Abstract
Continuous-flow left ventricular assist devices (CF-LVADs) have been clinically adopted as a long-term standard of care therapy option for patients with end-stage heart failure. For many patients, shared care between the care providers at the implanting center and care providers in the community in which the patient resides is a clinical necessity. The aims of this review are to (1) provide a rationale for the outpatient follow-up exam and surveillance testing used at our center to monitor patients supported by the HeartMate II® CF-LVAD (Thoratec Corporation, Pleasanton, CA) and (2) provide the protocol/algorithms we use for blood pressure, driveline exit site, LVAD alarm history, surveillance blood work, and echocardiography monitoring in this patient population. In addition, we define our partnership outpatient follow-up protocol and the “shared care” specific responsibilities we use with referring health care providers to best manage many of our patients.
Keywords: continuous flow LVAD, left ventricular assist device, heart failure, shared care

J. D. Estep, M.D.
Introduction
Over the past several years, axial and more recently centrifugal continuous-flow left ventricular assist devices (CF-LVADs) have become the devices of choice as bridge-to-transplantation or destination therapy due to significantly improved outcomes and fewer complications in patients with end-stage heart failure (Table 1).1 Historically, referring physicians and/or other care providers have deferred the bulk of post-CF-LVAD surveillance and management decisions to the implanting LVAD center heart team. With an increasing population of patients supported by CF-LVADs, especially as destination therapy with anticipated long-term support, the concept of “shared care” has evolved in the heart failure community. Shared care in part refers to the cared shared between the care providers at the implanting center and those in the community in which the patient resides. In our patient population, patients may live several hundred miles from our center, making frequent follow-up challenging due to travel and economic demands. Some of the other proposed benefits of shared care are listed in Table 2.
Table 1.
Approved adult durable continuous-flow left ventricular assist device types and characteristics. Photos reprinted with permission from Heartmate II (Thoratec Corp.) and HeartWare, Inc, Framingham, MA.
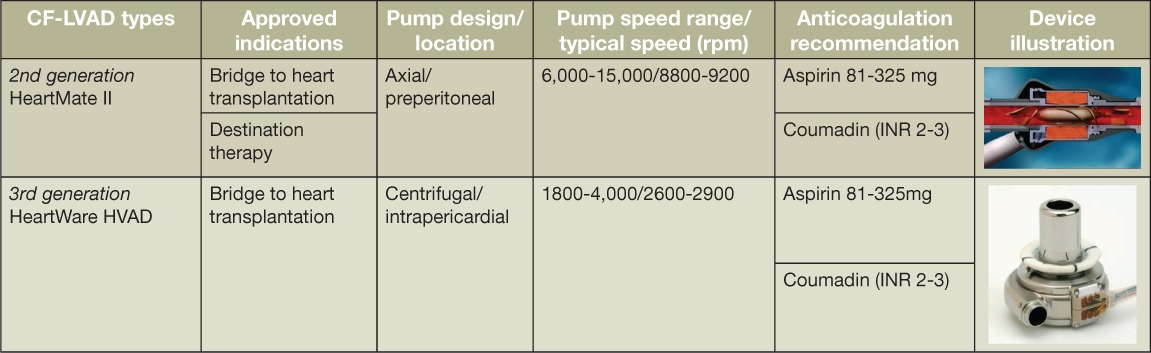
Table 2.
Benefits of establishing shared care responsibilities between the implanting left ventricular assist device (LVAD) center and the referring heath care provider/s.

This manuscript provides the rationale behind our outpatient follow-up exam at Houston Methodist Hospital and the surveillance testing used to monitor patients supported by the HeartMate II® CF-LVAD (Thoratec Corporation, Pleasanton, CA) (Figure 1). It also describes the protocol/algorithms we use for blood pressure (BP), driveline exit site, LVAD alarm history, surveillance blood work, and echocardiography monitoring in this patient population. In addition, we define our partnership outpatient follow-up protocol and the shared care-specific responsibilities we use with referring health care providers to best manage many of our patients.
Figure 1.
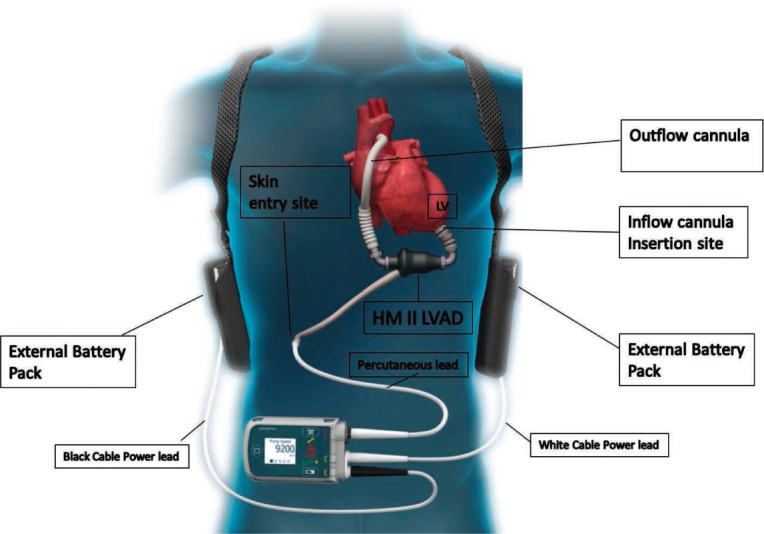
HeartMate II device and equipment illustration. HM: HeartMate II; LVAD: left ventricular assist device; LV: left ventricle.
Blood Pressure Monitoring
In addition to documenting routine vitals, careful BP assessment is necessary to minimize adverse events in patients supported with a CF-LVAD. It is critical for the provider to not only be familiar with BP goals in these patients but also understand the intricacies of measuring their BP. There are several advantages of maintaining BP in patients supported by CF-LVADs, including optimization of device pump function since all devices are sensitive to excess afterload (centrifugal devices to a greater extent than axial flow devices), optimization of LV unloading, minimizing left-sided heart failure symptoms, and preventing both hemorrhagic and nonhemorrhagic strokes.2 According to the most recent International Society of Heart and Lung Transplant (ISHLT) guidelines, mean BP in patients supported by CF-LVADs should be less than or equal to 80 mm Hg with a typical goal range between 70 and 80 mm Hg.3
In many patients, CF-LVADs are associated with a reduced pulse pressure, and the degree of this diminished pulsatility depends on the pump speed setting, underlying LV contractility, and preload and afterload pressures. The aortic valve in some patients may be open at a baseline set speed, but as the pump speed (revolutions per minute) is increased, the diastolic pressure increases to a point that exceeds the pressure gradient across the aortic valve (AV). This results in cessation of AV opening and a significantly reduced pulse pressure, which clinically equates to no palpable radial pulse. Patients supported by CF-LVADs may have complete AV opening (palpable pulse), absent AV opening (no palpable pulse), or partial and/or intermittent AV opening (intermittent and weak palpable pulse). Even at a fixed speed, the presence and degree of AV opening is dynamic and may change from one clinic visit to the next.
Due to the reduced pulse pressure common in this patient population, it can be difficult to record BP using traditional measurements (auscultation or automated cuff) because of the sensitivity range associated with these standard methods. In general, success rates in using automated BP monitors to obtain a BP in patients on CF-LVAD range between 17% and 63%.4–6 The current recommendation is to use a Doppler ultrasound probe that detects flow at any point during the cardiac cycle and measures an opening pressure in almost all CF-LVAD patients, with success rates between 94% and 100%.4,5
In a sample of 17 patients supported by a HeartMate II LVAD, Bennett et al. reported that the mean difference between Doppler and arterial-line mean arterial pressure (MAP) was 0.2 ± 10.5 mm Hg compared with a mean difference between Doppler and arterial-line systolic BP of 8.6 ± 9.5 mm Hg, thus suggesting that Doppler BP values more closely represent the true MAP as opposed to true systolic BP.4 In contrast, Lanier et al. demonstrated in 60 patients supported by the HeartMate II that the Doppler BP underestimates systolic BP by 4.1 ± 1.5 mm Hg and overestimates mean arterial BP by 9.5 ± 1.9 mm Hg, suggesting that Doppler better reflects systolic BP.5 For practical purposes, in patients with a significantly reduced pulse pressure (no palpable radial pulse), the recorded opening Doppler blood pressure reflects the systolic BP and approximates the mean BP.
Based on our observations and these reports, we measure blood pressure using the algorithm illustrated in Figure 2. If a radial pulse is present (reflective of a near normal pulse pressure), the typical Korotkoff sounds are present and systolic and diastolic blood pressure in this scenario is attainable. When the BP is attainable using standard methodologies (manual BP or an automated BP monitor), the recording remains accurate compared to arterial line measurement and can therefore be used to monitor patients.4,5
Figure 2.
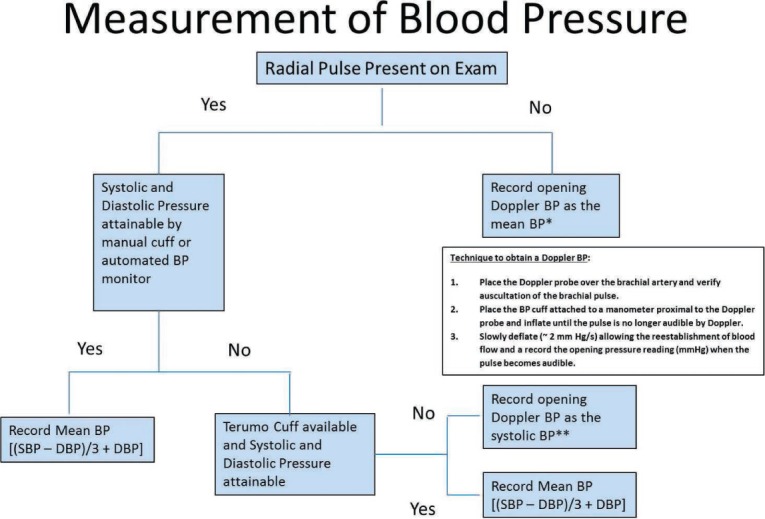
Measurement of blood pressure (BP) in patients on CF-LVADs.
*Doppler BP may be a slight overestimation compared to arterial line mean BP measurements.
**Doppler BP may be a slight underestimation compared to arterial line systolic BP measurements. BP: blood pressure; SBP: systolic blood pressure; DBP: diastolic blood pressure.
An acceptable alternative to an unattainable manual or automated BP monitor is to use the Terumo Elemano BP Monitor, which was designed with a slow-deflation capability to improve the sensitivity of BP measurements in patients with a reduced pulse pressure.7 Lanier et al. demonstrated that the Terumo Elemano BP Monitor was successful (91% success rate to attain BP reading), reproducible, and valid when compared with A-line (gold standard) and Doppler (clinical standard).5
An understanding of these important clinical nuances to BP monitoring in patients with CF-LVADs can curb outpatient overutilization of vasoactive medications to avoid adverse side effects of hypotension, which can manifest as dizziness or syncope. For those patients with true hypertension (mean BP > 80 mm Hg), the treatment recommendation is similar to the recommend use of agents indicated for heart failure patients.3 These include angiotensin converting enzymes or angiotensin receptor blockers and beta blockers. In a study by Lampert et al., 74% of patients who had received CF-LVAD required antihypertensive medications when using the commonly cited goal mean BP of less than 80 mm Hg. Of these, 88% required one or two antihypertensive medications, with no difference between axial and centrifugal flow devices in the percentage of those requiring BP mediations.8 The early initiation of beta-adrenergic receptor blockade in patients with a preimplant history of ventricular tachycardia or with early postimplant supraventricular tachycardia or ventricular tachycardia can be helpful. In keeping with heart failure guidelines, the use of heart rate-limiting calcium channel blockers is not recommended.3
Driveline Exit Site Inspection
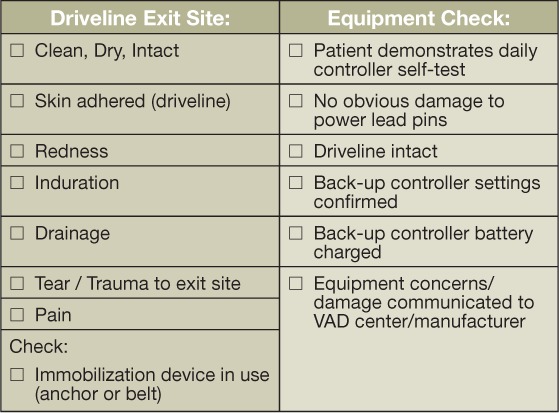
The most important factor in preventing the morbidity of infections is anchoring the device to help stabilize the driveline, thus minimizing trauma and tension at the exit site. Routine follow-up visits allow for continued education on driveline immobilization. The driveline exit site is examined for redness, drainage, tenderness, and open areas at the site. Figure 3 shows examples of a normal exit site and the variety of infections that may be seen there. When mild infections are encountered, such as scant drainage and limited redness without tenderness or induration, we obtain a culture and instruct the patient and/or caregivers to increase the frequency of local wound care (e.g., increase dressing changes to twice per day versus once). We ensure that the patient is following a sterile dressing care regimen in accord with our protocol requiring the use of chlorhexidine with exit site daily dressing changes. In patients showing signs of a complicated exit site infection, including moderate drainage and greater extent of redness and tenderness, additional treatment with oral antibiotics is recommended, and weekly clinic visits are planned to ensure exit site improvement. If the patient shows signs of systemic infection such as a fever greater than 101° F or an acquired leukocytosis and/or purulent drainage associated with subcutaneous induration, inpatient monitoring and treatment is recommended.
Figure 3.
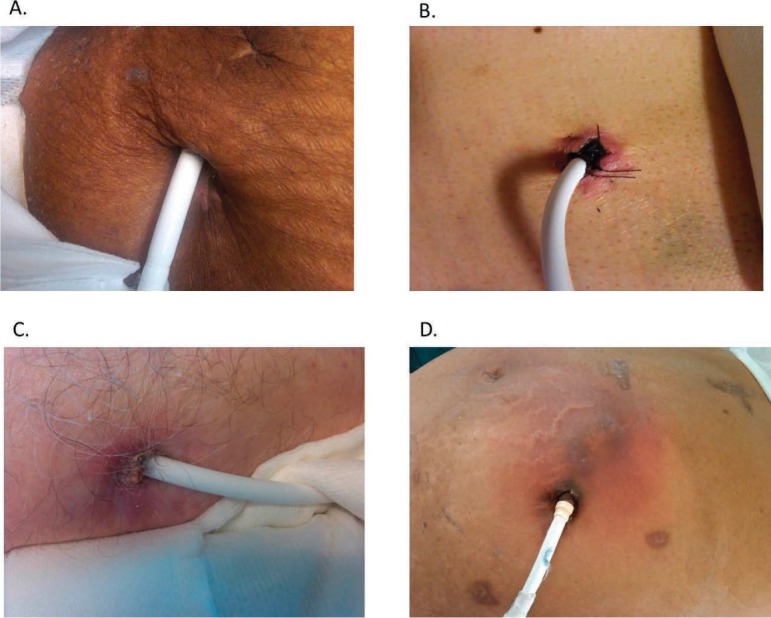
Driveline exit site examples showing (A) normal exit site with no associated redness or drainage; (B) mild redness around the exit site, no drainage; suture noted reflective of a recent implant (left in place for ~ 3 months); (C) mild to moderate redness with associated drainage (dry/crusted) around the exit site; and (D) extensive redness extending beyond the exit site consistent with cellulitis; typically associated with tenderness.
Outpatient LVAD Alarm Trouble Shooting
According to the most recent ISHLT guidelines, the alarm history and downloads should be obtained at regular intervals.3 It is our practice to review and document the alarm history (Figure 4) and pump parameters with every outpatient clinic visit. In the clinic, the patient's LVAD can be connected to the system monitor to permit LVAD setting review, documentation, and device interrogation. Displayed pump flow is an estimated value directly related to the selected speed (rotary pump rotation in revolutions per minute) and power. Typically, any increase in power will result in an increase in estimated flow.
Figure 4.
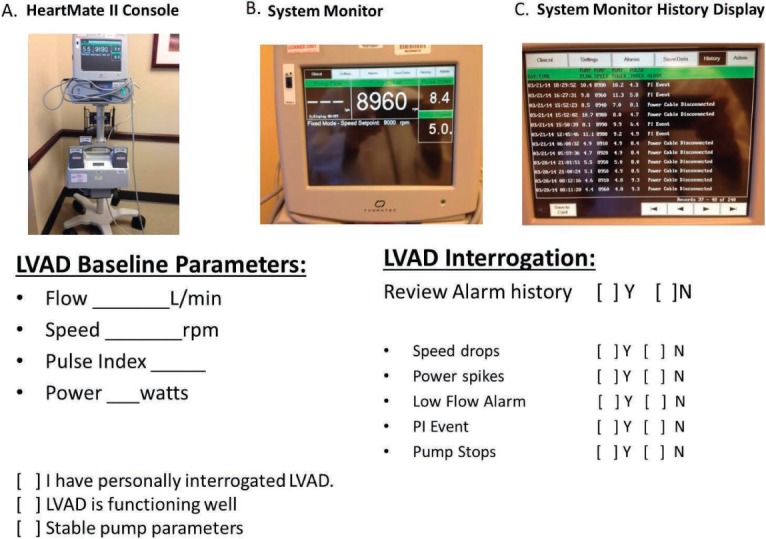
LVAD setting documentation/interrogation. (A) System monitor with power module (plugs into an AC outlet to provide power to the HeartMate II system) and power base unit including back up batteries. (B) System monitor displayed pump flow, pump speed, pulse index, and pump power. (C) System monitor alarm history display.
The displayed power is a direct measurement of pump motor voltage and current. Changes in pump speed, flow, or physiological demand can affect pump power. For the HeartMate II device, a power trend increase greater than 2 watts or a consistent reading greater than or equal to 10 watts in the setting of an unchanged pump speed is a red flag for possible rotor thrombosis. The displayed pulsatility index reflects contraction of the LV and hence LV contribution of flow into the pump. The magnitude of these flow pulses is measured by the pump and is averaged over a 15-s interval to produce the displayed pulsatility index (PI) value.
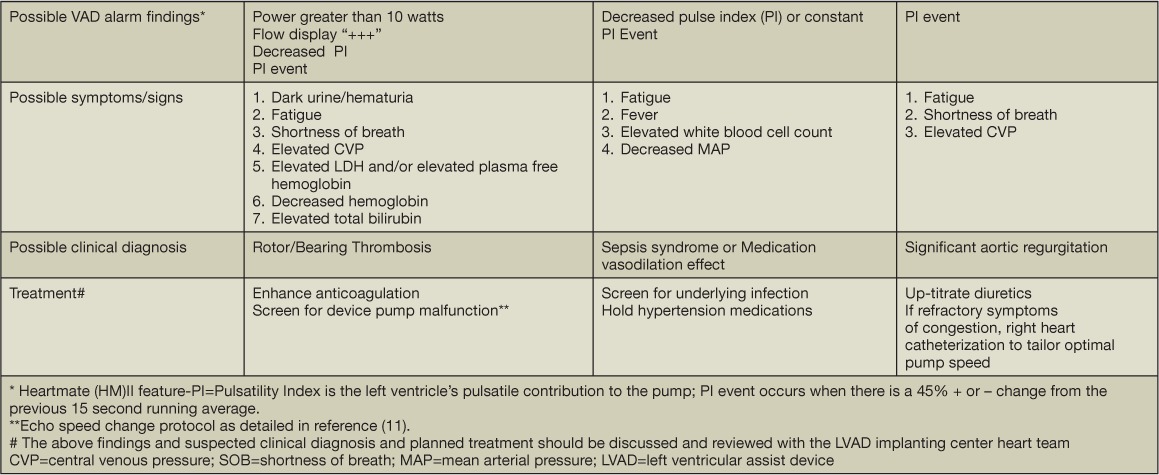
Pump parameter deviations and identified alarms should be placed in clinical context. The clinical symptoms and signs along with the type of LVAD alarm findings will guide the diagnosis and treatment recommendations. In general, alarms can be classified as either a low-flow or high-power (high-flow) alarm. For the HeartMate II device, low-flow alarms may be associated with or defined by one or more of the following signs: decreased pulsatility index (PI), PI event (a 45% ± change from the previous 15-s running average), low-flow alarm display, or flow display “---” noted as the flow estimate. LVAD suction events relate to contact of the inflow cannula and the LV endocardium, which results in a decrease in inflow cannula flow and can be associated with a change in clinical status and/or LVAD function. LVAD suction events can be seen with hypovolemia, right ventricular failure, cardiac tamponade (typically not seen in the outpatient setting), or inflow cannula malposition and may be associated with atrial and/or ventricular arrhythmias. The underlying treatment recommendations are specified in Tables 3 and 4.
Table 3.
HeartMate II left ventricular assist device (LVAD) “low flow” alarm trouble shooting.

Table 4.
HeartMate II left ventricular assist device (LVAD) “high flow” (high power) alarms.
Blood Work Surveillance Testing
As for any patient with underlying heart failure, we perform a thorough history, review of symptoms including device-specific questions (Table 5), and physical exam. We also obtain the following surveillance lab blood work with each clinic visit: complete blood count, complete metabolic profile, international normalized ratio, and lactate dehydrogenase (LDH). Patients on CF-LVAD support are at risk for infection, hemolysis due to rotor pump thrombosis, and gastrointestinal bleeding; therefore, it is important to screen for acquired leukocytosis, elevation in serum markers representing red blood cell disintegrity (e.g., LDH and/or serum free hemoglobin), and a decrement in hemoglobin.
Table 5.
Left ventricular assist device (LVAD)-specific review of symptoms and possible underlying diagnosis.
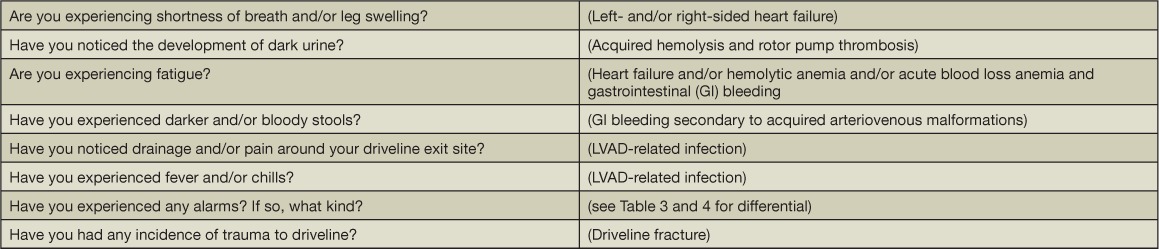
The clinical symptoms, signs, and other associated lab findings should help distinguish between hemolysis and gastrointestinal bleeding. Hemolysis may be characterized by dark urine, hemoglobinuria defined by urine analysis with large quantitative blood and few-to-no urine red blood cells, lactate dehydrogenase (LDH) level greater than 2.5-times upper limits of normal, increasing total bilirubin, and elevated serum free hemoglobin. Shah et al. showed that an elevated serum LDH was more predictive of device thrombosis than the INTERMACS-defined threshold of 40 mg/dL for serum free hemoglobin.9 Cowger et al. expanded these findings by demonstrating that an LDH-based definition of hemolysis (greater than 2.5-times reference range maximum) was also superior to the INTERMACS definition for predicting thrombus-related events.10 In our patient population, an LDH level greater than 1,050 IU/L was associated with a sensitivity and specificity of 80% and 86% to detect device thrombosis and malfunction and is similar to the LDH cutoff (1,103 IU/L) reported by Uriel et al. as an indicator for device thrombosis.11,12 Haptoglobin has been less useful as a parameter in that it is commonly low as demonstrated in our cohort of stable patients on CF-LVAD.11 In the absence of signs of hemolysis, a decrement in hemoglobin should prompt concern and an evaluation for a gastrointestinal bleed.
Echocardiography Surveillance (Image Acquisition and Reporting)
Echocardiography is the primary imaging modality to monitor patients supported by LVADs.13,14 Given the clinical implications of the type of LVAD and pump speed on heart function, these parameters should be recorded in the echo report. In our practice, the comprehensive LVAD exam (obtained at 1, 3, 6, and 12 months post-LVAD implant) consists of a standard echocardiographic examination (Table 6), recording several cardiac cycles to assess ventricular dimensions and AV function (Figure 5), visualization of apical inflow cannula with Doppler, assessment of peak systolic and diastolic inflow velocity (Figure 6), and a complete LV diastolic function assessment based on the transmitral Doppler inflow pattern (peak early and late velocity and deceleration time), septal and lateral mitral valve annular velocities, left atrial volume, and right atrial and systolic pulmonary artery pressure.15
Table 6.
Continuous-flow left ventricular assist device echocardiogram imaging protocol and reporting.

Figure 5.
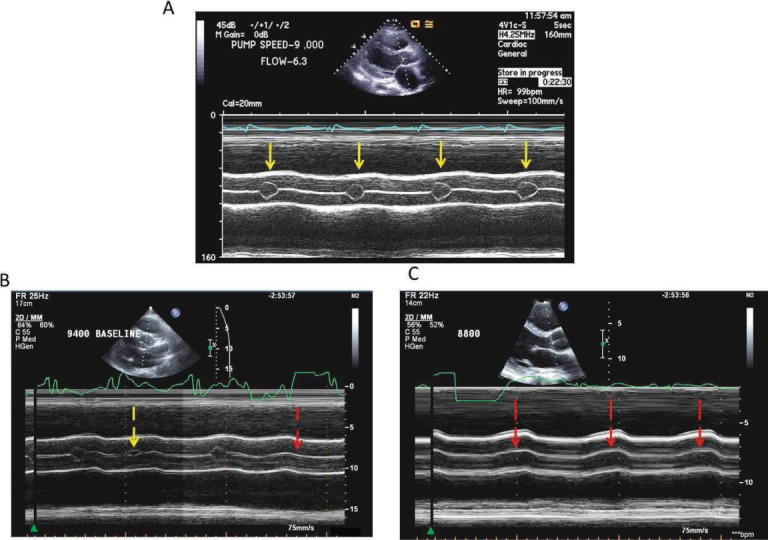
Echocardiographic aortic valve function assessment during continuous-flow left ventricular assist device (CF-LVAD) support. Various degrees of aortic valve (AV) opening with CF-LVAD support. M-mode images of aortic valve (AV) opening patterns in three different patients with HeartMate II devices. (A) Normal, consistent AV opening (solid yellow arrow). (B) Intermittent and variable partial AV opening (broken yellow arrow) and closure (broken red arrow). (C) Complete AV closure (red arrows). Reprinted from Estep et al. with permission of the publisher. Copyright ©2010 Elsevier.14
Figure 6.
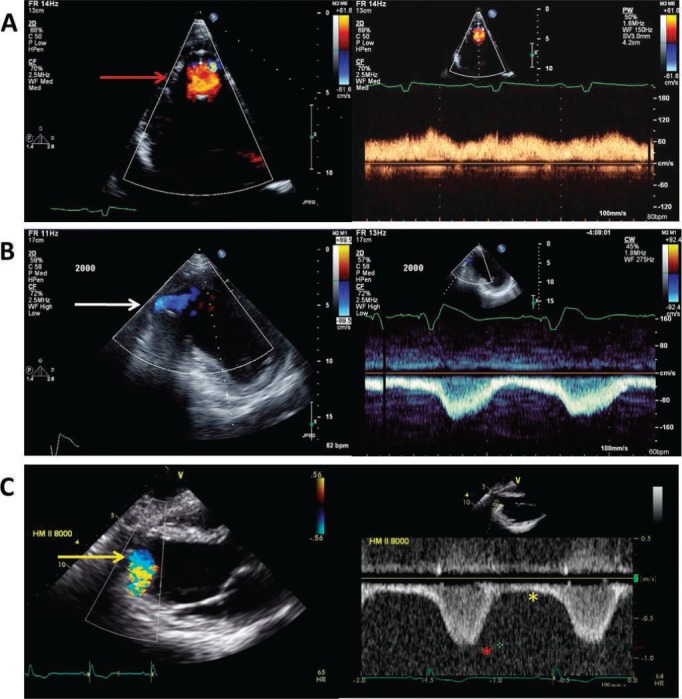
Normal continuous left ventricular assist device apical inflow cannula color and spectral Doppler. (A) Standard 4-chamber apical view illustration of nonturbulent, nonaliasing apical cannula inflow with color Doppler (red arrow) and slightly pulsatile, low peak velocity (< 1.5 m/s) continuous flow directed towards the apical transducer. (B) “Off axis” 2-chamber apical view of an inferiorly positioned apical inflow cannula (white arrow) with normal flow characteristics (slightly pulsatile, low peak velocity) similar to panel A with the exception of continuous flow directed away from the apical transducer. Red asterisk shows peak systolic apical inflow velocity; yellow asterisk shows peak diastolic apical inflow velocity. (C) Parasternal long-axis view of normal apical cannula inflow color Doppler (yellow arrow) and pulse-wave Doppler flow characteristics with continuous flow directed away from the apical transducer similar to panel B. Reprinted from Estep et al. with permission of the publisher. Copyright ©2010 Elsevier.14
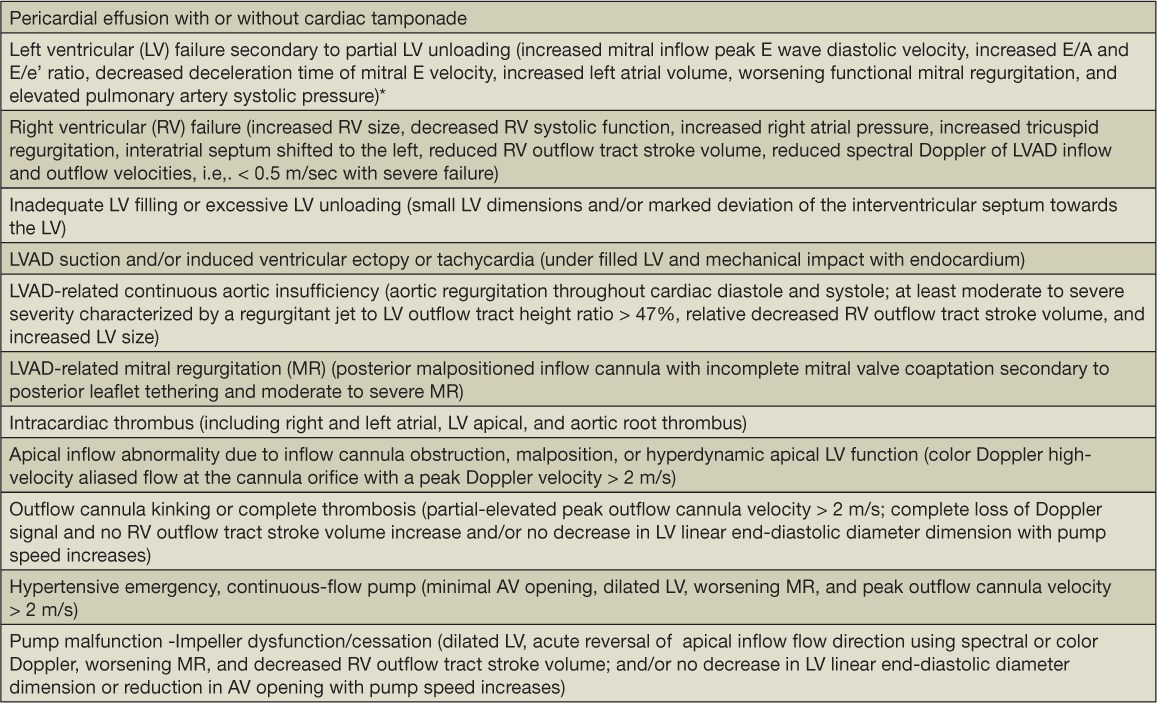
Our approach for assessing a patient on a CF-LVAD with recurrent HF symptoms, dysrhythmias, or a positive LVAD alarm history is based on an integrated clinical exam, device alarm interrogation, and device-specific echocardiographic findings. Table 7 lists CF-LVAD post-implantation complications (i.e., acquired continuous aortic insufficiency and LVAD-related suction events) and the associated echocardiographic criteria we use to guide echo reporting. Many of these clinical challenges can be significantly influenced by the underlying baseline pump speed setting, excessive afterload (occult hypertension), and the inflow cannula position/mal-position.
Table 7.
Continuous-flow LVAD post-implant complications and device dysfunction detected by echocardiography. Reprinted from Estep et al. with permission of the publisher. Copyright ©2010 Elsevier.14
Persistent or acquired HF symptoms (New York Heart Association class III/IV) while on CF-LVAD support may be due to partial LV unloading and/or right-sided HF. We recently demonstrated how Doppler echocardiography accurately estimated intracardiac hemodynamics in 50 patients supported with the HeartMate II LVAD.15 Our algorithm reliably distinguished normal from elevated LV filling pressures. The echo-based algorithm we derived to detect underlying partial LV unloading (i.e., pulmonary capillary wedge pressure > 15) is illustrated in Figure 7. We recommend that providers consult with the LVAD implanting center heart team if a patient has a clinical indication that warrants changing the underlying pump speed—for example, increasing the underlying pump speed if partial LV unloading or associated HF symptoms/signs are detected, or decreasing the pump speed if LVAD suction events are detected).
Figure 7.

Algorithm for continuous-flow left ventricular assist device (CF-LVAD) persistent heart failure symptom evaluation. *In the presence of fused or indistinct E and A signals, two of three parameters must be met and concordant. **Other echo findings are nondiagnostic (e.g., no pericardial effusion). Augmentation in heart failure medical therapy and/or increase pump speed should be discussed with the implanting LVAD heart team. Reproduced by permission Estep et al.15
Conclusions
Newer-generation CF-LVADs such as the HeartMate II device have gained widespread recognition and use. Given the continued support needed by patients on this type of device, standardized outpatient follow-up with clearly defined objectives (Table 8, 9) and shared care responsibilities between the implanting LVAD heart team and care providers in the community are necessary to facilitate the long-term management needed by many of these patients.
Table 8.
Houston Methodist Physician Partnership follow-up protocol. Orange highlight notes follow up schedule and physician partnership responsibilities.
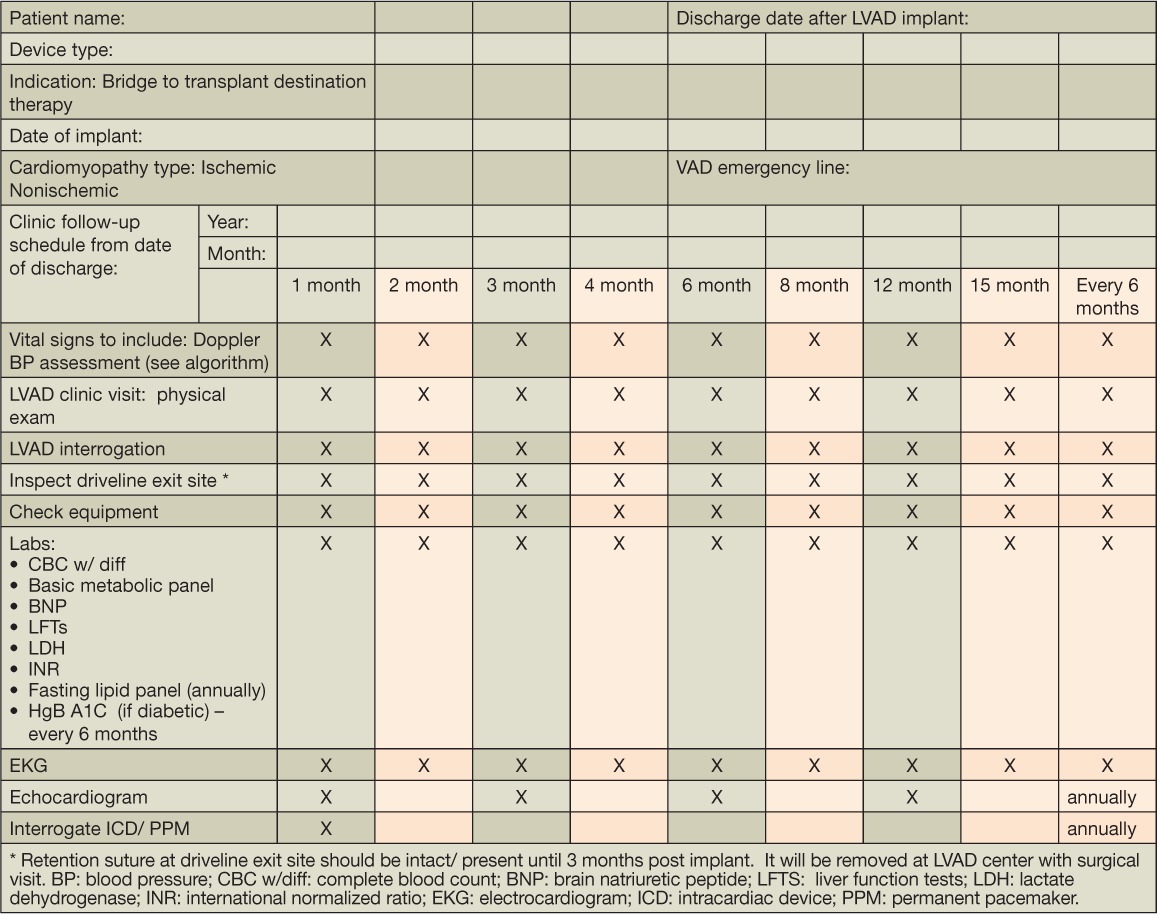
Table 9.
Clinic visit documentation.
Acknowledgments
Conflict of Interest Disclosure: The authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
Funding/Support: Dr. Estep is a consultant for and receives honoraria and grant-research support from Thoratec Corp.
Footnotes
Editor's Note: We are pleased to offer 1 credit of Continuing Medical Education for successfully completing an online quiz about this article. You may access the quiz at www.houstonmethodist.org/cme-online.
References
- 1.Kirklin JK, Naftel DC, Kormos RL et al. The Fourth INTERMACS Annual Report: 4,000 implants and counting. J Heart Lung Transplant. 2012 Feb;31(2):117–26. doi: 10.1016/j.healun.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Salamonsen RF, Mason DG, Ayre PJ. Response of rotary blood pumps to changes in preload and afterload at a fixed speed setting are unphysiological when compared with the natural heart. Artif Organs. 2011 Mar;35(3):E47–53. doi: 10.1111/j.1525-1594.2010.01168.x. [DOI] [PubMed] [Google Scholar]
- 3.Feldman D, Pamboukian SV, Teuteberg JJ et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant. 2013 Feb;32(2):157–87. doi: 10.1016/j.healun.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Bennett MK, Roberts CA, Dordunoo D, Shah A, Russell SD. Ideal methodology to assess systemic blood pressure in patients with continuous-flow left ventricular assist devices. J Heart Lung Transplant. 2010 May;29(5):593–4. doi: 10.1016/j.healun.2009.11.604. [DOI] [PubMed] [Google Scholar]
- 5.Lanier GM, Orlanes K, Hayashi Y et al. Validity and reliability of a novel slow cuff-deflation system for noninvasive blood pressure monitoring in patients with continuous-flow left ventricular assist device. Circ Heart Fail. 2013 Sep 1;6(5):1005–12. doi: 10.1161/CIRCHEARTFAILURE.112.000186. [DOI] [PubMed] [Google Scholar]
- 6.Myers TJ, Bolmers M, Gregoric ID, Kar B, Frazier OH. Assessment of arterial blood pressure during support with an axial flow left ventricular assist device. J Heart Lung Transplant. 2009 May;28(5):423–7. doi: 10.1016/j.healun.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 7.Tochikubo O, Nishijima K, Ohshige K, Kimura K. Accuracy and applicability of the Terumo ES-H55 double-cuff sphygmomanometer for hospital use. Blood Press Monit. 2003 Oct;8(5):203–9. doi: 10.1097/00126097-200310000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Lampert BC, Eckert C, Weaver S et al. Blood pressure control in continuous flow left ventricular assist devices: efficacy and impact on adverse events. Ann Thorac Surg. 2014 Jan;97(1):139–46. doi: 10.1016/j.athoracsur.2013.07.069. [DOI] [PubMed] [Google Scholar]
- 9.Shah P, Mehta VM, Cowger JA, Aaronson KD, Pagani FD. Diagnosis of hemolysis and device thrombosis with lactate dehydrogenase during left ventricular assist device support. J Heart Lung Transplant. 2014 Jan;33(1):102–4. doi: 10.1016/j.healun.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Cowger JA, Romano MA, Shah P et al. Hemolysis: a harbinger of adverse outcome after left ventricular assist device implant. J Heart Lung Transplant. 2014 Jan;33(1):35–43. doi: 10.1016/j.healun.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 11.Estep JD, Vivo RP, Cordero-Reyes AM et al. A simplified echocardiographic technique for detecting continuous-flow left ventricular assist device malfunction due to pump thrombosis. J Heart Lung Transplant. 2014 Jun;33(6):575–86. doi: 10.1016/j.healun.2014.01.865. [DOI] [PubMed] [Google Scholar]
- 12.Uriel N, Morrison KA, Garan AR et al. Development of a novel echocardiography ramp test for speed optimization and diagnosis of device thrombosis in continuous-flow left ventricular assist devices: the Columbia ramp study. J Am Coll Cardiol. 2012 Oct 30;60(18):1764–75. doi: 10.1016/j.jacc.2012.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Estep JD, Chang SM, Bhimaraj A, Torre-Amione G, Zoghbi WA, Nagueh SF. Imaging for ventricular function and myocardial recovery on nonpulsatile ventricular assist devices. Circulation. 2012 May 8;125(18):2265–77. doi: 10.1161/CIRCULATIONAHA.111.040238. [DOI] [PubMed] [Google Scholar]
- 14.Estep JD, Stainback RF, Little SH, Torre G, Zoghbi WA. The role of echocardiography and other imaging modalities in patients with left ventricular assist devices. JACC Cardiovasc Imaging. 2010 Oct;3(10):1049–64. doi: 10.1016/j.jcmg.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Estep JD, Vivo RP, Krim SR et al. Echocardiographic Evaluation of Hemodynamics in Patients With Systolic Heart Failure Supported by a Continuous-Flow LVAD. J Am Coll Cardiol. 2014 Sep 23;64(12):1231–41. doi: 10.1016/j.jacc.2014.06.1188. [DOI] [PubMed] [Google Scholar]


