Abstract
Since the advent of ventricular assist devices with smaller configurations and continuous-flow technology, survival rates for patients with end-stage heart failure have dramatically improved. While the burden of infectious complications is decreased in patients on continuous-flow ventricular assist devices compared to bulkier pulsatile-flow devices, infection remains one of the most common causes of morbidity and mortality. The majority of infections occur at the driveline exit site, beginning with a disruption or trauma to the barrier between the skin and driveline and sometimes spreading deeper. Once infections develop, they can be difficult to eradicate. Depending on the degree of infection, treatment options may include local wound care, antibiotics, or surgical treatment. Preventive strategies and careful surveillance are crucial to improve patient outcomes.
Keywords: left ventricular assist device, driveline, infection

B. H. Trachtenberg, M.D.
Introduction
The continued evolution of left ventricular assist devices has played a major role in the current treatment of patients with advanced heart failure. Newer and better devices have led to major improvements in survival and quality of life. As smaller continuous-flow left ventricular assist devices (CF-LVADs) have replaced bulkier pulsatile-flow devices, the rate of infections has decreased by as much as 50%.1 However, since the LVAD is a foreign body, and since all current long-term support devices depend upon a driveline exiting the body percutaneously to connect to a power source, the incidence of infectious complications remains considerable.
Complications of Infection
Infection is a common cause of morbidity and is the second most common cause of death in patients who survive the initial 6 months on CF-LVAD support (Figure 1).2 It is also one of the leading causes of readmission in these patients.3,4
Figure 1.
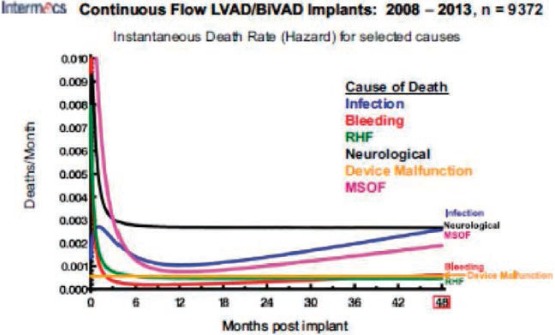
Figure shows INTERMACS report on cause of death of patients supported with LVADs; infection becomes the leading cause of mortality, along with neurological events, at 48 months post-implant.
While many small studies have reported on the epidemiology of infections in VAD patients, Goldstein et al. recently used INTERMACS registry data to characterize infections in the largest cohort to date. They reported that pneumonia and sepsis are the most common infectious complications in patients supported with CF-LVADs (23% and 20%, respectively), followed by percutaneous site infections (PSIs), which occur in approximately 19% of CFLVAD recipients by 1 year after implant and are associated with an increased risk of mortality.5 Young age is the only predictor of PSI; while the reason for this is unknown, it is hypothesized that young patients are more active and likely to have trauma at the driveline exit site. In addition, the greater the amount of time supported by a CF-LVAD, the greater the risk of developing a PSI.6
Several studies have reported an association between infection and cerebrovascular events (CVEs) in patients supported with LVADs, 7–8 and we recently reported that persistence of bacteremia greater than 72 hours is a crucial distinguishing factor.9 We recently reported a 7-fold increase in CVEs in patients with HeartMate-II (HMII) devices who have persistent Pseudomonas aeruginosa bloodstream infections. Interestingly, mycotic aneurysms can occur in these patients, with devastating consequences.10 Potential mechanisms wherein bacteria can lead to CVEs include platelet activation, alterations in endothelial function, systemic inflammation, and bacterial seeding of cerebral vasculature.11 Regarding infections related to LVADs, Staphylococcus species comprise the most common type of causative organism, followed by Pseudomonas species, which become even more prominent with longer time on VAD support and are very difficult to eradicate.6,12
Pathophysiology
Infections likely begin with a disruption or trauma to the barrier between the skin and driveline. It is commonly believed that PSIs involve the formation of a biofilm that make it difficult to eradicate bacteria,13 and both the Staphylococcus and pseudomonas species are among the bacteria known to produce biofilm. Infections can occur perioperatively, but most happen postoperatively, with Goldstein et al. reporting an average time-to-occurrence of a PSI at approximately 6 months.5 These infections may remain superficial, spread deeper along the driveline path and into the pocket or pump itself, or deepen within the abdominal wall to form an abscess.
Classification
Due to the heterogeneous definitions of infections in patients receiving LVAD support, the International Society for Heart and Lung Transplantation (ISHLT) established standard definitions for infections14 to aid in both clinical and investigational efforts. Infections are divided into VAD-specific, VAD-related, or non-VAD infections (Figure 2), and the criteria for classification is in part inspired by the modified Duke criteria for infective endocarditis. The ISHLT has also created a registry (http://www.ishlt.org/registries/mcsdDatabase.asp) to prospectively track details of all infections in VAD patients to better understand their risk factors and incidence.
Figure 2.
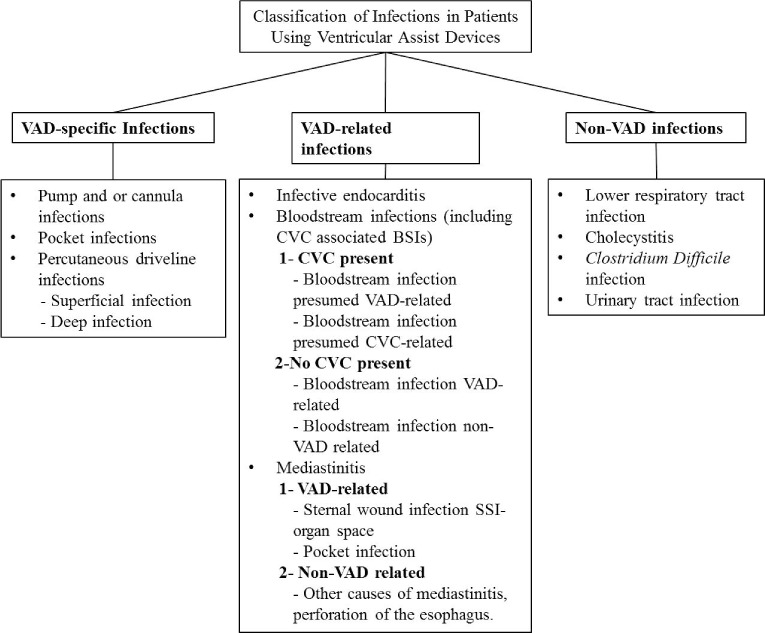
Modified from the International Society for Heart and Lung Transplantation working formulation for the standardization of definitions of infections in patients using ventricular assist devices. VAD: ventricular assist device; CVC: central venous catheter; BSI: blood stream infection; SSI: surgical site infection.
Diagnosis
Infections, particularly PSIs, can be very challenging to diagnose in patients on LVAD support. Recommendations for initial investigations in patients with suspected infections include prompt culture of drainage from the percutaneous exit site, three sets of blood cultures, chest radiography, and echocardiography (Figure 3).14
Figure 3.
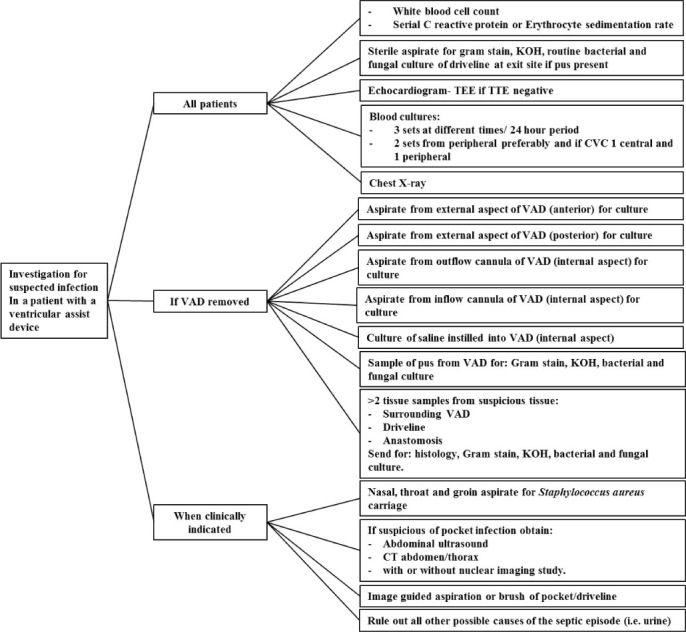
Modified from the International Society for Heart and Lung Transplantation working formulation for the standardization of definitions of infections in patients using ventricular assist devices. VAD: ventricular assist device; TEE: transesophageal echocardiogram; TTE: transthoracic echocardiogram; CT: contrast tomography; KOH: potassium hydroxide.
Various imaging techniques have been used to aid in the diagnosis of infections in patients with CF-LVADs. Transthoracic and transesophageal echocardiography are used to determine the presence of valvular endocarditis or device infections (particularly from pacemakers or defibrillator leads). Ultrasound or computed tomography are frequently used to diagnose collections of fluid around the driveline, pump, or pump pocket and may also be used to guide aspiration or debridement. Increasingly, some centers are using sophisticated techniques such as indium-labeled leukocyte scans or fluorodeoxyglucose positron emission tomography scans although these cannot presently be recommended for routine use.15,16
Prevention
Various preventive strategies have been attempted to reduce the burden of infections in patients on LVAD support. The use of perioperative antibiotics is standard practice. One of the most important factors in preventing the morbidity of infections is the use of various anchoring devices to help stabilize the driveline, thus minimizing trauma and tension at the exit site (Figure 4).17 In addition to education on driveline immobilization, patients are educated on routine driveline site care such as cleaning the exit site daily with chlorhexidine.18 One study examined the effect of chronic prophylactic antibiotic use (oral doxycycline and levofloxacin) and found no difference in the incidence of driveline infection or mortality compared to patients with usual care.19 Surgical techniques such as increasing intrafascial tunneling of the driveline may help reduce infections.20,21 Additionally, externalization of the silicone portion of the driveline (as opposed to the velour portion) also decreases infections.22
Figure 4.
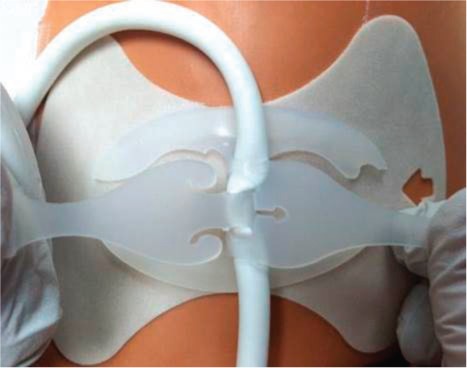
Example of an anchoring device that is used to stabilize driveline and help prevent infections.
Treatment
While there are standardized recommendations for the diagnosis and categorization of infections,14,18 no such guidelines exist for the treatment of infections once they occur. Antibiotics are used according to wound and blood cultures and antibacterial sensitivities. As opposed to infective endocarditis, for example, the choice of antibiotics and length of therapy are not standardized and are left to the discretion of the treating physicians.
Treatment of mild infections may include increasing the frequency of dressing changes, reviewing dressing change protocols to ensure compliance, and close monitoring. For moderate infections, which may involve local cellulitis and drainage, additional treatment may involve tailored antibiotic therapy, local debridement, and weekly clinic visits. If the patient has signs of systemic infection such as fever or leukocytosis, inpatient treatment should be considered. For severe infections that may involve more purulent drainage and subcutaneous induration, inpatient treatment is recommended. The treatment plan should target antimicrobial therapy under the guidance of an infectious disease specialist, imaging tests, and surgical intervention such as debridement and retunneling of the driveline (Figure 5). One method is to resect infected tissue and cover the driveline with well-perfused tissue such as rectus muscle. Additional tools may include the use of antimicrobial beads,23 wound vacuums, and novel therapies such as Mepilex™ or Aquacel® dressing changes. If all of these fail, another measure to combat prolonged infection is to move the driveline into the intraperitoneal space, wherein a completely new exit site is created and the driveline covered with omentum.
Figure 5.
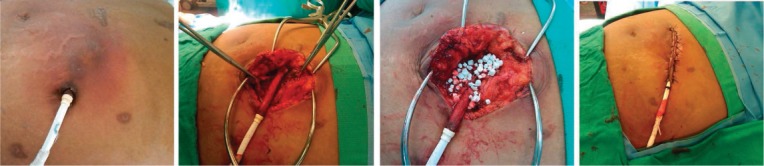
Example of driveline infection and the subsequent surgical treatment with beads and retunneling of driveline.
Chronic suppressive oral antibiotics are often used in patients with recurrent VAD-specific or VAD-related infections; however, studies indicate that approximately one-third of patients have recurrence despite antibiotic use.24 While device exchange can be performed for severe cases, recurrences are common with this treatment as well.12,25
Expediting heart transplant listing in patients with PSI may be a good option in appropriate candidates. Despite concerns about the effect of immunosuppression therapy in patients with prior driveline infections, studies have shown that patients have no increase in mortality post-transplant.12,26 However, patients with sepsis due to PSI are less likely to be bridged to transplant.
Conclusion
With an increasing number of patients on CF-LVAD support and an extended length of survival, infection remains one of the major contributors to morbidity. VAD-specific and VAD-related infections are associated with worsening mortality and can be difficult to eradicate. Therefore, preventive strategies and careful surveillance are crucial to improve patient outcomes.
Acknowledgments
Conflict of Interest Disclosure: The authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
Funding/Support: The authors have nothing to disclose.
References
- 1.Slaughter MS, Rogers JG, Milano CA et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009 Dec 3;361(23):2241–51. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 2.Kirklin JK, Naftel DC, Kormos RL et al. Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) analysis of pump thrombosis in the HeartMate II left ventricular assist device. J Heart Lung Transplant. 2014 Jan;33(1):12–22. doi: 10.1016/j.healun.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Patel CB, Cowger JA, Zuckermann A. A contemporary review of mechanical circulatory support. J Heart Lung Transplant. 2014 Jul;33(7):667–74. doi: 10.1016/j.healun.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Smedira NG, Hoercher KJ, Lima B et al. Unplanned hospital readmissions after HeartMate II implantation: frequency, risk factors, and impact on resource use and survival. JACC Heart Fail. 2013 Feb;1(1):31–9. doi: 10.1016/j.jchf.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein DJ, Naftel D, Holman W et al. Continuous-flow devices and percutaneous site infections: clinical outcomes. J Heart Lung Transplant. 2012 Nov;31(11):1151–7. doi: 10.1016/j.healun.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Sharma V, Deo SV, Stulak JM et al. Driveline infections in left ventricular assist devices: implications for destination therapy. Ann Thorac Surg. 2012 Nov;94(5):1381–6. doi: 10.1016/j.athoracsur.2012.05.074. [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal A, Gupta A, Kumar S et al. Are blood stream infections associated with an increased risk of hemorrhagic stroke in patients with a left ventricular assist device? ASAIO J. 2012 Sep–Oct;58(5):509–13. doi: 10.1097/MAT.0b013e318260c6a6. [DOI] [PubMed] [Google Scholar]
- 8.Kato TS, Schulze PC, Yang J et al. Pre-operative and postoperative risk factors associated with neurologic complications in patients with advanced heart failure supported by a left ventricular assist device. J Heart Lung Transplant. 2012 Jan;31(1):1–8. doi: 10.1016/j.healun.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trachtenberg BH, Cordero-Reyes AM, Aldeiri M et al. Persistent Blood Stream Infection in Patients Supported with a Continuous Flow Left Ventricular Assist Device is Associated with an Increased Risk of Cerebrovascular Accidents. J Card Fail. 2014 Nov 6 doi: 10.1016/j.cardfail.2014.10.019. pii: S1071-9164(14)01274-3. [DOI] [PubMed] [Google Scholar]
- 10.Motomura T, Bruckner B, Leon-Becerril J et al. Superior mesenteric artery mycotic aneurysm in patients with left ventricular assist device support and intravenous drug abuse. Artif Organs. 2011 Jul;35(7):E164–7. doi: 10.1111/j.1525-1594.2011.01250.x. [DOI] [PubMed] [Google Scholar]
- 11.Dalager-Pedersen M, Søgaard M, Schønheyder HC, Nielsen H, Thomsen RW. Risk for myocardial infarction and stroke after community-acquired bacteremia: a 20-year population-based cohort study. Circulation. 2014 Apr 1;129(13):1387–96. doi: 10.1161/CIRCULATIONAHA.113.006699. [DOI] [PubMed] [Google Scholar]
- 12.Koval CE, Thuita L, Moazami N, Blackstone E. Evolution and impact of drive-line infection in a large cohort of continuous-flow ventricular assist device recipients. J Heart Lung Transplant. 2014 Jun 5 doi: 10.1016/j.healun.2014.05.011. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Padera RF. Infection in ventricular assist devices: the role of biofilm. Cardiovasc Pathol. 2006 Sep–Oct;15(5):264–70. doi: 10.1016/j.carpath.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Hannan MM, Husain S, Mattner F et al. Working formulation for the standardization of definitions of infections in patients using ventricular assist devices. J Heart Lung Transplant. 2011 Apr;30(4):375–84. doi: 10.1016/j.healun.2011.01.717. [DOI] [PubMed] [Google Scholar]
- 15.Tlili G, Picard F, Pinaquy JB, Domingues-Dos-Santos P, Bordenave L. The usefulness of FDG PET/CT imaging in suspicion of LVAD infection. J Nucl Cardiol. 2014 Aug;21(4):845–8. doi: 10.1007/s12350-014-9872-x. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, Feller ED, Chen W, Dilsizian V. FDG PET/CT imaging for LVAD associated infections. JACC Cardiovasc Imaging. 2014 Aug;7(8):839–42. doi: 10.1016/j.jcmg.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Baronetto A, Centofanti P, Attisani M et al. A simple device to secure ventricular assist device driveline and prevent exit-site infection. Interact Cardiovasc Thorac Surg. 2014 Apr;18(4):415–7. doi: 10.1093/icvts/ivt549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feldman D, Pamboukian SV, Teuteberg JJ et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant. 2013 Feb;32(2):157–87. doi: 10.1016/j.healun.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Stulak JM, Maltais S, Cowger J et al. Prevention of percutaneous driveline infection after left ventricular assist device implantation: prophylactic antibiotics are not necessary. ASAIO J. 2013 Nov–Dec;59(6):570–4. doi: 10.1097/MAT.0b013e3182a9e2a5. [DOI] [PubMed] [Google Scholar]
- 20.Fleissner F, Avsar M, Malehsa D, Strueber M, Haverich A, Schmitto JD. Reduction of driveline infections through doubled driveline tunneling of left ventricular assist devices. Artif Organs. 2013 Jan;37(1):102–7. doi: 10.1111/aor.12036. [DOI] [PubMed] [Google Scholar]
- 21.Schibilsky D, Benk C, Haller C et al. Double tunnel technique for the LVAD driveline: improved management regarding driveline infections. J Artif Organs. 2012 Mar;15(1):44–8. doi: 10.1007/s10047-011-0607-3. [DOI] [PubMed] [Google Scholar]
- 22.Singh A, Russo MJ, Valeroso TB et al. Modified HeartMate II Driveline Externalization Technique Significantly Decreases Incidence of Infection and Improves Long-Term Survival. ASAIO J. 2014 Jul 28 doi: 10.1097/MAT.0000000000000121. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Kretlow JD, Brown RH, Wolfswinkel EM et al. Salvage of infected left ventricular assist device with antibiotic beads. Plast Reconstr Surg. 2014 Jan;133(1):28e–38e. doi: 10.1097/01.prs.0000436837.03819.3f. [DOI] [PubMed] [Google Scholar]
- 24.Jennings DL, Chopra A, Chambers R, Morgan JA. Clinical Outcomes Associated With Chronic Antimicrobial Suppression Therapy in Patients With Continuous-Flow Left Ventricular Assist Devices. Artif Organs. 2014 Jan 20 doi: 10.1111/aor.12254. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Levy DT, Guo Y, Simkins J et al. Left ventricular assist device exchange for persistent infection: a case series and review of the literature. Transpl Infect Dis. 2014 Jun;16(3):453–60. doi: 10.1111/tid.12207. [DOI] [PubMed] [Google Scholar]
- 26.Schulman AR, Martens TP, Russo MJ et al. Effect of left ventricular assist device infection on post-transplant outcomes. J Heart Lung Transplant. 2009 Mar;28(3):237–42. doi: 10.1016/j.healun.2008.12.007. [DOI] [PubMed] [Google Scholar]


