Abstract
Posttraumatic stress disorder (PTSD) symptoms can result in functional impairment among service members (SMs), even in those without a clinical diagnosis. The variability in outcomes may be related to underlying catecholamine mechanisms. Individuals with PTSD tend to have elevated basal catecholamine levels, though less is known regarding catecholamine responses to trauma-related stimuli. We assessed whether catecholamine responses to a virtual combat environment impact the relationship between PTSD symptom clusters and elements of functioning. Eighty-seven clinically healthy SMs, within 2 months after deployment to Iraq or Afghanistan, completed self-report measures, viewed virtual-reality (VR) combat sequences, and had sequential blood draws. Norepinephrine responses to VR combat exposure moderated the relationship between avoidance symptoms and scales of functioning including physical functioning, physical-role functioning, and vitality. Among those with high levels of avoidance, norepinephrine change was inversely associated with functional status, whereas a positive correlation was observed for those with low levels of avoidance. Our findings represent a novel use of a virtual environment to display combat-related stimuli to returning SMs to elucidate mind-body connections inherent in their responses. The insight gained improves our understanding of post-deployment symptoms and quality of life in SMs and may facilitate enhancements in treatment. Further research is needed to validate these findings in other populations and to define the implications for treatment effectiveness.
Keywords: norepinephrine, posttraumatic stress, PTSD, functional status, virtual reality
Introduction
Symptoms of posttraumatic stress disorder (PTSD) are common in the aftermath of war, and the recent wars in Iraq and Afghanistan are no exception (Veterans Health Administration, 2014). Exposure to traumatic experiences such as being attacked, ambushed, or in an accident, or witnessing physical devastation during these campaigns has been associated with PTSD, depressive symptoms, hazardous drinking, and relationship stress, which persist for at least 9 months after returning home (Cigrang et al., 2014). PTSD symptoms resulting from combat-related events can impair quality of life and functional status (e.g., degree of disability across domains of health and behavior) among service members (SMs; Schnurr et al., 2009), including those who are subthreshold, or lacking a sufficient number or distribution of symptoms to meet full criteria for PTSD (Magruder et al., 2004; Grubaugh et al., 2005; Cukor et al., 2010). PTSD symptom clusters include re-experiencing (e.g., intrusive memories and bad dreams), avoidance (e.g., avoidance of trauma-related thoughts, feelings, activities, as well as a loss of social connection, and interest in activities), and hyperarousal (e.g., strong startle response, sleep problems, angry outbursts, hypervigilance), with dysphoric mood recently added. While PTSD is associated with poorer functional outcomes overall, this relationship may vary according to particular symptom clusters and domains of functional status. The intent of this report is to explore the relationship between PTSD symptom clusters and functional impairment through an assessment of blood catecholamine responses to a combat-related virtual environment in a sample of U.S. military SMs recently returned from deployment.
Posttraumatic stress disorder symptoms are multifaceted and the number and intensity of symptoms can vary considerably between individuals resulting in variable patterns of functional impairment (Norrholm and Jovanovic, 2010). Previous studies suggest the importance of examining PTSD symptom clusters separately, rather than one amalgamated score. In studies of veterans of the Iraq and Afghanistan wars, dysphoria has been linked to a multitude of poor outcomes including mental health function, problematic alcohol use, and greater psychosocial, and work difficulties (Pietrzak et al., 2010), as well as greater propensity to seek mental health care (Blais et al., 2014). Re-experiencing symptoms have been positively associated with alcohol use problems (Pietrzak et al., 2010), healthcare utilization (Blais et al., 2014), reduced health functioning, and elevated bodily pain (Asnaani et al., 2014); while avoidance was linked with greater psychosocial difficulties (Pietrzak et al., 2010) and lower healthcare utilization (Blais et al., 2014). Hyperarousal symptoms have been independently associated with lower vitality and emotional functioning (Asnaani et al., 2014). Though PTSD symptom clusters have sometimes been associated with functional deficits, they are not invariably associated with impaired function, and psychophysiological factors may represent important moderators.
The sympathetic nervous system stimulates the release of the catecholamines epinephrine, norepinephrine, and dopamine to mediate adaptive responses to acute stressors (Southwick et al., 2002; Cahill and Alkire, 2003), but they are also linked with long-term memory of events that induce strong emotions such as fear (Soeter and Kindt, 2011). Though adaptive for acute stress, chronic stress, and associated repetitive catecholamine-system activation leads to damaging biopsychosocial outcomes (Mead et al., 2010). In a community sample, individuals with PTSD had higher 24-h levels of catecholamines compared to both those without trauma exposure, as well as those exposed to trauma who did not develop PTSD (Young and Breslau, 2004). In fact, those with trauma exposure who did not develop PTSD actually had lower catecholamine levels than those without trauma exposure (Young and Breslau, 2004), indicating a potential mechanism for resilience. Though catecholamine levels are related to PTSD symptomatology, research on catecholamine responses to acute combat-related cues among post-deployment SMs is limited. In a small study of veterans with and without PTSD, those with PTSD had exaggerated epinephrine and norepinephrine responses to combat-sounds than those without PTSD (Liberzon et al., 1999). Recent technological advances make it possible to present salient combat-related stimuli and monitor individualized psychophysiological reactions, providing the potential to elucidate the complexities of trauma-related symptomology and functioning outcomes.
Virtual reality exposure therapy (VRET) has shown efficacy in the treatment of combat-related PTSD (Roy et al., 2010; McLay et al., 2011; Reger et al., 2011; Goncalves et al., 2012; McLay et al., 2014). A study conducted in U.S. veterans with recent-onset combat-related PTSD found both that virtual reality (VR) was acceptable to them, and that the psychological, physiological, and behavioral responses it elicited were similar to those they had experienced during deployment (Kramer et al., 2013). However, there is relatively little in the medical literature regarding the use of VR in PTSD assessment. Recently, one study examined responses to neutral computer-generated characters using head-mounted display VR in participants who had experienced a physical assault 4 weeks prior, which found that reactions to the neutral stimuli predicted paranoia and PTSD 6 months later (Freeman et al., 2014). We previously documented a relationship between physiologic responses to combat-related VR scenarios and PTSD symptoms (Roy et al., 2013; Costanzo et al., 2014), but we believe that incorporation of catecholamine measurements in the analysis can further improve our understanding of the impact of exposure to virtual combat sequences. We therefore examine catecholamine levels (epinephrine, norepinephrine) before and after exposure to virtual combat sequences in relation to PTSD symptom clusters (e.g., re-experiencing, avoidance, hyperarousal) and multiple functional domains (e.g., mental health, physical health, vitality). We hypothesize that catecholamine responses to virtual combat sequences will positively correlate with PTSD symptom clusters and that each will be inversely correlated with functional status.
Materials and Method
Participants and Procedures
The present study was approved by the institutional review boards at the Walter Reed National Military Medical Center, Uniformed Services University of the Health Sciences, and the National Institutes of Health, and adhered to the Declaration of Helsinki. Clinically healthy SMs within 2 months of return from deployment to either Iraq or Afghanistan first completed self-report questionnaires, then viewed VR combat sequences, and completed baseline and post-VR blood draws for catecholamines. Participants viewed 3 sequential 2-min Virtual Iraq prescripted sequences (Rizzo et al., 2010) on a computer screen, which were separated by 30-s presentations of a blue square; the sequences, respectively, depicted a first person view at the gunner position of a Humvee, within the cabin of a Humvee, and walking through the streets of a middle eastern city. The scenarios included smoke, gunfire, explosions, and overhead aircraft.
Blood was drawn from each participant prior to 9:00 AM (baseline), as well as immediately after exposure to the three virtual combat sequences. Plasma samples were isolated from whole blood in sodium heparin preserved collection tubes with centrifugation for 10 min at room temperature. Samples were stored at -70°C, and all samples were then run in a single batch. Following manufacturer’s instructions, a commercially available enzyme immunoassay was used to measure plasma catecholamine levels, including epinephrine and norepinephrine. First, catecholamines were extracted with a cis-diol-affinity specific gel, followed by acetylation and enzymatic conversion. Then, the separate microtiter plates bound with specific catecholamine antigen were processed with a competitive enzyme linked immunosorbent assays. Standards, controls, samples, and their respective antiserum were incubated for 20 h, followed by washing with the automated plate washer to remove free antigen-antiserum complexes. An anti-rabbit IgG-peroxidase conjugate using 3.3′, 5.5′ Tetramethylbenzidine (TMB) as the substrate detected antibody, and a plate reader then detected absorbance at 450 nm. Next, sample and standard curve analysis was calculated. A non-linear regression, 4-parameter logistic curve fitting method calculated the standard curve and sample concentrations (Molecular Devices Corporation, 2012). The equation used to generate this curve fit is: y = ((A - D) / (1 + (× /C) B)) + D.
Measures
Posttraumatic stress disorder symptom clusters were assessed with the 17-item PTSD Checklist Military Version (PCL-M), for which the total score may range from 17 to 85, with the various items measuring symptoms within each of the 3 clusters. The PCL-M compares favorably with the gold-standard Clinician Administered PTSD Scale (Forbes et al., 2001). Functional status was assessed using the widely used and well-validated 36-item Short Form Health Survey (SF-36; Ware and Sherbourne, 1992). Epinephrine and norepinephrine values at baseline were subtracted from values after VR so that “catecholamine responses” represent the difference in values between the two time points.
Analyses
Demographic characteristics and biopsychosocial factors were assessed by univariate analysis to obtain frequencies for categorical variables and means and standard deviations for continuous variables. We then examined the relationships between PTSD symptom clusters, catecholamine responses, and functional status subscales in a multivariate fashion with a series of multiple linear regressions. A PCL-M cluster (centered) was placed in Block 1 and a catecholamine response value (centered) was placed in Block 2. To examine moderating effects, the respective PCL-M cluster X catecholamine response interaction term was placed in Block 3. To control for the risk of false positive findings, we utilized an online False Discovery Rate calculator (http://sdmproject.com/utilities). All statistical analyses were completed using IBM SPSS (version 22).
Results
Univariate and Bivariate Findings
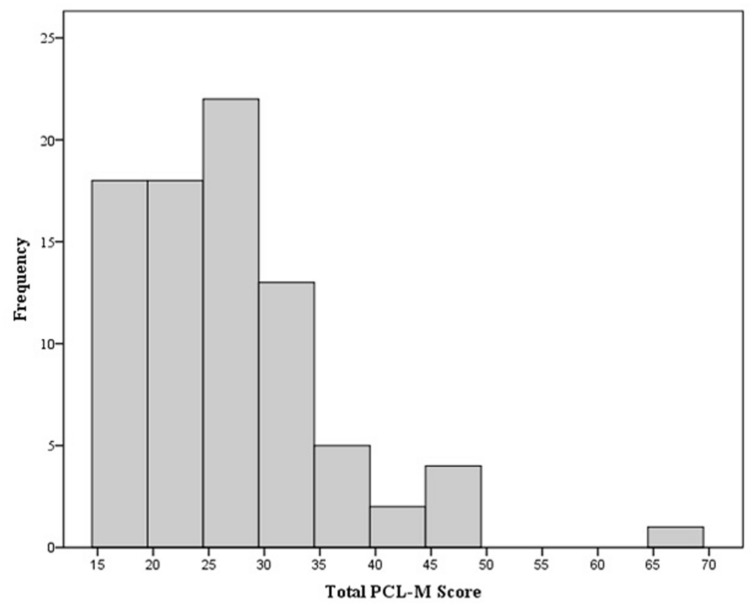
Study participants (N =87) were predominantly male (80%) and self-identified as white (73%); all were either active or reserve component SMs representing multiple military service branches. On average, participants were 30.0 (SD = 8.0) years of age, had 9.4 (SD = 5.9) years of service, and had been deployed 1.7 (SD = 0.95) times. The average PCL-M total score was 26.93 (SD = 8.74); one participant had a score > 50 and was excluded from the physiological analysis, 50 is a widely used cut-off for identifying a likely diagnosis, while another two had a PCL-M score within 5% of that cut-off. The frequency of PCL-M scores are shown in a histogram (Figure 1). On average, epinephrine levels increased by 2.2% after VR, whereas norepinephrine levels decreased by 11.2%. Means and SD are shown in Table 1.
FIGURE 1.
Frequency of PCL-M total scores.
Table 1.
Means and SD for catecholamine, trauma symptoms, and functioning scores.
| Variable | Mean (SD) |
|---|---|
| Baseline epinephrine | 51.30 (25.57) |
| Epinephrine response | 1.20 (36.13) |
| Baseline norepinephrine | 274.77 (173.50) |
| Norepinephrine response | -38.50 (200.30) |
| PCL-M total | 26.93 (8.74) |
| PCL re-experiencing | 7.22 (2.47) |
| PCL avoidance | 8.92 (2.30) |
| PCL hyperarousal | 8.76 (3.52) |
| Bodily pain | 77.73 (16.93) |
| General health | 82.32 (13.83) |
| Mental health | 79.71 (11.38) |
| Physical functioning | 94.69 (11.56) |
| Emotional role functioning | 85.37 (27.27) |
| Physical role functioning | 92.68 (16.89) |
| Social functioning | 83.02 (17.17) |
| Vitality | 63.90 (15.34) |
PCL-M = Posttraumatic stress disorder (PTSD) checklist – military.
There were several significant correlations among predictor and outcome variables. When looking at the relationship between PCL-M total score and outcomes, we found that higher PCL-M scores were associated with more bodily pain (r = -0.37, p < 0.01), and poorer general health (r = -0.24, p < 0.05), mental health (r = -0.38, p < 0.01), emotional role functioning (r = -0.24, p < 0.05), social functioning (r= -0.57, p< 0.01), and vitality (r = -0.40, p < 0.01). When examining these correlations on a symptom cluster level, higher levels of hyperarousal was related to more bodily pain (r = 0.26, p < 0.05) and poorer general health (r= -0.35, p < 0.01). There were no direct linear effects observed between catecholamine responses and functioning status subscales.
Linear Regression
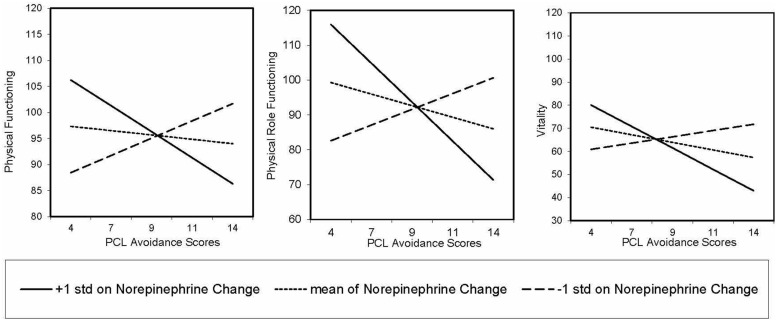
A series of simple and multiple linear regressions examined the relationship between PCL-M symptom clusters and catecholamine responses with functional status subscales. We used a false discovery rate correction for multiple comparisons (q-values) to determine significance (i.e., p < 0.05 and q< 0.10). First, we assessed whether PCL-M symptom clusters and catecholamine responses were independently associated with functional status subscales using several simple linear regressions. We found a significant main effect for hyperarousal scores on general health (β = -0.35, p = 0.001, q = 0.01), such that as hyperarousal increased, reported general health decreased. No other main effects were significant. Next, we examined the independent and interactive effects of PCL-M symptom clusters and catecholamine responses on functional status subscales using several multiple linear regressions. Consistent with our hypothesis, norepinephrine responses to VR combat sequences significantly moderated the relationship between avoidance and functional status subscales, including physical functioning (β = 0.53, p < 0.001, q = 0.001), physical role functioning (β = 0.36, p = 0.002, q = 0.02), and vitality (β = 0.36, p = 0.002, q =0.02; Figure 2). For individuals with lower avoidance symptoms, increased norepinephrine responses were associated with increased functional status subscale scores, while participants with higher avoidance symptoms, increased norepinephrine responses were associated with decreased functional status subscale scores.
FIGURE 2.
Pattern of relationship between PCL-M avoidance and physical functioning, physical role functioning, and vitality as moderated by norepinephrine response from baseline to post-virtual reality.
Discussion
Norepinephrine release in response to virtual combat sequences modulates the relationship between the avoidance symptoms of PTSD and multiple domains of functional status in a population of recently deployed U.S. military SMs. For individuals with lower levels of avoidance symptoms, a significant catecholamine response was indicative of better functional status. However, for SMs reporting more severe avoidance symptoms, an amplified catecholamine response was associated with poorer functional status across multiple domains. From the perspective of emotional processing theory, avoidance reduces opportunities to dispel trauma-related, maladaptive beliefs, enabling the persistence of hyperarousal and re-experiencing symptoms that may have been normal initial reactions (Rauch and Foa, 2006). Participants having both higher avoidance symptoms and high catecholamine responses to trauma-related stimuli may be disengaging from day-to-day experiences, which may in turn adversely impact their physical function, physical role function, and vitality. This pattern of response may signal the differentiation between adaptive, resilient coping responses and less adaptive responses that impact functional status.
Our identification of a link between PTSD symptom clusters and functional status is consistent with a previous report (Pietrzak et al., 2010), though our findings significantly expand upon the prior work both by examining the impact of psychophysiological responses and by looking at a more broadly representative population of SMs, all of whom fell below diagnostic criteria, as opposed to solely focusing on those meeting diagnostic criteria for PTSD (Rauch and Foa, 2006). Our findings complement previous studies that have shown that combat-related VR engenders significant psychophysiological reactions among SMs (Kramer et al., 2013; Costanzo et al., 2014) and decrease with treatment (Rothbaum et al., 2014), as well as that catecholamines play an important role in PTSD and functional status (Young and Breslau, 2004). Similar to more recent work (Freeman et al., 2014), our findings also demonstrate the assessment utility of VR in relation to PTSD. We are able to expand significantly upon the prior literature by exploring relationships between individual catecholamines, PTSD symptom clusters, and various functional domains in a sample of SM with a wide range of symptom severity. We report that norepinephrine modulates the relationship between avoidance and markers of physical functioning (e.g., general physical functioning, physical role functioning, vitality). One previous report of a SM with post-deployment PTSD documented greater epinephrine and norepinephrine responses to combat sounds than was seen in controls (Liberzon et al., 1999), which is not surprising, but our study includes larger numbers and assesses the full spectrum of PTSD symptoms and reveals a far more nuanced picture. Our results support a role for VR in making such assessments, with further studies needed in order to refine its full value.
In the present study, we included SM with a wide range of traumatic symptoms. In doing so, we were able to capture a population of SM who may already be engaging in resilient coping techniques, marked by their capacity to successfully adapt in the face of stress and adversity (Jensen and Fraser, 2005). One subset of our study population of particular interest is a group that had low norepinephrine responses and relatively good functional status despite relatively high avoidance scores. Whereas resilience has more commonly been used to refer to those reporting relatively few symptoms after significant trauma, it may be that we are distinguishing another pattern of resilience in this subset in which they acknowledge symptoms but are not necessarily as troubled by them, either with regard to the autonomic nervous system, or in terms of functional status. Although PTSD had not been labeled as such at the time of World War I or II, it is clear that there are many SMs, as well as such groups as Holocaust survivors, who may have exhibited a similar pattern, with sleep disruptions and other symptoms, yet they were somehow able to function relatively well, and it would certainly have been interesting to see what pattern their catecholamine responses displayed. There are many avenues for future research to investigate regarding resilience in a biopsychosocial framework. Because kernels of resilience are embedded within an individual prior to a trauma (Feder et al., 2009), long-term prospective studies would be especially helpful to further assess the trajectory of catecholamine levels in the face of trauma. Such investigations could help to elucidate why one person develops PTSD and another does not following a traumatic experience.
There are several limitations inherent in our study. First, the cross-sectional nature of the study does not allow us to draw causal inferences. We cannot rule out common method variance that may result with the use of a single questionnaire, and there may also be extraneous variables of significance that were not measured. Therefore, alternative explanations are plausible (e.g., responses to PCL-M items may be influenced by salient events and influence the reportable time-frame). Though our findings require replication in other populations, our results represent a foundational step in utilizing catecholamine response to VR combat scenarios in order to improve our understanding of the relationship between PTSD symptom clusters and functional status.
In recent years, VR has progressed from the realm of science fiction to an accepted and proven method for delivering treatment for PTSD (Goncalves et al., 2012) and advances in inexpensive hardware may soon make it a staple for video gaming. VR has already been utilized to improve our understanding of prospective memory (Gonneaud et al., 2012), attentional processes (Parsons and Rizzo, 2008), attention bias among smokers (Marra et al., 2009), and food cravings (Ledoux et al., 2013), and our work here is in keeping with such intellectual explorations. We demonstrate that the measurement of catecholamine responses to traumatic or stressful virtual presentations can enhance our understanding of PTSD symptomatology, and what really matters most, how well someone is functioning in the various domains of life. Translational approaches to PTSD treatment focused on individual symptom clusters hold promise (Norrholm and Jovanovic, 2010), and we believe that elucidation of the significance of the clusters can be improved by exploring sympathetic nervous system and consequent physiologic responses to VR. Our report represents an early step on the path to improved understanding and more individually tailored approaches to PTSD symptoms.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was supported by a grant from the Center for Neuroscience and Regenerative Medicine (CNRM), Uniformed Services University of the Health Sciences, Bethesda, MD, USA Any opinions, views, or assertions expressed are solely those of the authors and do not necessarily represent those of CNRM, Uniformed Services University, the Department of Defense, Department of Army/Navy/Air Force, or the U.S. Government.
REFERENCES
- Asnaani A., Reddy M. K., Shea M. T. (2014). The impact of PTSD symptoms on physical and mental health functioning in returning veterans. J. Anxiety Disord. 28 310–317 10.1016/j.janxdis.2014.01.005 [DOI] [PubMed] [Google Scholar]
- Blais R. K., Hoerster K. D., Malte C., Hunt S., Jakupcak M. (2014). Unique PTSD clusters predict intention to seek mental health care and subsequent utilization in US veterans with PTSD symptoms. J. Trauma Stress 27 168–174 10.1002/jts.21898 [DOI] [PubMed] [Google Scholar]
- Cahill L., Alkire M. T. (2003). Epinephrine enhancement of human memory consolidation: interaction with arousal at encoding. Neurobiol. Learn. Mem. 79 194–198 10.1016/S1074-7427(02)00036-9 [DOI] [PubMed] [Google Scholar]
- Cigrang J. A., Talcott G. W., Tatum J., Baker M., Cassidy D., Sonnek S., et al. (2014). Impact of combat deployment on psychological and relationship health: a longitudinal study. J. Trauma Stress 27 58–65 10.1002/jts.21890 [DOI] [PubMed] [Google Scholar]
- Costanzo M. E., Leaman S., Jovanovic T., Norrholm S. D., Rizzo A. A., Taylor P., et al. (2014). Psychophysiological response to virtual reality and subthreshold posttraumatic stress disorder symptoms in recently deployed military. Psychosom. Med. 76 670–677 10.1097/PSY.0000000000000109 [DOI] [PubMed] [Google Scholar]
- Cukor J., Wyka K., Jayasinghe N., Difede J. (2010). The nature and course of subthreshold PTSD. J. Anxiety Disord. 24 918–923 10.1016/j.janxdis.2010.06.017 [DOI] [PubMed] [Google Scholar]
- Feder A., Nestler E. J., Charney D. S. (2009). Psychobiology and molecular genetics of resilience. Nat. Rev. Neurosci. 10 446–457 10.1038/nrn2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes D., Creamer M., Biddle D. (2001). The validity of the PTSD checklist as a measure of symptomatic change in combat-related PTSD. Behav. Res. Ther. 39 977–986 10.1016/S0005-7967(00)00084-X [DOI] [PubMed] [Google Scholar]
- Freeman D., Antley A., Ehlers A., Dunn G., Thompson C., Vorontsova N., et al. (2014). The use of immersive virtual reality (VR) to predict the occurrence 6 months later of paranoid thinking and posttraumatic stress symptoms assessed by self-report and interviewer methods: a study of individuals who have been physically assaulted. Psychol. Assess. 26 841–847 10.1037/a0036240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves R., Pedrozo A. L., Coutinho E. S., Figueira I., Ventura P. (2012). Efficacy of virtual reality exposure therapy in the treatment of PTSD: a systematic review. PLoS ONE 7:e48469 10.1371/journal.pone.0048469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonneaud J., Piolino P., Lecouvey G., Madeleine S., Orriols E., Fleury P., et al. (2012). “Assessing prospective memory in young health adults using virtual reality,” in Proceedings of the International Conference on Disability, Virtual Reality, and Associated Technologies (Reading: The University of Reading; ). [Google Scholar]
- Grubaugh A. L., Magruder K. M., Waldrop A. E., Elhai J. D., Knapp R. G., Frueh B. C. (2005). Subthreshold PTSD in primary care: prevalence, psychiatric disorders, healthcare use, and functional status. J. Nerv. Ment. Dis. 193 658–664 10.1097/01.nmd.0000180740.02644.ab [DOI] [PubMed] [Google Scholar]
- Jensen J. M., Fraser M. W. (2005). “A risk and resilience framework for child, youth, and family policy,” in Social Policy for Children and Families: A Risk and Resilience Perspective, eds. Jensen J. M., Fraser M. W. (Thousand Oaks, CA: Sage Publications; ). [Google Scholar]
- Kramer T. L., Savary P. E., Pyne J. M., Kimbrell T. A., Jegley S. M. (2013). Veteran perceptions of virtual reality to assess and treat posttraumatic stress disorder. Cyberpsychol. Behav. Soc. Netw. 16 293–301 10.1089/cyber.2013.1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledoux T., Nguyen A. S., Bakos-Block C., Bordnick P. (2013). Using virtual reality to study food cravings. Appetite 71 396–402 10.1016/j.appet.2013.09.006 [DOI] [PubMed] [Google Scholar]
- Liberzon I., Abelson J. L., Flagel S. B., Raz J., Young E. A. (1999). Neuroendocrine and psychophysiologic responses in PTSD: a symptom provocation study. Neuropsychopharmacology 21 40–50 10.1016/S0893-133X(98)00128-126 [DOI] [PubMed] [Google Scholar]
- Magruder K. M., Frueh B. C., Knapp R. G., Johnson M. R., Vaughan J. A., III, Carson T. C., et al. (2004). PTSD symptoms, demographic characteristics, and functional status among veterans treated in VA primary care clinics. J. Trauma Stress 17 293–301 10.1023/B:JOTS.0000038477.47249.c8 [DOI] [PubMed] [Google Scholar]
- Marra C., Ogilvie G., Gastonguay L., Colley L., Taylor D., Marra F. (2009). Patients with genital warts have a decreased quality of life. Sex. Transm. Dis. 36 258–260 10.1097/OLQ.0b013e318191a55e [DOI] [PubMed] [Google Scholar]
- McLay R., Ram V., Murphy J., Spira J., Wood D. P., Wiederhold M. D., et al. (2014). Effect of virtual reality PTSD treatment on mood and neurocognitive outcomes. Cyberpsychol. Behav. Soc. Netw. 17 439–446 10.1089/cyber.2013.0383 [DOI] [PubMed] [Google Scholar]
- McLay R. N., Wood D. P., Webb-Murphy J. A., Spira J. L., Wiederhold M. D., Pyne J. M., et al. (2011). A randomized, controlled trial of virtual reality-graded exposure therapy for post-traumatic stress disorder in active duty service members with combat-related post-traumatic stress disorder. Cyberpsychol. Behav. Soc. Netw. 14 223–229 10.1089/cyber.2011.0003 [DOI] [PubMed] [Google Scholar]
- Mead H. K., Beauchaine T. P., Shannon K. E. (2010). Neurobiological adaptations to violence across development. Dev. Psychopathol. 22 1–22 10.1017/S0954579409990228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molecular Devices Corporation. (2012). Molecular Devices SoftMax Pro Software User Guide - 0112-0140 Rev A. Sunnyvale, CA: Molecular Devices Corporation. [Google Scholar]
- Norrholm S. D., Jovanovic T. (2010). Tailoring therapeutic strategies for treating posttraumatic stress disorder symptom clusters. Neuropsychiatr. Dis. Treat. 6 517–532 10.2147/NDT.S10951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons T. D., Rizzo A. (2008). Neuropsychological assessment of attentional processing using virtual reality. Annu. Rev. Cyber. Telemed. 6 23–28. [Google Scholar]
- Pietrzak R. H., Goldstein M. B., Malley J. C., Rivers A. J., Southwick S. M. (2010). Structure of posttraumatic stress disorder symptoms and psychosocial functioning in veterans of operations enduring freedom and Iraqi freedom. Psychiatry Res. 178 323–329 10.1016/j.psychres.2010.04.039 [DOI] [PubMed] [Google Scholar]
- Rauch S., Foa E. B. (2006). Emotional processing theory (EPT) and exposure therapy for PTSD. J. Contemp. Psychol. 36 61–65 10.1007/s10879-006-9008-y [DOI] [Google Scholar]
- Reger G. M., Holloway K. M., Candy C., Rothbaum B. O., Difede J., Rizzo A. A., et al. (2011). Effectiveness of virtual reality exposure therapy for active duty soldiers in a military mental health clinic. J. Trauma Stress 24 93–96 10.1002/jts.20574 [DOI] [PubMed] [Google Scholar]
- Rizzo A. S., Difede J., Rothbaum B. O., Reger G., Spitalnick J., Cukor J., et al. (2010). Development and early evaluation of the virtual Iraq/Afghanistan exposure therapy system for combat-related PTSD. Ann. N. Y. Acad. Sci. 1208 114–125 10.1111/j.1749-6632.2010.05755.x [DOI] [PubMed] [Google Scholar]
- Rothbaum B. O., Price M., Jovanovic T., Norrholm S. D., Gerardi M., Dunlop B., et al. (2014). A randomized, double-blind evaluation of D-cycloserine or alprazolam combined with virtual reality exposure therapy for posttraumatic stress disorder in Iraq and Afghanistan War Veterans. Am. J. Psychiatry 171 640–648 10.1176/appi.ajp.2014.13121625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M. J., Costanzo M. E., Jovanovic T., Leaman S., Taylor P., Norrholm S. D., et al. (2013). Heart rate response to fear conditioning and virtual reality in subthreshold PTSD. Stud. Health Technol. Inform. 191 115–119. [PubMed] [Google Scholar]
- Roy M. J., Francis J., Friedlander J., Banks-Williams L., Lande R. G., Taylor P., et al. (2010). Improvement in cerebral function with treatment of posttraumatic stress disorder. Ann. N. Y. Acad. Sci. 1208 142–149 10.1111/j.1749-6632.2010.05689.x [DOI] [PubMed] [Google Scholar]
- Schnurr P. P., Lunney C. A., Bovin M. J., Marx B. P. (2009). Posttraumatic stress disorder and quality of life: extension of findings to veterans of the wars in Iraq and Afghanistan. Clin. Psychol. Rev. 29 727–735 10.1016/j.cpr.2009.08.006 [DOI] [PubMed] [Google Scholar]
- Soeter M., Kindt M. (2011). Noradrenergic enhancement of associative fear memory in humans. Neurobiol. Learn. Mem. 96 263–271 10.1016/j.nlm.2011.05.003 [DOI] [PubMed] [Google Scholar]
- Southwick S. M., Davis M., Horner B., Cahill L., Morgan C. A., III, Gold P. E., et al. (2002). Relationship of enhanced norepinephrine activity during memory consolidation to enhanced long-term memory in humans. Am. J. Psychiatry 159 1420–1422 10.1176/appi.ajp.159.8.1420 [DOI] [PubMed] [Google Scholar]
- Veterans Health Administration. (2014). Analysis of VA Health Care Utilization Among Operation Enduring Freedom, Operation Iraqi Freedom, and Operation New Dawn Veterans. Washington, DC: Office of Public Health. [Google Scholar]
- Ware J. E., Jr., Sherbourne C. D. (1992). The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 30 473–483 10.1097/00005650-199206000-00002 [DOI] [PubMed] [Google Scholar]
- Young E. A., Breslau N. (2004). Cortisol and catecholamines in posttraumatic stress disorder: an epidemiologic community study. Arch. Gen. Psychiatry 61 394–401 10.1001/archpsyc.61.4.394 [DOI] [PubMed] [Google Scholar]