Abstract
Seed dormancy controls germination and plays a critical role in regulating the beginning of the life cycle of plants. Seed dormancy is established and maintained during seed maturation and is gradually broken during dry storage (after-ripening). The plant hormone abscisic acid (ABA) and DELAY OF GERMINATION1 (DOG1) protein are essential regulators of seed dormancy. Recent studies revealed that chromatin modifications are also involved in the transcription regulation of seed dormancy. Here, we showed that two Arabidopsis histone demethylases, LYSINESPECIFIC DEMETHYLASE LIKE 1 and 2 (LDL1 and LDL2) act redundantly in repressing of seed dormancy. LDL1 and LDL2 are highly expressed in the early silique developing stage. The ldl1 ldl2 double mutant displays increased seed dormancy, whereas overexpression of LDL1 or LDL2 in Arabidopsis causes reduced dormancy. Furthermore, we showed that LDL1 and LDL2 repress the expression of seed dormancy-related genes, including DOG1, ABA2 and ABI3 during seed dormancy establishment. Furthermore, genetic analysis revealed that the repression of seed dormancy by LDL1 and LDL2 requires DOG1, ABA2, and ABI3. Taken together, our findings revealed that LDL1 and LDL2 play an essential role in seed dormancy.
Keywords: histone demethylase, seed dormancy, ABA, gene expression, DOG1
Introduction
Accurate timing of seed germination requires a reliable control mechanism. Seed dormancy is a major factor in this control, which refers to the seed property that incapacitates seed germination even under optimal conditions (Hilhorst, 2007). Seed dormancy prevents or delays the germination of maturated seed until conditions are favorable for starting a new life cycle. Seed dormancy is established during seed maturation, and dormancy has been shown to be imposed by the embryo, testa, endosperm or combinations of these tissues (Kim et al., 2013). Seed dormancy can be broken after a period of seed after-ripening or on seed stratification, that is, exposure to cold and moist conditions.
Diverse endogenous and environmental factors including phytohormones, nutrients, temperature and light affect seed dormancy through different pathways (Finkelstein et al., 2008). Emerging evidences have shown that abscisic acid (ABA) plays a critical role in the establishment and maintenance of seed dormancy (Finch-Savage and Leubner-Metzger, 2006; Holdsworth et al., 2008; North et al., 2010). Genetic studies demonstrate that loss-of-function mutants of ABA biosynthesis genes in Arabidopsis such as ABA1, ABA2, ABA3, and NCED6/9 show reduced seed dormancy (Koornneef et al., 1982; Giraudat et al., 1992; Leon-Kloosterziel et al., 1996; Lefebvre et al., 2006; Okamoto et al., 2006), whereas loss-of-function mutants of ABA catabolism genes such as CYP707A1, CYP707A2, and CYP707A3 display enhanced seed dormancy, supporting an essential role of ABA for seed dormancy (Kushiro et al., 2004; Okamoto et al., 2006; Finkelstein et al., 2008; Holdsworth et al., 2008) Furthermore, many components involved in ABA signaling transduction also influence the degree of seed dormancy. abi1-1, a gain-of-function mutant of ABI1 encoding a member of group A protein phosphatase 2Cs (group A PP2Cs), shows reduced seed dormancy (Koornneef et al., 1984; Finkelstein, 1994). Furthermore, ABI3, a seed-specific B3 domain-containing transcription factor, was revealed to be necessary for the establishment of seed dormancy (Sugliani et al., 2009).
Seed dormancy is a typical quantitative trait. In Arabidopsis thaliana, the Columbia-0 (Col-0) and Landsberg erecta (Ler) ecotypes display relatively weak seed dormancy, whereas the Cape Verde Islands (Cvi) ecotype shows strong seed dormancy (Alonso-Blanco et al., 2003). Analysis of quantitative trait loci (QTL) using recombinant inbred lines between Ler and Cvi identified several Delay of Germination (DOG) genes (Alonso-Blanco et al., 2003). Among them, DOG1 is the master regulator, which is only expressed in seeds and its expression level is increased during seed maturation (Bentsink et al., 2006). In freshly harvested seeds, the time required for dormancy release is determined by the DOG1 protein level. Furthermore, it was proposed that DOG1 acts independent of ABA signaling (Nakabayashi et al., 2012). Collectively, these findings suggested that both ABA and DOG1 are required for seed dormancy.
Recent studies suggested an involvement of epigenetic regulators in seed dormancy and germination (Liu et al., 2014). REDUCED DORMANCY 4 (RDO4)/HISTONE MONOUBIQUITINATION 1 (HUB1) and its homolog HUB2 influence seed dormancy through ubiquitination of H2B, leading to changes in histone H3 methylation of the seed dormancy-related genes (Liu et al., 2007). FERTILIZATION INDEPENDENT ENDOSPERM (FIE), an essential component of the polycomb repressive complex 2 (PRC2,) reduces seed dormancy by repressing ABI3, ABA/GA signaling factors and DOG1 expression (Bouyer et al., 2011). Furthermore, the histone methyltransferases KRYPTONITE (KYP)/SUVH4 and SUVH5 repress DOG1 and ABI3 expression during seed maturation (Zheng et al., 2012). More recently, it was reported that histone deacetylase9 (hda9) mutants display reduced seed dormancy. Transcriptome analysis revealed that HDA9 repressed the expression of photosynthesis and photoautotrophic growth-related genes in dry seeds (van Zanten et al., 2014). Collectively, these findings revealed that chromatin modifications, including histone acetylation, methylation and ubiquitination are required for the transcriptional regulation of seed dormancy.
LDL1 and LDL2, two Arabidopsis homolog of the human LYSINESPECIFIC DEMETHYLASE 1 (LSD1), have been reported to play an important role in control of flowering. LDL1 and LDL2 reduce the histone H3-Lys 4 methylation levels in chromatin of the floral repressors FLOWERING LOCUS C (FLC) and FWA. Loss-of-function mutants of LDL1 and LDL2 show increased expression levels of FLC and FWA and late flowering phenotype (Jiang et al., 2007). Here in our present work, we showed that LDL1 and LDL2 act redundantly in repressing of seed dormancy. The ldl1 or ldl2 single mutant do not change the seed dormancy level, while the ldl1 ldl2 double mutants display strong increased seed dormancy, what's more, overexpression of LDL1 or LDL2 in Arabidopsis causes reduced seed dormancy. Furthermore, LDL1 and LDL2 repress the expression levels of dormancy-related genes, including DOG1, ABA2, and ABI3 in maturating seeds. Our studies suggest that LDL1 and LDL2 play an essential role in regulating primary seed dormancy by mediating DOG1 expression and ABA signaling pathway.
Results
Subcellular localization of LDL1 and LDL2
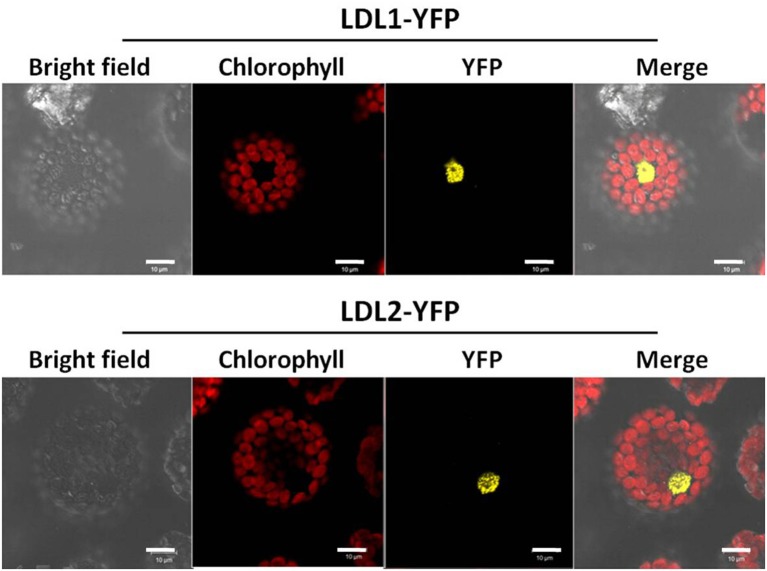
To investigate the subcellular localization of LDL1 and LDL2 proteins, full-length coding sequences of LDL1 and LDL2 fused with yellow fluorescent protein (YFP) were delivered to the Arabidopsis protoplasts. LDL1-YFP and LDL2-YFP proteins were observed to be localized in the nucleus of the protoplast (Figure 1).
Figure 1.
Subcellular localization analysis of LDL1 and LDL2. Constructs of LDL1-YFP and LDL2-YFP were transfected into Arabidopsis protoplasts. The fluorescence signal was detected with a laser scanning confocal microscope. YFP indicates fluorescence of YFP, and the red color indicates the auto-fluorescence of chlorophyll. The length of the bar is 10 μm.
Expression patterns of LDL1 and LDL2 during seed maturation
To characterize the roles of LDL1 and LDL2 in plant development, we first checked their expression patterns through the public Arabidopsis microarray database (http://www.bar.utoronto.ca/efp/cgi-bin/efpWeb.cgi). LDL1 and LDL2 are highly expressed in seed development stage (Supplemental Figure 1). We further investigated the expression patterns of LDL1 and LDL2 by quantitative RT-PCR assays. Consistently, relatively higher expression levels of LDL1 and LDL2 were detected at 3 and 6 DPA (days post-anthesis) siliques, whereas the expression levels of LDL1 and LDL2 were gradually decreased from 9 DPA (Figure 2). Our data reveal a possible role of LDL1 and LDL2 in seed development.
Figure 2.
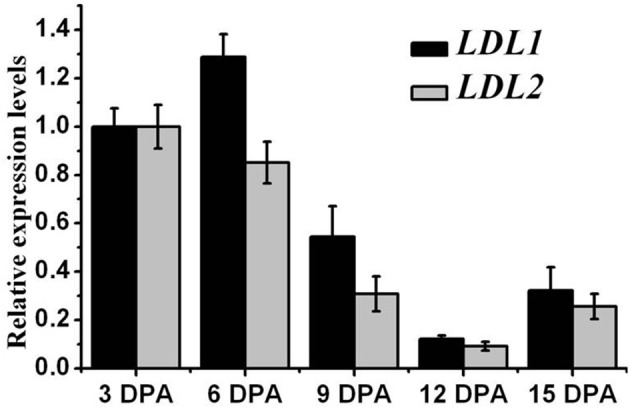
Expression patterns of LDL1 and LDL2 during seed maturation. DPA, days post-anthesis. UBQ10 was used as an internal control. At least three biological replicates were conducted. The average (±SD) values are shown.
Mutations in LDL1 and LDL2 increase primary seed dormancy
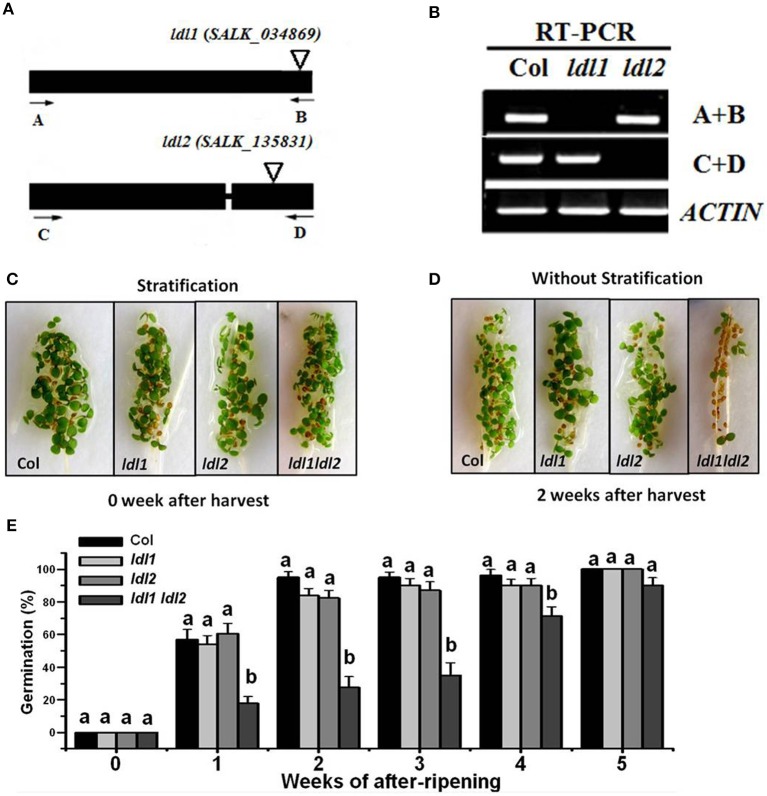
To study the function of LDL1 and LDL2, two T-DNA insertion mutants, ldl1 (SALK_034869) and ldl2 (SALK_135831) were analyzed (Figure 3A). RT-PCR analyses showed that the transcripts of the full-length LDL1 and LDL2 were disrupted in ldl1 and ldl2 mutants (Figure 3B). The ldl1 ldl2 double mutant was also generated by genetic crossing. The freshly harvested seeds of Col, ldl1, ldl2, and ldl1 ldl2 with stratification treatment were all well germinated (Figure 3C). Then, the germination rates of the ldl1, ldl2, and ld1 ldl2 mutants were scored after stored in dry conditions for different times. After 1–5 weeks of storage, the ldl1 and ldl2 single mutants display no significant effect on seed germination, however, the ldl1 ldl2 double mutant shows a significant decrease of seed germination (Figures 3D,E), suggesting that LDL1 and LDL2 may be functionally redundant in repressing of seed dormancy.
Figure 3.
ldl1 ldl2 double mutant shows increased primary seed dormancy. (A) The gene structures and T-DNA insertion sites of ldl1 and ldl2. The black boxes, lines and triangles indicate exons, introns and T-DNA insertions, respectively. (B) RT-PCR analysis of the expression levels of LDL1 and LDL2 in ldl1 and ldl2 alleles. Leaves of 15-day-old plants were harvested for analysis. The sites of the primer pairs used for RT-PCR analysis were indicated with arrows in (A). ACTIN was used as a loading control. (C) Visualization of seed germination of ldl1, ldl2, and ldl1 ldl2 siliques 0 week after harvest. The siliques were stratified and sown on water-saturated filter papers for 7 d. (D) Visualization of seed germination of ldl1, ldl2, and ldl1 ldl2 siliques 2 week after harvest. The siliques were sown on water-saturated filter papers for 7 d without stratification. (E) Quantification of germination rates of non-stratified seeds of ldl1, ldl2, and ldl1 ldl2 with different periods of after-ripening. The germination rates were scored 5 d after plated on 1/2 MS medium. The average (±SD) values are shown. One-Way ANOVA (Tukey-Kramer test) analysis was performed, and statistically significant differences (P < 0.01) were indicated by different lowercase letters (a, b). Equivalent means have the same letter; different letters indicate statistically significant differences.
Overexpression of LDL1 or LDL2 reduces primary seed dormancy
To further investigate the effect of LD1 and LDL2 on seed dormancy, we generated LDL1 and LDL2 overexpression plants. The coding regions of LDL1 and LDL2 were introduced to the vector pCAMBIA-1302 under the control of the Cauliflower Mosaic Virus (CaMV) 35S promoter, separately. Increased expression levels of LDL1 and LDL2 were detected in the transgenic plants (Figure 4A). The LDL1 and LDL2 overexpression lines display enhanced seed germination compared with the wild-type (Figures 4B,C), confirming a negative role of LDL1 and LDL2 in primary seed dormancy. Collectively, our findings suggest that LDL1 and LDL2 may function redundantly in the repression of primary seed dormancy in Arabidopsis.
Figure 4.
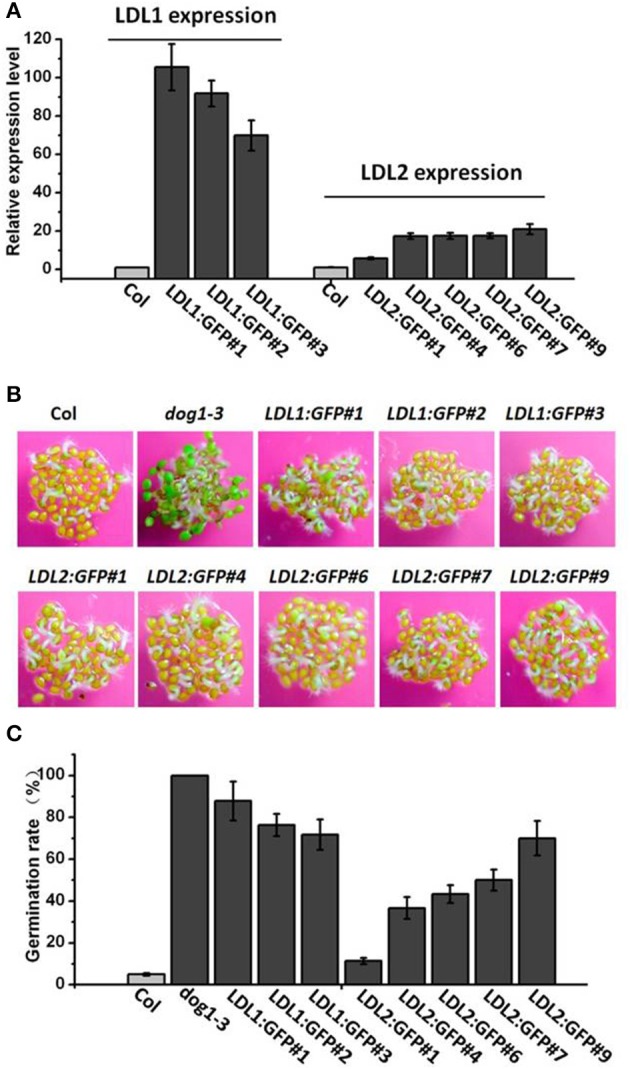
Overexpression of LDL1 or LDL2 in Arabidopsis reduces seed dormancy. (A) Quantitative analysis of the expression levels of LDL1 and LDL2 in the GFP-tagged transgenic lines. Leaves of 15-day-old plants were harvested for analysis. UBQ10 was used as an internal control. (B) Visualization of seed germination of LDL1 and LDL2 overexpression lines. (C) Quantification of germination rates of with 4 days' after-ripening after 3 days on 1/2 MS. The average (±SD) values are shown. The seeds of 4 d after-ripening were plated on 1/2 MS medium for 3 d.
ldl1 ldl2 mutant shows increased ABA sensitivity during seed germination
Previous works reported that the mutants with a deep degree of seed dormancy such as the histone methyltransferase mutant kyp/suvh4 are hypersensitive to ABA. We further tested the sensitivity of ldl1 ldl2 mutant to ABA during germination. The ABA insensitive mutants, abi3-sk11 (Park et al., 2011) and dog1-3, were also analyzed. Here dog1-3 used in our study shown different to dog1, which is a sensitive ABA mutant (Bentsink et al., 2006), this might be explained by two Arabidopsis accessions were used, dog1 mutant is in Ler background, whereas dog1-3 is in Columbia background. Opposite to abi3-sk11 and dog1-3, the ldl1 ldl2 seeds plated on 1/2 MS medium supplemented with ABA display enhanced sensitivity compared with the wild-type (Figure 5), suggesting that LDL1 and LDL2 might be involved in ABA signaling pathway.
Figure 5.
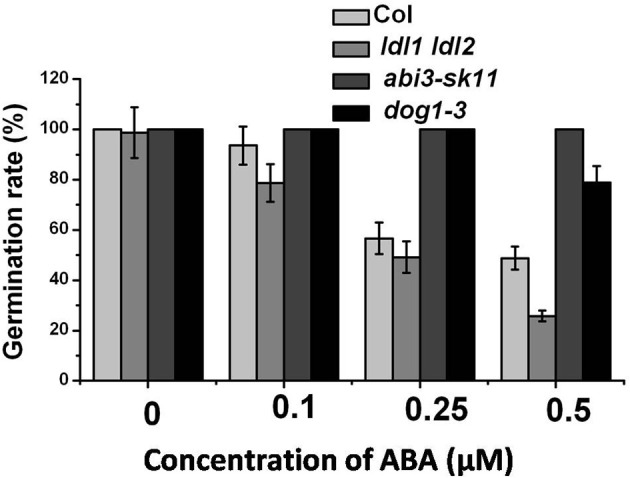
ldl1 ldl2 increases ABA sensitivity during seed germination. The seeds of 10 d after-ripening were stratified and plated on 1/2 MS medium supplemented with various concentrations of ABA. The germination rates were scored 3 d after plating. The average (±SD) values are shown.
LDL1 and LDL2 repress ABA2, ABI3 and ABI5 expression
Previous reports demonstrated that the ABA biosynthesis genes, ABA1, ABA2, ABA3, AAO3, NCED3, and NCED9, and the ABA signal transduction-related factors, ABI3, ABI4, and ABI5, play key roles in seed dormancy (Seo et al., 2000, 2006; Iuchi et al., 2001; Lopez-Molina et al., 2001; Xiong et al., 2001; Gonzalez-Guzman et al., 2002; Sugliani et al., 2010). We further detected the expression levels of these genes in ldl1 ldl2 mutants. Among the tested ABA biosynthesis genes, the transcript of ABA2 was significantly increased at 3 DPA and 6 DPA in ldl1 ldl2 mutants (Figure 6A). Notably, the expression levels of the ABA signaling transduction components, ABI3 and ABI5, were significantly up-regulated at 12 and 15 DPA, respectively, in ldl1 ldl2 mutants (Figure 6B). Taken together, the above results indicated that LDL1 and LDL2 may decrease primary seed dormancy by repressing ABA biosynthesis and signaling transduction related gene expression.
Figure 6.
Expression levels of ABA biosynthesis and signal transduction-related genes in ldl1 ldl2 during seed maturation. (A) Transcription analysis of genes related to ABA biosynthesis in Col and ldl1 ldl2. (B) Transcription analysis of genes related to ABA signal transduction in Col and ldl1 ldl2. UBQ10 was used as an internal control. At least three biological replicates were conducted. The average (±SD) values are shown. One-Way ANOVA (Tukey-Kramer test) analysis was performed, and statistically significant differences (P < 0.01) were indicated by different lowercase letters (a, b). Equivalent means have the same letter; different letters indicate statistically significant differences.
LDL1 and LDL2 repress DOG1 expression
Previous work has demonstrated that DOG1 is the master regulator of seed dormancy which is only expressed in seed and its expression level increases during seed maturation (Bentsink et al., 2006). We further detect the expression level of DOG1 in ldl1 ldl2 mutant. A previous report revealed that the transcription levels of DOG1 reach the peak at around 16 DPA (Nakabayashi et al., 2012). In present work, the DOG1 expression accumulates the highest at 9 DPA (Figure 7), this may due to a different maturation speed of siliques under the specific environmental conditions in which the plants were growing. In present work, the seed development from pollination until fully mature ripe seeds needs 15 days. The transcription of DOG1 was significantly enhanced in ldl1 ldl2 compared with the wild-type at 9 and 12 DPA (Figure 7).
Figure 7.
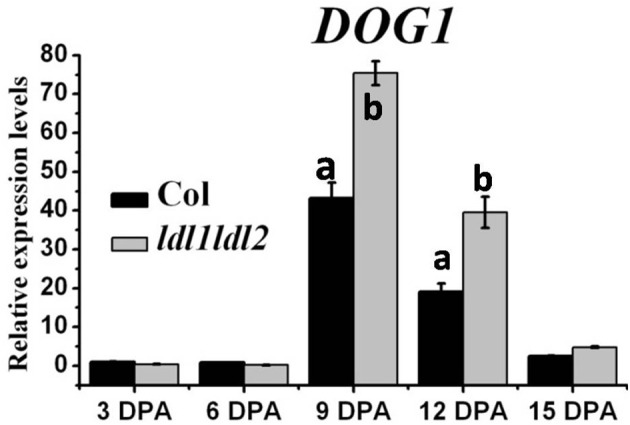
Transcription analysis of DOG1 expression in Col and ldl1 ldl2 during seed maturation. UBQ10 was used as an internal control. At least three biological replicates were conducted. The average (±SD) values are shown. One-Way ANOVA (Tukey-Kramer test) analysis was performed, and statistically significant differences (P < 0.01) were indicated by different lowercase letters (a, b). Equivalent means have the same letter; different letters indicate statistically significant differences.
Genetic relationship of LDL1 and LDL2 with DOG1, ABA2 and ABI3
Elevated ABA2, ABI3 and DOG1 expression in ldl1 ldl2 mutants during seed maturation prompted us to analyze the genetic relationship between LDL1,LDL2 and ABA2, ABI3 and DOG1. We crossed ldl1 ldl2 with dog1-3, aba2-1 and abi3-sk11 mutants, respectively. As results, similar to dog1-3, ldl1 ldl2 dog1-3 seeds were completely non-dormant (Figures 8A,B). Furthermore, the triple mutants ldl1 ldl2 aba2-1 and ldl1 ldl2 abi3-sk11 show an increase of germination rate compared with ldl1 ldl2 mutants, respectively (Figures 8A,B). These data suggested that DOG1, ABA2 and ABI3 are required for LDL1 and LDL2-mediated repressing of seed dormancy.
Figure 8.
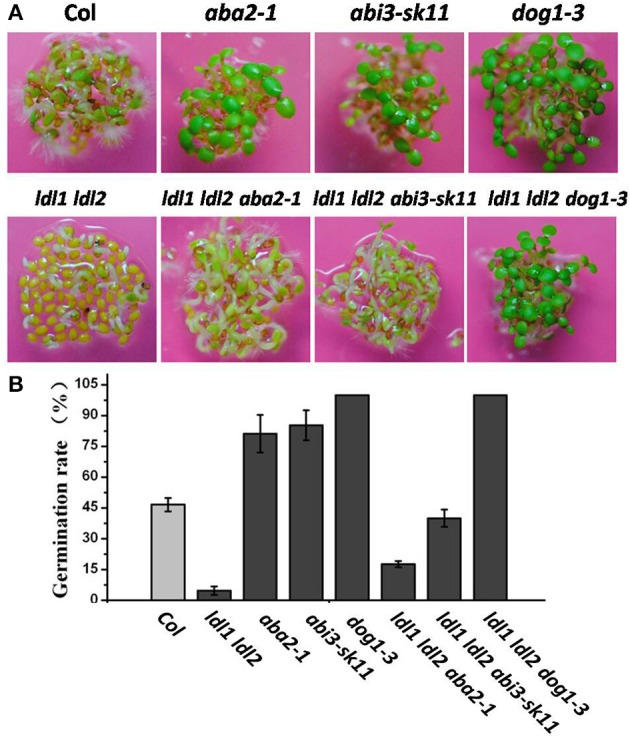
Genetic analysis of the seed dormancy levels of ldl1 ldl2 in dog1-3, abi3-sk11 or aba2-1 backgrounds. (A) Visualization of seed germination of the mutants. (B) Quantification of the germination rates of the mutants. The average (±SD) values are shown. The seeds of 10 d after-ripening were used for analysis. The germination rates were scored 3 d after plated on 1/2 MS medium.
Discussion
In present work, we provided evidences that histone demethylases LDL1 and LDL2 are required for seed dormancy in Arabidopsis. Mutation in LDL1 or LDL2 do not change seed dormancy level, whereas ldl1 ldl2 double mutant displays strong increased seed dormancy. In contrast, overexpression of LDL1 or LDL2 in Arabidopsis strongly decreases seed dormancy level. Taken together, our findings indicated that LDL1 and LDL2 play an essential role in seed dormancy.
ABA has been proved to play a critical role in establishment of seed dormancy. During seed maturation, the expression levels of the genes involved in ABA biosynthesis are increased and ABA signaling responses are enhanced (Xiong and Zhu, 2003). Consistently, loss-of-function mutants of ABA biosynthesis and signaling transduction components, such as ABA2, a key ABA biosynthetic gene, and ABI3 which encodes a seed-specific B3 domain-containing DNA binding protein, show reduced primary seed dormancy levels in Arabidopsis (Lopez-Molina et al., 2002). In this study, loss-of-function and gain-of-function analysis revealed a negative role of LDL1 and LDL2 in primary seed dormancy. Increased ABA sensitivity of ldl1 ldl2 seeds during germination indicates that LDL1 and LDL2 may regulate seed dormancy through ABA signaling pathway. Transcriptional analysis showed that the expression levels of ABA2 and ABI3 are increased in ldl1 ldl2, suggesting that LDL1 and LDL2 may control seed dormancy level by regulating both the ABA biosynthesis and signaling transduction. Additionally, the expression level of ABI5, a downstream target of ABI3, was also elevated in ldl1 ldl2 mutants, confirming the effect of LDL1 and LDL2 in ABA signaling transduction. Genetic analysis indicated that mutation of either aba2 or abi3 in ldl1 ldl2 background resulted in reduced seed dormancy compared with ldl1 ldl2, suggesting that ABA2 and ABI3 are required for LDL1 and LDL2 repressed seed dormancy. Since endogenous ABA level changes also affect the expression level of ABI3 (Zhang et al., 2005), the increased expression level of ABI3 and ABI5 may be pronounced by elevated ABA2 transcription level in ldl1 ldl2. Further study is required to determine whether these genes are direct targets of LDL1 and LDL2 in maturating seeds.
DOG1 was recently identified as a major regulator of seed dormancy independent of ABA in Arabidopsis thaliana (Nakabayashi et al., 2012). The protein level of DOG1 in freshly harvested seeds highly correlate with dormancy. The transcription and protein levels of DOG1 gradually increase with the seed maturation (Nakabayashi et al., 2012). In the present study, we found that the transcription level of DOG1 was significantly up-regulated in the ldl1 ldl2 mutant, indicating LDL1 and LDL2 regulate DOG1 expression. Furthermore, the genetic analysis showed that the triple mutant ldl1 ldl2 dog1 is completely non-dormant, suggesting that dog1 mutation is epistatic to ldl1 ldl2 double mutant in seed dormancy.
Recent studies of natural variation in Arabidopsis showed that late flowering is correlated with higher seed dormancy. The late flowering mutants constans (co) and flowering locus t (ft) display strong increased seed dormancy (Penfield and Hall, 2009; Debieu et al., 2013). Furthermore, lower temperature during the vegetative phase delays flowering time and causes a large increase in the dormancy of seeds produced later on the plants. It was found that maternal past and current temperature experience are transduced to the FT locus in silique phloem. In turn, FT controls seed dormancy through inhibition of proanthocyanidin synthesis in fruits, resulting in altered seed coat tannin content (Chen et al., 2014). Similar to co and ft mutants, we showed that the late flowering mutant ldl1 ldl2 also displays strong increased seed dormancy. Since LDL1 and LDL2 repress FLC expression (Jiang et al., 2007) and FT acts downstream of FLC (Helliwell et al., 2006) in control of flowering, LDL1 and LDL2 may also regulate seed dormancy through regulating FLC and FT expression.
Interestingly, mutations of the histone methyltransferase SUVH4 also lead to increased seed dormancy. The expression levels of DOG1, ABI3, and ABI4 were elevated in suvh4 (kyp-2) mutant in maturation seeds (Zheng et al., 2012). In present work, an increase of expression of DOG1 and ABI3 was detected in ldl1 ldl2 mutant, whereas the transcription of ABI4 was not significantly altered. Further research is required to determine whether LDL1, LDL2, and SUVH4 repress seed dormancy through regulating the same target genes such as DOG1 and ABI3.
Materials and methods
Plant materials
Arabidopsis ecotype Col-0 was used in all experiments, the abi3-sk11 (SALK_023411) (Park et al., 2011), ldl1 (SALK_034869), ldl2 (SALK_135831), dog1-3 (SALK_000867), and aba2-1 (CS156), which are in the Col background, were obtained from Nottingham Arabidopsis Stock Centre (NASC). The ldl1 ldl2 double mutant was generated by crossing ldl1 with ldl2, and the ldl1 ldl2 dog1-3, ldl1 ldl2 abi3-sk11, and ldl 1ldl2 aba2-1 triple mutants were generated by crossing dog1-3, abi3-sk11, and aba2-1 plants with ldl1 ldl2 plants, respevtively. For generation of the LDL1 or LDL2 overexpression lines, the full-length open reading frame (ORF) of LDL1 or LDL2 was subcloned to pCAMBIA1302 vector under the control of the CaMV 35S promoter with specific primers (Supplemental Table 1), then these constructs were transformed to Col plants following the floral dip assay (Clough and Bent, 1998). The T3 homozygous transgenic plants were used for phenotypic analysis. All the Arabidopsis plants were grown at 22°C under long-day (16 h light/8 h dark) conditions. To reduce variations, all genotypes tested in each experiment were grown together and harvested at the same time when most siliques turn brown.
Subcellular localization analysis
The coding sequences of LDL1 and LDL2 without the stop codon were amplified by PCR primers (listed in Supplemental Table 1) and then subcloned into the pSAT6-EYFP-N1 vector and fused in-frame with the Yellow Fluorescent Protein (YFP) sequence under the control of the CaMV 35S promoter. The fusion constructs were introduced into Arabidopsis protoplasts by using 40% polyethylene glycol (PEG) as described previously (Yoo et al., 2007). YFP fluorescence was observed with a laser scan confocal microscope (Leica TCS SP2, Leica Microsystems, Wetzlar, Germany). The transient expression assay was repeated three times.
Germination assay
For the seed dormancy analysis, to make sure that all freshly harvested seeds mature at the same time, we carefully selected plants with siliques that matured at the same time. For the time course of after-ripening germination assay, the seeds or siliques were directly sown on 1/2 MS medium without stratification. For the ABA sensitivity germination assay, only seeds that matured at the same time were selected. After 2 weeks of after-ripening, seeds were sown on 1/2 MS medium supplemented with or without ABA, and then incubated at 4°C for 4 d for stratification or without stratification. The seeds were then placed in a growth chamber at 22°C under long day conditions. Seeds were counted as germinated when the radicle tip had fully penetrated the seed coat (radicle protrusion), and germinated seeds were scored at the indicated times. The statistical analyses were performed with three biological replicates.
RNA isolation and real-time PCR analysis
Total RNA was isolated from developing siliques (20 siliques) or germination seeds (0.2 g) using 1 mL extraction buffer [0.1 M Tris-HCl, pH 8.0, 0.05 M ethylenediaminetetraacetic acid (EDTA) (pH 8.0), 2% (wt/vol) hexadecyltrimethylammonium bromide (CTAB), 2% (wt/vol) polyvinylpyrrolidone (PVP), 2 M NaCl, 3% β-mercaptoethanol (vol/vol)]. The first strand cDNA synthesis was generated using 2 μg total RNA according to the manufacturer's instructions of TransScript™ One-Step gDNA Removal and cDNA Synthesis SuperMix Kit (TransGen, Beijing). 100 ng synthesized cDNA was used as a template to perform real-time RT-PCR analysis. PCR reactions were performed in the total volume of 20 μL, with 0.5 μL for each primer (10 mM, final concentration 100 nM) and 10 μL for SYBR® Green PCR Supermix (Bio-Rad Laboratories) on a ABI7500 Real-Time PCR System (Applied Biosystems). The PCR program included an initial denaturation step at 94°C for 3 min, followed by 40 cycles of 5 s at 94°C and 1 min at 60°C. Each sample was quantified at least triplicate and normalized using Ubiquitin 10 (UBQ) as an internal control. The gene-specific primer pairs for quantitative Real-Time PCR were listed in Supplemental Table 1. All PCR reactions were normalized using Ct value corresponding to the reference gene UBQ. The relative expression levels of target genes were calculated with formula 2−ddCt (Livak and Schmittgen, 2001). Values represented the average of three biological replicates.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by grants from the National Basic Research Program of China (973 program No. 2012CB910900), the National Natural Science Foundation of China (No. 31301056, No. 31128001, and No. 31371308). This work was also supported by the National Science Council of Taiwan (101-2923-B-002-005-MY3, 101-2311-B-002-012-MY3 and 102-2321-B-002-074-) and National Taiwan University (101R892005).
Supplementary material
The Supplementary Material for this article can be found online at: http://www.frontiersin.org/journal/10.3389/fpls.2015.00159/abstract
Expression patterns of LDL1 (left) and LDL2 (right) through the public Arabidopsis microarray database (http://www.bar.utoronto.ca/efp/cgi-bin/efpWeb.cgi).
References
- Alonso-Blanco C., Bentsink L., Hanhart C. J., Blankestijn-de Vries H., Koornneef M. (2003). Analysis of natural allelic variation at seed dormancy loci of Arabidopsis thaliana. Genetics 164, 711–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentsink L., Jowett J., Hanhart C. J., Koornneef M. (2006). Cloning of DOG1, a quantitative trait locus controlling seed dormancy in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 103, 17042–17047. 10.1073/pnas.0607877103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouyer D., Roudier F., Heese M., Andersen E. D., Gey D., Nowack M. K., et al. (2011). Polycomb repressive complex 2 controls the embryo-to-seedling phase transition. PLoS Genet. 7:e1002014. 10.1371/journal.pgen.1002014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., MacGregor D. R., Dave A., Florance H., Moore K., Paszkiewicz K., et al. (2014). Maternal temperature history activates Flowering Locus T in fruits to control progeny dormancy according to time of year. Proc. Natl. Acad. Sci. U.S.A. 111, 18787–18792. 10.1073/pnas.1412274111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S. J., Bent A. F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743. 10.1046/j.1365-313x.1998.00343.x [DOI] [PubMed] [Google Scholar]
- Debieu M., Tang C., Stich B., Sikosek T., Effgen S., Josephs E., et al. (2013). Co-variation between seed dormancy, growth rate and flowering time changes with latitude in Arabidopsis thaliana. PLoS ONE 8:e61075. 10.1371/journal.pone.0061075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch-Savage W. E., Leubner-Metzger G. (2006). Seed dormancy and the control of germination. New Phytol. 171, 501–523. 10.1111/j.1469-8137.2006.01787.x [DOI] [PubMed] [Google Scholar]
- Finkelstein R. R. (1994). Mutations at 2 new Arabidopsis ABA response loci are similar to the ABI3 mutations. Plant J. 5, 765–771 10.1046/j.1365-313X.1994.5060765.x [DOI] [Google Scholar]
- Finkelstein R., Reeves W., Ariizumi T., Steber C. (2008). Molecular aspects of seed dormancy. Annu. Rev. Plant Biol. 59, 387–415. 10.1146/annurev.arplant.59.032607.092740 [DOI] [PubMed] [Google Scholar]
- Giraudat J., Hauge B. M., Valon C., Smalle J., Parcy F., Goodman H. M. (1992). Isolation of the Arabidopsis-ABI3 gene by positional cloning. Plant Cell 4, 1251–1261. 10.1105/tpc.4.10.1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Guzman M., Apostolova N., Belles J. M., Barrero J. M., Piqueras P., Ponce M. R., et al. (2002). The short-chain alcohol dehydrogenase ABA2 catalyzes the conversion of xanthoxin to abscisic aldehyde. Plant Cell 14, 1833–1846. 10.1105/tpc.002477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell C. A., Wood C. C., Robertson M., James Peacock W., Dennis E. S. (2006). The Arabidopsis FLC protein interacts directly in vivo with SOC1 and FT chromatin and is part of a high-molecular-weight protein complex. Plant J. 46, 183–192. 10.1111/j.1365-313X.2006.02686.x [DOI] [PubMed] [Google Scholar]
- Hilhorst H. W. M. (2007). Are dormant seeds lazy and germinating seeds not? in Seeds: Biology, Development and Ecology, eds Adkins S. W., Navie S. V., Ashmore S. (Cambridge: CABI; ), 88–194. [Google Scholar]
- Holdsworth M. J., Bentsink L., Soppe W. J. J. (2008). Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination. New Phytol. 179, 33–54. 10.1111/j.1469-8137.2008.02437.x [DOI] [PubMed] [Google Scholar]
- Iuchi S., Kobayashi M., Taji T., Naramoto M., Seki M., Kato T., et al. (2001). Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J. 27, 325–333. 10.1046/j.1365-313x.2001.01096.x [DOI] [PubMed] [Google Scholar]
- Jiang D., Yang W., He Y., Amasino R. M. (2007). Arabidopsis relatives of the human lysine-specific demethylase1 repress the expression of FWA and FLOWERING LOCUS C and thus promote the floral transition. Plant Cell 19, 2975–2987. 10.1105/tpc.107.052373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W., Lee Y., Park J., Lee N., Choi G. (2013). HONSU, a protein phosphatase 2C, regulates seed dormancy by inhibiting ABA signaling in Arabidopsis. Plant Cell Physiol. 54, 555–572. 10.1093/pcp/pct017 [DOI] [PubMed] [Google Scholar]
- Koornneef M., Jorna M. L., Derswan D., Karssen C. M. (1982). The isolation of abscisic-acid (ABA) deficient mutants by selection of induced revertants in non-germinating gibberellin sensitive lines of Arabidopsis thaliana (L) heynh. Theor. Appl. Genet. 61, 385–393. [DOI] [PubMed] [Google Scholar]
- Koornneef M., Reuling G., Karssen C. M. (1984). The isolation and characterization of abscisic-acid insensitive mutants of Arabidopsis thaliana. Physiol. Plant. 61, 377–383 10.1111/j.1399-3054.1984.tb06343.x [DOI] [Google Scholar]
- Kushiro T., Okamoto M., Nakabayashi K., Yamagishi K., Kitamura S., Asami T., et al. (2004). The Arabidopsis cytochrome P450 CYP707A encodes ABA 8′-hydroxylases: key enzymes in ABA catabolism. Embo J. 23, 1647–1656. 10.1038/sj.emboj.7600121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre V., North H., Frey A., Sotta B., Seo M., Okamoto M., et al. (2006). Functional analysis of Arabidopsis NCED6 and NCED9 genes indicates that ABA synthesized in the endosperm is involved in the induction of seed dormancy. Plant J. 45, 309–319. 10.1111/j.1365-313X.2005.02622.x [DOI] [PubMed] [Google Scholar]
- Leon-Kloosterziel K. M., Gil M. A., Ruijs G. J., Jacobsen S. E., Olszewski N. E., Schwartz S. H., et al. (1996). Isolation and characterization of abscisic acid-deficient Arabidopsis mutants at two new loci. Plant J. 10, 655–661. 10.1046/j.1365-313X.1996.10040655.x [DOI] [PubMed] [Google Scholar]
- Liu X., Yang S., Zhao M., Luo M., Yu C. W., Chen C. Y., et al. (2014). Transcriptional repression by histone deacetylases in plants. Mol. Plant 7, 764–772. 10.1093/mp/ssu033 [DOI] [PubMed] [Google Scholar]
- Liu Y., Koornneef M., Soppe W. J. J. (2007). The absence of histone H2B monoubiquitination in the Arabidopsis hub1 (rdo4) mutant reveals a role for chromatin remodeling in seed dormancy. Plant Cell 19, 433–444. 10.1105/tpc.106.049221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lopez-Molina L., Mongrand S., Chua N. H. (2001). A postgermination developmental arrest checkpoint is mediated by abscisic acid and requires the AB15 transcription factor in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 98, 4782–4787. 10.1073/pnas.081594298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Molina L., Mongrand S., McLachlin D. T., Chait B. T., Chua N. H. (2002). ABI5 acts downstream of ABI3 to execute an ABA-dependent growth arrest during germination. Plant J. 32, 317–328. 10.1046/j.1365-313X.2002.01430.x [DOI] [PubMed] [Google Scholar]
- Nakabayashi K., Bartsch M., Xiang Y., Miatton E., Pellengahr S., Yano R., et al. (2012). The time required for dormancy release in arabidopsis is determined by DELAY OF GERMINATION1 protein levels in freshly harvested seeds. Plant Cell 24, 2826–2838. 10.1105/tpc.112.100214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- North H., Baud S., Debeaujon I., Dubos C., Dubreucq B., Grappin P., et al. (2010). Arabidopsis seed secrets unravelled after a decade of genetic and omics-driven research. Plant J. 61, 971–981. 10.1111/j.1365-313X.2009.04095.x [DOI] [PubMed] [Google Scholar]
- Okamoto M., Kuwahara A., Seo M., Kushiro T., Asami T., Hirai N., et al. (2006). CYP707A1 and CYP707A2, which encode abscisic acid 8′-hydroxylases, are indispensable for proper control of seed dormancy and germination in Arabidopsis. Plant Physiol. 141, 97–107. 10.1104/pp.106.079475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Lee N., Kim W., Lim S., Choi G. (2011). ABI3 and PIL5 collaboratively activate the expression of SOMNUS by directly binding to its promoter in imbibed Arabidopsis seeds. Plant Cell 23, 1404–1415. 10.1105/tpc.110.080721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield S., Hall A. (2009). A role for multiple circadian clock genes in the response to signals that break seed dormancy in Arabidopsis. Plant Cell 21, 1722–1732. 10.1105/tpc.108.064022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo M., Hanada A., Kuwahara A., Endo A., Okamoto M., Yamauchi Y., et al. (2006). Regulation of hormone metabolism in Arabidopsis seeds: phytochrome regulation of abscisic acid metabolism and abscisic acid regulation of gibberellin metabolism. Plant J. 48, 354–366. 10.1111/j.1365-313X.2006.02881.x [DOI] [PubMed] [Google Scholar]
- Seo M., Peeters A. J. M., Koiwai H., Oritani T., Marion-Poll A., Zeevaart J. A. D., et al. (2000). The Arabidopsis aldehyde oxidase 3 (AA03) gene product catalyzes the final step in abscisic acid biosynthesis in leaves. Proc. Natl. Acad. Sci. U.S.A. 97, 12908–12913. 10.1073/pnas.220426197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugliani M., Brambilla V., Clerkx E. J. M., Koornneef M., Soppe W. J. J. (2010). The conserved splicing factor SUA controls alternative splicing of the developmental regulator ABI3 in Arabidopsis. Plant Cell 22, 1936–1946. 10.1105/tpc.110.074674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugliani M., Rajjou L., Clerkx E. J. M., Koornneef M., Soppe W. J. J. (2009). Natural modifiers of seed longevity in the Arabidopsis mutants abscisic acid insensitive3-5 (abi3-5) and leafy cotyledon1-3 (lec1-3). New Phytol. 184, 898–908. 10.1111/j.1469-8137.2009.03023.x [DOI] [PubMed] [Google Scholar]
- van Zanten M., Zoell C., Wang Z., Philipp C., Carles A., Li Y., et al. (2014). HISTONE DEACETYLASE 9 represses seedling traits in Arabidopsis thaliana dry seeds. Plant J. 80, 475–488. 10.1111/tpj.12646 [DOI] [PubMed] [Google Scholar]
- Xiong L. M., Ishitani M., Lee H., Zhu J. K. (2001). The Arabidopsis LOS5/ABA3 locus encodes a molybdenum cofactor sulfurase and modulates cold stress- and osmotic stress-responsive gene expression. Plant Cell 13, 2063–2083. 10.1105/tpc.13.9.2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L., Zhu J. K. (2003). Regulation of abscisic acid biosynthesis. Plant Physiol. 133, 29–36. 10.1104/pp.103.025395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S.-D., Cho Y.-H., Sheen J. (2007). Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat. Protoc. 2, 1565–1572. 10.1038/nprot.2007.199 [DOI] [PubMed] [Google Scholar]
- Zhang X., Garreton V., Chua N. H. (2005). The AIP2 E3 ligase acts as a novel negative regulator of ABA signaling by promoting ABI3 degradation. Genes Dev. 19, 1532–1543. 10.1101/gad.1318705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J., Chen F., Wang Z., Cao H., Li X., Deng X., et al. (2012). A novel role for histone methyltransferase KYP/SUVH4 in the control of Arabidopsis primary seed dormancy. New Phytol. 193, 605–616. 10.1111/j.1469-8137.2011.03969.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expression patterns of LDL1 (left) and LDL2 (right) through the public Arabidopsis microarray database (http://www.bar.utoronto.ca/efp/cgi-bin/efpWeb.cgi).