Abstract
Objective:
To evaluate the distribution and pattern of neural tube defects in Saudi Arabia by creating a hospital based registry.
Methods:
All cases registered in the King Faisal Specialist Hospital and Research Center (KFSH&RC) neural tube defect (NTD) registry since it was established in October 2000 until December 2012 were studied through active surveillance comprising a registrar who collects NTD information by reviewing the patient’s medical records, and interviewing patient’s families.
Results:
The total number of patients registered from October 2000 to December 2012 was 718 patients. There were more females (417, 58%) than males (301, 42%). Of 620 mothers who underwent antenatal ultrasonography; 392 (63%) were diagnosed at birth, and 204 (33%) were diagnosed with antenatal hydrocephalus. In our registry sample, most mothers (95%) did not take folic acid 3 months prior to pregnancy, and 76% did not take folic acid during the 3 months after conception with the affected child. Only 5% received folic acid prior to conception.
Conclusions:
The KFSH&RC-NTD registry has met its objectives as a source of data that may significantly contribute to the prevention of NTDs, and improving quality of care for NTD patients through active publication of registry findings and management approaches.
Neural tube defects (NTDs) are serious birth defects of the spine and the brain and they cause substantial mortality and morbidity worldwide. There are a number of different types of NTDs, including anencephaly, spina bifida, and encephalocele. Infants born with anencephaly usually die within a few days of birth, and those with spina bifida have lifelong disabilities with varying degrees of paralysis. Each year, in the USA, 3000 pregnancies are affected by NTDs,1 and more than 300,000 worldwide.2 For a child born with spina bifida, the total lifetime cost of care is estimated to be $560,000.3 Both genetic and environmental risk factors are known to be involved in the etiology of NTDs. Factors that increase the risk of an NTD affected pregnancy include: Previous pregnancy affected by NTD; anti-seizure medications: valproic acid or carbamazepine; having diabetes before the beginning of pregnancy (not gestational diabetes); inadequate folic acid intake; pre-pregnancy obesity; and high temperatures early in pregnancy. The King Faisal Specialist Hospital & Research Centre (KFSH&RC) - NTDs Registry was established in March 2000 through the joint efforts of the departments of Neurosciences, Biostatistics, Epidemiology and Scientific Computing (BESC), Pediatrics, Orthopedics, Urology, and Obstetrics and Gynecology. The registry is designed for the collection, management, and analysis of data belonging to patients who present to KFSH&RC with a NTD. Our objective is to evaluate the pattern and distribution of this disease in Saudi Arabia, as there is a lack of hospital based referral center registries for Saudi Arabia, and to use these findings for the prevention of NTD’s, and the improvement of quality of care of NTD patients.
Methods
All cases registered in the KFSH&RC - NTD registry (Riyadh, Saudi Arabia) from October 2000 to December 2012 were studied by active surveillance comprising a registrar who collects NTD information by reviewing the patient’s medical records, and interviewing patient’s families residing throughout the Kingdom. The diagnosis of NTD was confirmed by clinical and MRI findings. This registry includes all NTD patients presenting to KFSH&RC, with no age, gender, or nationality exclusion criteria. The registry includes Saudi and non-Saudi patients. Incomplete data of other risk factors were not included in the study; for example, mother’s weight, and comorbid medical problems.
All the historical data on NTD registry patients were entered into a web-based database. Data quality assurance was performed to assure eligibility, as well as completeness, and consistency of data items in all sections of the registry. Data security and confidentiality is essential to the credibility of the registry. Data on all personal and medical identifiable information in the registry is considered confidential. Data is only released to authorized individuals following registry committee, and ethics committee approval.
Patients were labeled disabled when they have neurological or mental deficits, while called handicapped if they have physical or mental disabilities and are unable to function within their community. Therefore, not all disabled patients are labeled handicapped.
Results
This report is based on the cumulative data from KFSH&RC combined with data from actively collaborating hospitals, in particular the Disabled Children’s Association in Riyadh and Jeddah cities, King Saud Medical Complex, KFSH&RC, Jeddah, and Al-Qunfudah General Hospital, Al-Qunfudah, Saudi Arabia. A total of 718 patients were registered in this study. Among them, females outnumbered males with 417 (58%) females compared with 301 (42%) males. A total of 175 (24%) patients were attending school and performing well, whereas 36 (5%) patients never attended school for various reasons, and 15 (2%) are disabled. Almost one third of the patients (216, 30%) are handicapped, while 89 (12%) can walk around independently in the community. Ninety infants (12.7%) have not yet reached walking age. Patients are considered ambulatory when they are able to walk with or without appliances at 2 years of age or older. In those <2 years of age, ambulatory was defined as at least 4/5 lower extremity muscle strength.4 Among 620 mothers who had antenatal ultrasonography, 392/620 (63%) claimed that their babies were diagnosed only at birth despite ultrasonography, and 7/620 (1%) were diagnosed after birth. One hundred and twenty-seven patients (20.5%) were diagnosed in the third trimester of pregnancy, and only 6/620 (0.8%) patients were diagnosed during the first trimester (Figure 1).
Figure 1.
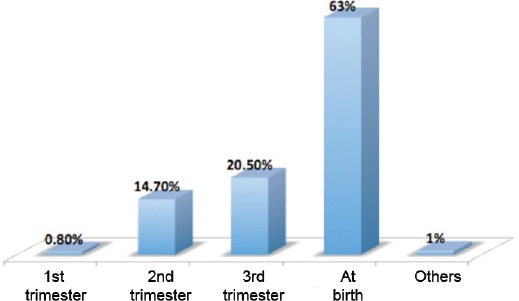
Mothers with ultrasonography and stage of neural tube defect diagnosis.
Most spina bifida patients have myelomeningoceles (MMC). There were 408/718 (57.6%) cases with MMC and hydrocephalus, and 188/718 (26%) cases without hydrocephalus. Among the patients who were born with hydrocephalus, 204/408 (50%) were diagnosed in utero by ultrasound, and 174/408 (24.2%) were diagnosed after birth. The distribution of NTDs in our patients by the time of diagnosis is shown in (Figure 1).
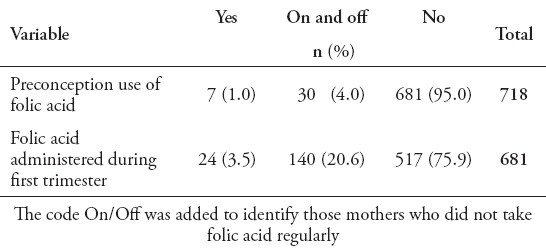
Most patients underwent primary operation within the first 3 days of life (313, 43.6%). A significant number of patients (420, 58.5%), required ventriculoperitoneal shunt (VPS) insertion. Among those, 310 (74%) had VPS inserted after repair of MMC, and only 30 (7%) had their shunt inserted before the repair. A significant number of mothers (681, 95%), did not take folic acid preconception or during the first trimester, and 521 (72.6%) when conceiving with the affected child (Table 1). Many parents declared that they were not related (383, 53.3%), while 329 (46%) had positive consanguinity. Most patients (696, 97%), did not have first-degree relatives affected with NTD. Also, a substantial number of patients (665, 92.6%), did not have blood relatives with NTD. Approximately half of the patients (369, 51%), were born to mothers between 21-30 years of age, and 246 (34%) between 31-40 years of age.
Table 1.
Folic acid use preconception and during first trimester among mothers of children with neural tube defects.
Discussion
There is increasing evidence that the gender of a child affects the risk of developing a NTD, and females are more afflicted by the disease than males. In our registry data this is shown clearly although the difference is not significant. Kanit et al4 also found a remarkable female predominance in the incidence of NTDs in their series. A number of factors associated with spina bifida, like the extent of disability, remedial surgery, age, and the opportunity to develop may affect individuals’ ability to participate in education. Children with spina bifida often have learning difficulties depending on the level of spinal cord affected. In spite of the different physical, mental, and cognitive impairments, a considerable number of our patients go to school.
Patients affected by spina bifida get around in different ways. These include walking without assistance, walking with braces, crutches, or walkers, and using wheelchairs. Patients with spina bifida located high on the spine (thoracic, or upper lumbar) are paraplegic and use wheelchairs. Those with spina bifida lower on the spine (mid lumbar or low lumbar and sacral) might have more use of their legs and use crutches, braces, or walkers, or they might be able to walk without these devices.5,6 After being treated and regularly followed up in the hospital, almost half of the patients are handicapped and cannot move around.
Advances in prenatal diagnosis allowed detection of spina bifida as early as the first trimester.7 Many studies were conducted to determine the accuracy of ultrasound in prenatal diagnosis of NTDs. Ultrasound scanning has 98% sensitivity, and 100% specificity for the detection of neural tube defects.8 Over the last 12 years, the prenatal detection rate has not improved, which is most likely due to the fact that not all women undergo a detailed anomaly scan, but rather receive a social scan (looking at presentation and viability only). The education of the appropriate health providers in conducting detailed ultrasound examination, or asking them to refer women to centers that can provide such services is very important. Most of the cases registered are MMC and the highest percentage is spina bifida aperta with hydrocephalus.
The standard procedure of repairing the exposed lesion is usually initiated within the first few days of life. Most of our registry sample underwent the primary operation within the first 3 days of life. In over 75% of cases, some degree of hydrocephalus is seen on prenatal ultrasound. In most those who have no ventricular dilatation before birth, hydrocephalus develops soon after the open NTD is closed. The mere presence of hydrocephalus does not mean that treatment with a shunting device will be necessary. However, even in the presence of the Arnold Chiari II malformation, approximately 10-15% of those with spina bifida will not develop a degree of hydrocephalus requiring treatment.9 Similarly, 58% of our patients with hydrocephalus diagnosed antenatally, and 26% of our registered cases with spina bifida did not develop hydrocephalus.
If hydrocephalus is left untreated, it can lead to serious illness and neurological problems; this includes brain damage. Hydrocephalus is usually treated by surgically inserting a VPS, which usually redirects the CSF from the brain to the abdomen, and also controls the pressure by draining the excess fluid. These operations are usually carried out before, at, or after the primary surgical repair. A significant number of patients required VPS insertion, and among those patients most had their VPS inserted after the repair.
In 1992, the U.S. Public Health Service recommended that all women of childbearing age consume 400 micrograms (mcg) of folic acid daily for the aim of lowering the risk of having spina bifida or other NTDs.10 In January 1998, the U.S. Food and Drug Administration mandated adding folic acid to all enriched cereal grain products.11 The prevalence rate of spina bifida dropped by 31% from a rate of 5.04 per 10,000 before the fortification period (1995-1996) to a rate of 3.49 per 10,000 after fortification took place in 1998-2006.1 Consequently, each year an estimated average of 1,000 and more babies are saved from an NTD since food fortification began.1
Women’s age was not a significant factor for NTD in their children, as half of them were aged between 21 and 30, and one third between 31 and 40 (p>0.0005). Our data shows that the percentage of women taking folic acid preconception has not increased over the last 12 years. This could be due to inadequate patient education on the importance of such supplementation, or simply due to lack of planning of a pregnancy on the women’s side. On the other hand, the use of folic acid during pregnancy has increased over the last 12 years, although still mostly not regular but on and off. This could reflect that knowledge of the importance of folic acid is either there, or is stressed by the health care givers once they see a pregnant woman; however, it is inadequate to actually reduce the incidence of the disorder due to late initiation of the intervention. Caregivers should be more proactive in conducting educational campaigns for the public.
With respect to consanguinity, the NTD rate has been found to be higher when parents are related.12 Studies indicate that consanguinity is a major risk factor for congenital anomalies.9,13 In the registry data, a minimal association between consanguinity and NTDs is encountered, and the percentage of nonrelated parents exceeds that of related ones. Women who have had an infant with NTD run the risk of having another affected baby. However, more than 95% of infants with NTDs are born to parents with no family history of the defect.14 We find that most patients do not have a first-degree relative with NTD; this includes “mother, father, sibling, half-sibling, and child.”
In conclusion, the NTD Registry aims to provide data to conduct research studies to provide optimal treatment and support services, study risk factors of NTDs, and to evaluate treatment for NTDs. The evidence that folic acid taken before and during the first 3 months of pregnancy reduces the occurrence of NTDs is so strong, that public health strategies concerning food fortification have been implemented worldwide. Hopefully, the KFSH&RC-NTD registry will serve as a model for the establishment of a nationwide registry. The members of the KFSH&RC NTD registry are looking forward to implementing more activities around the registry on a nationwide basis to support the health and well being of children, and to participate in outreach activities to educate mothers, and increase public awareness.
Footnotes
Disclosure.
References
- 1.Centers for Disease Control and Prevention (CDC). Spina bifida and anencephaly before and after folic acid mandate--United States, 1995-1996 and 1999-2000. MMWR Morb Mortal Wkly Rep. 2004;53:362–365. [PubMed] [Google Scholar]
- 2.Shibuya K, Murray CJ. Congenital anomalies. In Health dimensions of sex and reproduction: the global burden of sexually transmitted diseases, HIV, maternal conditions, perinatal disorders, and congenital anomalies. In: Murray CJ, Lopez AD, editors. Boston (MA): Harvard School of Public Health on behalf of the World Health Organization and the World Bank; 1998. pp. 455–512. [Google Scholar]
- 3.Grosse SD, Ouyang L, Collins JS, Green D, Dean JH, Stevenson RE. Economic evaluation of a neural tube defect recurrence-prevention program. Am J Prev Med. 2008;35:572–577. doi: 10.1016/j.amepre.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Kanit H, Özkan AA, Öner SR, Ispahi C, Endrikat JS, Ertan K. Chromosomal abnormalities in fetuses with ultrasonographically detected neural tube defects. Clin Dysmorphol. 2011;20:190–193. doi: 10.1097/MCD.0b013e328348d99d. [DOI] [PubMed] [Google Scholar]
- 5.Assaad A, Mansy A, Kotb M, Hafez M. Spinal dysraphism: experience with 250 cases operated upon. Childs Nerv Syst. 1989;5:324–329. doi: 10.1007/BF00274523. [DOI] [PubMed] [Google Scholar]
- 6.Biggio JR, Jr, Owen J, Wenstrom KD, Oakes WJ. Can prenatal ultrasound findings predict ambulatory status in fetuses with open spina bifida? Am J Obstet Gynecol. 2001;185:1016–1020. doi: 10.1067/mob.2001.117676. [DOI] [PubMed] [Google Scholar]
- 7.Botto LD, Moore CA, Khoury MJ, Erickson JD. Neural-tube defects. N Engl J Med. 1999;341:1509–1519. doi: 10.1056/NEJM199911113412006. [DOI] [PubMed] [Google Scholar]
- 8.Morrow RJ, McNay MB, Whittle MJ. Ultrasound detection of neural tube defects in patients with elevated maternal serum alpha-fetoprotein. Obstet Gynecol. 1991;78:1055–1057. [PubMed] [Google Scholar]
- 9.Shaer CM, Chescheir N, Schulkin J. Myelomeningocele: a review of the epidemiology, genetics, risk factors for conception, prenatal diagnosis, and prognosis for affected individuals. Obstet Gynecol Surv. 2007;62:471–479. doi: 10.1097/01.ogx.0000268628.82123.90. [DOI] [PubMed] [Google Scholar]
- 10.Wilson RD, Davies G, Désilets V, Reid GJ, Summers A, Wyatt P, et al. The use of folic acid for the prevention of neural tube defects and other congenital anomalies. J Obstet Gynaecol Can. 2003;25:959–973. doi: 10.1016/s1701-2163(16)30248-1. [DOI] [PubMed] [Google Scholar]
- 11.Honein MA, Paulozzi LJ, Mathews TJ, Erickson JD, Wong LY. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA. 2001;285:2981–2986. doi: 10.1001/jama.285.23.2981. [DOI] [PubMed] [Google Scholar]
- 12.Jaber L, Karim IA, Jawdat AM, Fausi M, Merlob P. Awareness of folic acid for prevention of neural tube defects in a community with high prevalence of consanguineous marriages. Ann Genet. 2004;47:69–75. doi: 10.1016/j.anngen.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 13.Sheridan E, Wright J, Small N, Corry PC, Oddie S, Whibley C, et al. Risk Factors for Congenital Anomaly in a Multiethnic Birth Cohort: An Analysis of the Born in Bradford Study. Obstetrical & Gynecological Survey. 2014;69:75–77. doi: 10.1016/S0140-6736(13)61132-0. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell LE. Epidemiology of neural tube defects. Am J Med Genet C Semin Med Genet. 2005;135C:88–94. doi: 10.1002/ajmg.c.30057. [DOI] [PubMed] [Google Scholar]


