ABSTRACT
Objectives:
To assess the fluoride concentration of different commercially available mouthrinses in central Saudi Arabia, and compare the obtained measurements with label values.
Methods:
This cross-sectional study identified 25 brands of mouthrinses in the markets of Riyadh city between August and September 2013. Nineteen brands of mouthrinses whose labels indicate the percentage of sodium fluoride (NaF) and 6 brands not indicating the fluoride percentage were included in the study. Three bottles of 2 manufacturing batches of each brand were acquired, coded, and analyzed after dilution using specific electrodes for fluoride and an ion analyzer at the College of Science, King Saud University, Riyadh, Saudi Arabia.
Results:
The average fluoride concentrations in the tested mouthrinses ranged from 8.4 ppm (Voza) to 448.7 ppm (Sensodyne “Pronamel”). Analysis of variance showed a statistically significant difference (p<0.05) in the fluoride concentration between the studied 25 brands. Almost 60% of the brands’ fluoride concentrations were significantly different (mainly lower) from the label value. However, only 5 brands contain fluoride at a concentration not significantly different from the recommended fluoride concentration in daily mouthrinses 0.05% (225 ppm).
Conclusion:
Most of the studied commercially available mouthrinses contain topical fluoride at concentrations below the manufacturers’ label value, but above the recommended 0.05%.
Due to the fluoride’s cariostatic and remineralizing properties, it has been advocated as a major contributor in the treatment and prevention of dental caries.1 The use of fluoride containing mouthrinses has been recommended in conditions such as the presence of carious lesions, orthodontic appliances, or prostheses, gingival recessions, chemotherapy or radiotherapy treatment, decreased salivary flow, and patients with physical or psychological disability. The daily rinse with a 0.05% sodium fluoride (NaF) mouthrinse has been reported to be very effective for orthodontic patients with fixed appliances.2 Aminabadi et al’s3 study showed that weekly use of 0.2% NaF mouthwash significantly decreased dental caries after 3 years of use. The incidence of dental caries has been reduced by using 0.2% NaF for 6 years in south Africa.4 Furthermore, Marinho et al’s5 meta-analysis showed that fluoride mouthrinse was effective in the prevention of carious surfaces in permanent dentition. In Brazil,6 50% of the tested 14 mouthrinses showed statistically significant higher F concentrations than the manufactures’ label values. Moreover, Pizzatto et al7 found that all the studied solutions had fluoride concentrations above that required in the dentist’s prescription. Dental professionals must be aware of the exact concentration of F available in solutions, gels, and varnishes to avoid the risk of toxicity and to insure the effectiveness of the prescribed mouthrinses. In the literature, there is a scarcity of published papers in regards to the fluoride concentration of commercially available mouthrinses in Saudi Arabia. Therefore, the aim of the current study was to analyze the F concentration of different available mouthrinses in Riyadh, and compare the values obtained with the manufacturers’ labels.
Methods
The study was performed at the Chemistry Laboratory, College of Science, King Saud University, Riyadh, Saudi Arabia and it was registered at the College of Dentistry Research Center, Riyadh, Saudi Arabia.
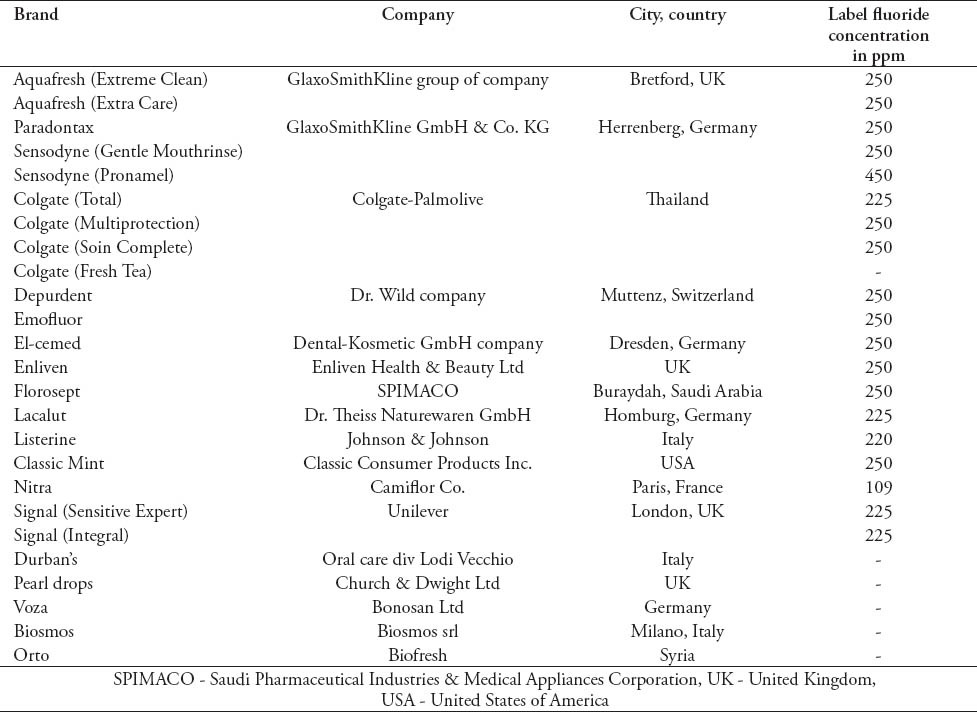
The following 25 brands of mouthrinses that are commercially available in pharmacies and supermarkets in Riyadh, Saudi Arabia were included in the study: Aquafresh (Extreme Clean)®, Aquafresh (Extra Care)®, Colgate (Total)®, Colgate (Multiprotection)®, Colgate (Soin Complete)®, Depurdent®, El-cemed®, Enliven®, Emofluor®, Florosept®, Lacalut®, Listerine®, Classic Mint®, Nitra®, Paradontax®, Sensodyne (Pronamel)®, Signal (Sensitive Expert)®, Signal (Integral)®, Sensodyne (Gentle Mouthrinse)®, Colgate (Fresh Tea)®, Durban’s®, Pearl drops®, Voza®, Biosmos®, Orto® (Table 1). For the first 19 brands that show the F concentration on their label, 3 bottles of the same manufacturing batch were acquired. In addition, 3 bottles of a second batch were purchased yielding a total of 6 samples of each brand. For the remaining 6 brands that do not show the F concentration, 2 bottles were tested. All the products were coded to allow a blind analysis of F concentration and pH values, and each bottle was measured 3 times.
Table 1.
List of the 25 mouthrinses, brands, and the name of the manufacturing company included in the study.
Instruments and chemicals used for F and pH assessment
A calibration curve was preliminary made, which ranged from 1.0 to 10.0 ppm fluoride for calculation of the concentration in ppm fluoride. The solutions were coded, and 1 mL of each product was placed by pipette into a 100-mL volumetric flask and the volume was made up with deionized water. Then a pH/ion-meter (Metrohm Model 781, Herisau, Switzerland) with a combination of F electrodes has been used to determine the fluoride concentration in the mouthrinses samples, which were mixed with total ionic strength adjustment buffer (TISAB) solution 1:1 (v/v). These solutions containing 25 ml of the sample and 25 ml of TISAB solutions were then mixed for approximaltey 3 minutes. Following this, the electrode potentials of these solutions were compared with those of fluoride standard solutions. The results were shown directly on the ion analyzer monitor in millivolts unit, awaiting stabilization, which varied according to the fluoride concentration of the sample. The data obtained using 3 replicates were converted into ppm of fluoride. The pH was determined through the electrometric method by means of the apparatus pH Meter, Thermo Scientific Orion 5-Star, Thermal Scientific Inc., Wlatham, USA, that was calibrated with a standard solution (pH 4.0 and pH 7.0). Table 1 summarizes the list of the mouthrinses and their manufacturers included in the study.
Analysis of the data
Data were analyzed using IBM® Statistical Package for Social Science® Version 20 (International Business Machines Corporation, Armonk, New York, USA), and the level of significance was set at p<0.05. The following tests were used: analysis of variance (ANOVA) to test the differences in the fluoride concentration between the 3 samples of the same batch, and between the means of the brands with Post-Hoc (Tukey HSD test) and t-test to analyze the difference between the 2 batches, and the measured F concentration with the stated manufacturer’s fluoride concentration value on the label and the recommended concentration (0.05%, 225 ppm) for daily use.
Results
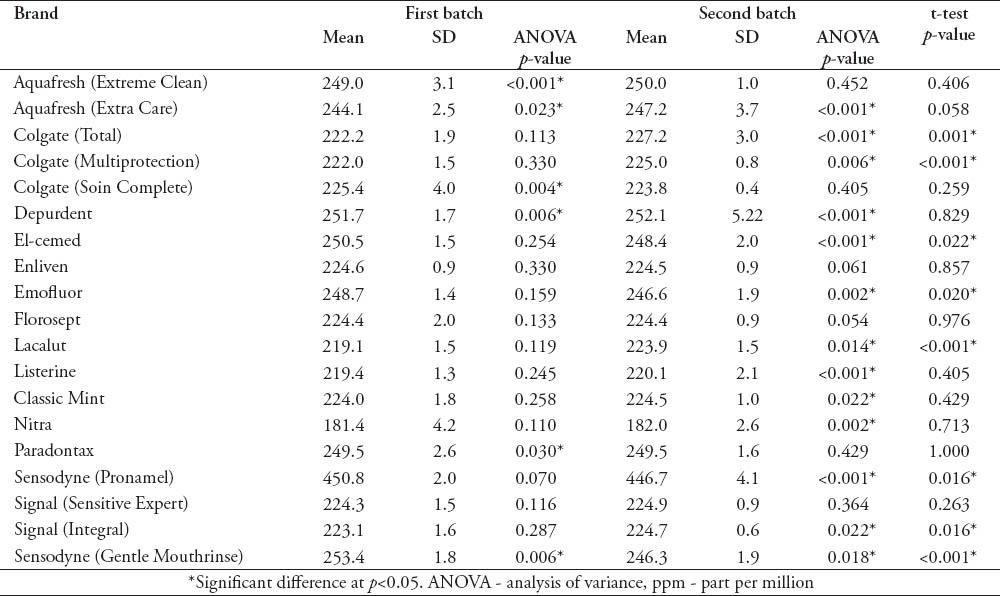
The means of the F concentration obtained from the 6 samples (2 batches) of the brands with fluoride listed on the label appear in Table 2. The ANOVA results were also reported in the table. Three brands (Aquafresh “Extra Care”®, Depurdent®, Sensodyne “Gentle Mouthrinse”®) showed significant differences in the F concentrations between the bottles in both batches. While 3 brands (Enliven®, Florosept®, Signal “Sensitive Expert”®) did not show any statistically significant differences in the F concentration between the 6 samples. When the 2 batches were compared using t-test, 11 out of the tested 25 brands showed a statistically significant difference in the fluoride concentration (Table 2).
Table 2.
Means of the 6 samples of the 19 fluoride labeled brands in ppm, the ANOVA comparisons, and t-test comparisons of the fluoride concentration in the 2 batches.
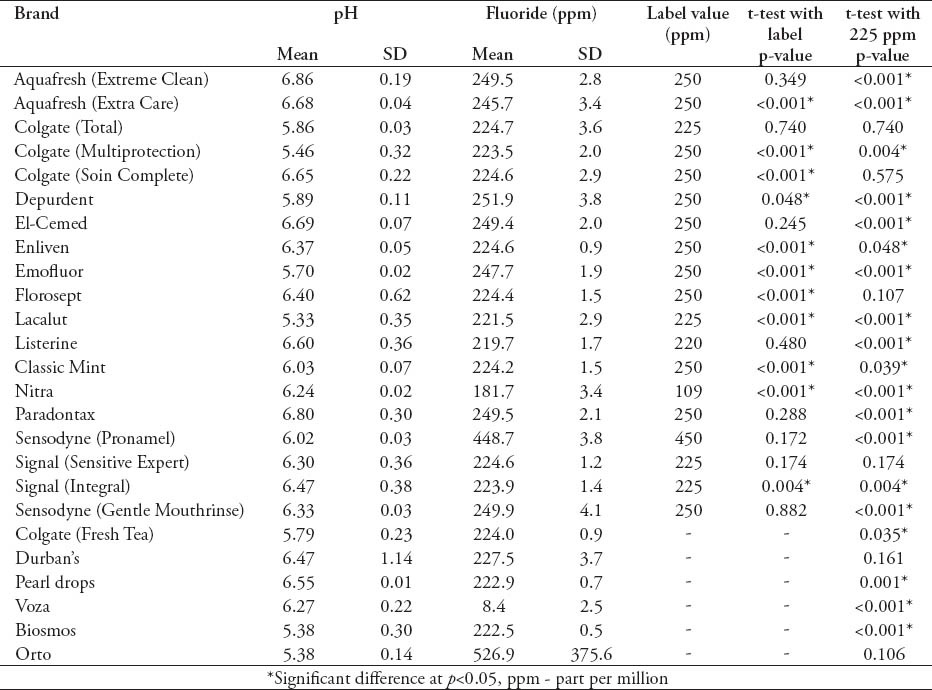
The average fluoride concentrations in the tested mouthrinses ranged from 8.4 ppm (Voza®) to 448.7 ppm (Sensodyne “Pronamel”®) (Table 3). All the brands’ pH values were below 7 and ranged between 5.38 and 6.86. Most of the tested brands showed relatively small standard deviations with the exception of the brands “Voza®” and “Orto®”. The t-test also shows discrepancies between the measured fluoride concentrations and the labeled values. Almost 60% of the brands’ fluoride concentrations were significantly different (mainly lower) from the label value. The brand “Nitra®” had a significantly higher concentration (181.7 ppm) compared with the label value (109 ppm). The same test was used and revealed that only 5 brands (Colgate “Total”®, Colgate “Soin Complete”®, Florosept®, Signal “Sensitive Expert”®, Durban’s®) contained fluoride at a concentration that is not significantly different from the recommended concentration in daily mouthrinses 0.05% (225 ppm) (Table 3).
Table 3.
Means of the pH values of the studied brands, and t-test comparisons between the mean fluoride concentrations and the labeled values and the recommended concentration (225 ppm).
The analysis of variance was used to compare the means of the fluoride concentrations in the 19 brands with fluoride listed in their labels, and it shows that the differences are statistically significant (p<0.001). Pot-hoc comparison results indicate that the brands Nitra® and Sensodyne “Pronamel”® are significantly different from other brands.
Discussion
Systematic reviews of clinical trials concluded that NaF mouthrinses may have an anti-caries effect in children with a limited background of fluoride exposure.8 Similar conclusions were reported by 2 Cochrane reviews: there were some evidence that daily NaF mouthrinses (0.05%, 225 ppm) could reduce the occurrence and severity of white spot lesions during orthodontic treatment, and topical fluorides (mouthrinses, gels, or varnishes) used in addition to fluoride toothpaste achieve the greatest reduction in caries compared with toothpaste used alone.5 Almost all the studied fluoride mouthrinses contain fluoride of approximately 225 ppm. This is the recommended amount for rinsing once a day, for 1-2 minutes, before spitting out. It is also recommended to rinse at a different time to tooth brushing. This is to increase the frequency of exposure of dental plaque to fluoride.9 Although clinical trials showed the effectiveness of the weekly use of 0.2% NaF mouthrinse in improving the oral and dental health among children of school age,3 mouthrinses that contain such concentration are not available in Saudi Arabia. The highest concentration of fluoride tested was approximately 0.1% (450 ppm) found in Sensodyne “Pronamel”®. Governmental initiatives to implement the fluoride 0.2% mouthrinse programs (on a weekly basis) should be studied to aid in the prevention of dental caries.
Concerns over fluoride toxicity frequently arise especially for use by children. The approximate toxic dose of fluoride has been estimated to be 5 mg/kg. Therefore, accurate labeling and controlled production of the prescribed concentration of fluoride by the manufactures, and supervised use by the parents is essential to prevent any toxic reactions.
In this study, the average fluoride concentration of the tested brands presented with a wide range (8.4-448.7 ppm). This clearly illustrates the significance of this investigation for dental professionals. Knowledge of the available products and the range of fluoride concentration in the market is critical for accurate oral health promotion recommendations. Significant discrepancies in the fluoride concentrations observed in some brands “Nitra®, Voza®” reveals that more regulations and monitoring by the responsible agencies are needed to control these health-related products, especially that 5 out of the 6 non-labeled brands contain fluoride.
Few studies have investigated the discrepancy in the fluoride concentration in mouthrinses; Delbem et al6 found that the fluoride concentrations are rarely coincident with the values on the label. Moreover, Pizzatto et al7 stated that all the tested solutions presented fluoride concentration above the required concentration in the dentist’s prescription. However, Tabchoury et al10 found that most of the mouthrinses prepared in 5 pharmacies indicated a fluoride concentration close to the expected value. The pH of the 25 brands analyzed varied from 5.38-6.86 and none of them indicated the pH value on the label. The current study supports the earlier conclusions on the discrepancy between the measured fluoride concentration, and the amount indicated in the manufactures’ labels. Although Delbem et al6 reported that most of the tested mouthrinses actually had concentrations of fluoride above the manufactures’ label value. The present study found that most of the mouthrinses provided fluoride at concentrations below the manufacturers’ label values. Every attempt was made to include all the available mouth rinse brands; however, there is a possibility that other brands in the market were not tested. More powerful statistical analyses could have been performed if more bottles were purchased. However, the sample size of 3 bottles per batch and 2 batches per brand was considered appropriate, especially that each bottle was tested 3 times. Future studies can be designed to test the caries prevention clinical effectiveness, and the risks associated with the daily use of different fluoride containing mouthrinses (225 ppm and 450 ppm).
In conclusion, most of the commercially available mouthrinses provide topical fluoride to the consumers at concentrations below the manufacturers’ label value, but above the recommended 0.05%. One product contained only 0.2% fluoride, and 2 brands provide significantly low concentrations. Discrepancies in the fluoride concentration exist within the same batch and between batches of some brands.
Footnotes
Disclosure.
New Peer Reviewers.
Join our team of expert peer reviewers for the Saudi Medical Journal by sending an enquiry and summarized CV to info@smj.org.sa. Note that SMJ reviewers, whose reviews are returned on time and are judged satisfactory by the Editors, may receive 1 CME credit per review, with a maximum of 5 credits per year, from the Saudi Council for Health Specialties.
References
- 1.Lobo PL, de Carvalho CB, Fonseca SG, de Castro RS, Monteiro AJ, Fonteles MC, et al. Sodium fluoride and chlorhexidine effect in the inhibition of mutans streptococci in children with dental caries: a randomized, double-blind clinical trial. Oral Microbiol Immunol. 2008;23:486–491. doi: 10.1111/j.1399-302X.2008.00458.x. [DOI] [PubMed] [Google Scholar]
- 2.Benson PE, Shah AA, Millett DT, Dyer F, Parkin N, Vine RS. Fluorides, orthodontics and demineralization: a systematic review. J Orthod. 2005;32:102–114. doi: 10.1179/146531205225021033. [DOI] [PubMed] [Google Scholar]
- 3.Aminabadi NA, Balaei E, Pouralibaba F. The Effect of 0.2% Sodium Fluoride Mouthwash in Prevention of Dental Caries According to the DMFT Index. J Dent Res Dent Clin Dent Prospects. 2007;1:71. doi: 10.5681/joddd.2007.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonald RE, Avery DR, Dean JA. 8th ed. St. Louis (MO): Mosby; 2004. Dentistry for the Child and Adolescent. [Google Scholar]
- 5.Marinho VC, Higgins JP, Logan S, Sheiham A. Topical fluoride (toothpastes, mouthrinses, gels or varnishes) for preventing dental caries in children and adolescents. Cochrane Database Syst Rev. 2003;4:CD002782. doi: 10.1002/14651858.CD002782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delbem AC, Sassaki KT, Castro AM, Pinto LM, Bergamaschi M. Assement of the fluoride concentration and pH in different mouthrinses on the brazilian market. J Appl Oral Sci. 2003;11:319–323. doi: 10.1590/s1678-77572003000400008. [DOI] [PubMed] [Google Scholar]
- 7.Pizzatto E, Losso EM, Miranda MC, de Souza VD, Archetti FB. Analysis of fluoride concentration in solutions prepared at dispensing pharmacies. RSBO Revista Sul-Brasileira de Odontologia. 2011;8:294–298. [Google Scholar]
- 8.Twetman S, Petersson L, Axelsson S, Dahlgren H, Holm AK, Kallestal C, et al. Caries-preventive effect of sodium fluoride mouthrinses: a systematic review of controlled clinical trials. Acta Odontol Scand. 2004;62:223–230. doi: 10.1080/00016350410001658. [DOI] [PubMed] [Google Scholar]
- 9.Rugg-Gunn A, Banoczy J. Fluoride toothpastes and fluoride mouthrinses for home use. Acta Med Acad. 2013;42:168–178. doi: 10.5644/ama2006-124.84. [DOI] [PubMed] [Google Scholar]
- 10.Tabchoury CPM, Pierobon CN, Cury JA. Concentration and bioavailability of fluoride in mouthrinses prepared in dispensing pharmacies. Journal of Applied Oral Science. 2005;13:41–46. doi: 10.1590/s1678-77572005000100009. [DOI] [PubMed] [Google Scholar]


