Google Scholar]
- 17.Mendis D, Vesely M. Improvements in the management of trauma patients with the introduction of a lower limb trauma coordinator. Trauma Mon. 2012;16(4):201–2. doi: 10.5812/kowsar.22517464.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pozza M, Millner RW. Celox (chitosan) for haemostasis in massive traumatic bleeding: experience in Afghanistan. Eur J Emerg Med. 2011;18(1):31–3. doi: 10.1097/MEJ.0b013e32833a5ee4. [DOI] [PubMed] [Google Scholar]
- 19.Millner RW, Lockhart AS, Bird H, Alexiou C. A new hemostatic agent: initial life-saving experience with Celox (chitosan) in cardiothoracic surgery. Ann Thorac Surg. 2009;87(2):e13–4. doi: 10.1016/j.athoracsur.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 20.Bostrom L, Heinius G, Nilsson B. Trends in the incidence and severity of stab wounds in Sweden 1987-1994. Eur J Surg. 2000;166(10):765–70. doi: 10.1080/110241500447380. [DOI] [PubMed] [Google Scholar]
- 21.Pallett JR, Sutherland E, Glucksman E, Tunnicliff M, Keep JW. A cross-sectional study of knife injuries at a London major trauma centre. Ann R Coll Surg Engl. 2014;96(1):23–6. doi: 10.1308/003588414X13824511649616. [DOI] [PMC free article] [PubMed] [Google Scholar]