Abstract
The invention of beta (β)-blockers culminated in a new era in the treatment of cardiovascular diseases (CD), and changed the course of pharmacology research for years to come. Since the introduction of propranolol into clinical practice in 1964, β-blockers enjoyed a special place in the clinicians’ armamentarium against CDs, especially for patients with ischemic heart diseases, and are still one of the most extensively used therapeutic drugs in both cardiac and non-cardiac ailments. Current uses of β-blockers in CDs include ischemic heart diseases, hypertension, cardiac arrhythmias, and heart failure. Other substantial non-cardiac uses include glaucoma, migraine, situational anxiety, benign essential tremors, and cardiac symptoms of thyrotoxicosis. This review covers some of the evolutionary changes of clinical uses of β-blockers, the rationale for their use, some recent controversies surrounding their use for treatment of hypertension, and advantages of newer additions to the group.
The purpose of this review was to determine the current consensus, or at least the difference of opinions of various investigators regarding the use of beta (β)-blockers in the treatment of hypertension, in light of the recent clinical findings. It can safely be stated that there is no other class of pharmacological agents, such as β-blockers that revolutionized the treatment of cardiovascular diseases (CDs) so much, and changed the course of pharmacology research for years to come. Beta-blockers are still one of the most extensively used therapeutic drugs in both cardiac and non-cardiac ailments, but they have also been the subject of debates for different reasons in past decades. Since the introduction of propranolol into clinical medicine in 19641 (propranolol was only approved by the US Food and Drug Administration (FDA) for treatment of angina in 1973),2 a number of β-blockers have been added to the clinicians’ arsenal to treat a myriad of diseases. However, the primary targets of β-blockers are CDs. Current uses of β-blockers in CDs include ischemic heart diseases (IHDs), hypertension, cardiac arrhythmias, and heart failure (HF), although they remained contraindicated in patients with HF for approximately a quarter of a century after their introduction into clinical practice. Other substantial non-cardiac uses include glaucoma, migraine headaches, situational anxiety, benign essential tremors, and cardiac symptoms of thyrotoxicosis. The prototype propranolol was developed when even the physiological/pharmacological roles of β-adrenergic receptors (β-ARs), let alone their subtypes, had not been clearly delineated. Propranolol was developed to primarily treat angina pectoris, and to reduce morbidity and mortality associated with it.1 Nevertheless, the underlying mechanism for this effect soon led to include its use for the treatment of hypertension,2 and cardiac arrhythmias.3 Since then a number of β-blockers, both selective and non-selective for β-ARs, and some with additional properties have been developed, and their uses expanded. This review covers some of the evolutionary changes of clinical uses of β-blockers, some recent controversies surrounding their use for treatment of hypertension, and the advantages and disadvantages of newer additions to the group.
Autonomic control of the heart
Sympathetic autonomic control of the heart is exerted through G-protein-coupled β-ARs. Stimulation of β-ARs by endogenous ligands, norepinephrine and epinephrine, results in increased cardiac contractility and heart rate (HR), via G-protein/adenylyl cyclase transduction pathways that are important factors in the maintenance of blood pressure (BP). An increase in HR and contractility enhances oxygen consumption by the heart muscle, a factor that plays a critical role in the causation of angina in the presence of coronary insufficiency. The concept of a and β-ARs originally proposed by Ahlquist in 19484 lay unrecognized for more than a decade before the development of β-blockers. Sir James Black,5 the inventor of β-ARs antagonists, defines receptors as “any devices that receive information, signals, and so forth.” The heart expresses both α-and β-ARs families, the role of cardiac β3- and α-adrenergic receptors in cardiac diseases is still in the evolutionary stage, and is being delineated. In a normal non-failing heart, approximately 80% of the expressed receptors are β1-ARs and 20% β2-ARs; the ratio becomes almost equal in a failing heart, when β1-ARs are down-regulated correlating with the severity of the heart disease.6 The density of β1-ARs also declines with old age due to increased sympathetic activity.7 Myocardial β3-adrenergic receptors are found both in the atria and ventricles, and are over-expressed in HF and hypertension.8 They may be coupled through the inhibitory G (Gi) protein, or through the stimulatory Gs proteins.9 The β3-AR stimulation results in the decrease of cAMP generation, which is in contrast to the stimulation of β1- and β2-ARs that increase the formation of cAMP, and in decreased cardiac contractility via release of nitric oxide (NO).10 The β3-ARs inhibit hypertrophic response to neurohormonal stimulation through NO synthase-mediated mechanism.11 Despite the potential for α-and β-ARs exploitation for many heart diseases, for all practical purposes, so far β1-AR blockers have been the only target for clinical applications.
Hypertension
Use of β-blockers to treat hypertension started in the 1960's, as these agents were enormous improvement in terms of adverse effects over the existing antihypertensive drugs in vogue at the time, such as ganglionic blockers, guanethidine, or methyldopa.3 However, since the introduction of newer classes of antihypertensive drugs, such as angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), and calcium channel blockers (CCBs), β-blockers have been subjected to a more stringent scrutiny and their performance is usually compared with these new agents. The β-blockers are still preferred in hypertensive patients who have suffered from myocardial infarction (MI), or other forms of IHDs, and HF due to systolic dysfunction,12 but not in hypertensive patients without comorbidities.13 Beta-blockers are usually avoided in patients suffering from bronchial asthma, or with airway hyper-reactivity. Their use as first-line therapy for hypertension first came under criticism in the 1990's when it was shown by meta-analyses of clinical trials that β-blockers did not prevent coronary heart disease (CHD), or significantly reduce cardiac and all-cause mortality. Propranolol showed little benefit against stroke and none on coronary events in elderly British patients.14 Beta-blockers were also found less effective in lowering systolic blood pressure (SBP) and diastolic blood pressure (DBP) in hypertensive patients than those treated with ACEIs, ARBs, and CCBs, and significantly less patients continued their treatment with β-blockers.15
The American Heart Association/American College of Cardiology (AHA/ACC), however, continued to recommend β-blockers as first-line drugs in patients with CHD to increase exercise tolerance and reduce morbidity and mortality.16 Another adverse observation against β-blockers was that the use of β-blockers with diuretics produced more new diabetes than other antihypertensive drugs, such as CCBs or ACEIs.17 In the meantime, the United Kingdom's National Institute of Clinical Excellence (NICE) Guidelines in 200618 recommended not using β-blockers as first-line treatment for hypertension that brought this important group of drugs to the forefront of academic discussion. Some cohort studies suggested that hypertensive patients with a high resting HR, and free from other overt heart ailments, are at an increased risk for all-cause and cardiovascular death. However, the use of β-blockers to reduce HR was not superior to other antihypertensive drug classes in reducing all-cause and cardiovascular mortality.18 In fact, a lower HR in β-blockers users was found associated with increased all-cause and cardiovascular mortality, MI, and HF.19 An overall U-shaped relationship between HRs and the prognosis was reported, after a prospective study of 528 patients with resistant hypertension monitored for ambulatory BP for a median of 4.8 years. Both fast (>75 bpm or >70 bpm for nighttime), and slow (<60 bpm or <55 bpm for nighttime) HRs were predictors of mortality; while a fast HR was a significant predictor in patients using β-blockers, the slow HR was a more important predictor in those not using β-blockers.20 The 7th report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) found atenolol lacking in protection against stroke,21 and meta-analyses of other clinical trials reported it less effective against cardiovascular outcomes compared with other antihypertensive drugs.22 Other reports also did not support using β-blockers as first-line treatment as they showed relatively weak effect on reducing stroke, especially the non-selective β-blockers,23 or reduce the incidence of CHD.24 Use of first (propranolol) and second generation β-blockers (atenolol) as initial therapy for hypertension causes a modest reduction in cardiovascular events, but they are not credited with reducing all-cause mortality.25
Despite the disagreements, most national guidelines still do not forbid the use of β-blockers as first-line drugs for the initial treatment of hypertension. The European Society of Hypertension (ESH) Task Force dismissed the classification and ranking of antihypertensive drugs into first, second, or third-line drugs, as the classification is not justified on scientific and practical basis.26 The ESH contends that the 5 major antihypertensive drug classes, that is, CCBs, ACEIs, ARBs, β-blockers, and diuretics, do not differ significantly in their ability to lower BP, or offer an unequivocal proof of protection against overall cardiovascular risks, such as stroke, or MI.27 A similar outcome was reported by Fretheim et al,28 after a comparative meta-analysis of 25 clinical trials of these major classes of antihypertensive drugs for their effectiveness and ability for primary prevention of cardiovascular events. None of these 5 major antihypertensive drug classes was consistently superior to another class across different outcomes. While ARBs were superior to β-blockers in reducing all-cause mortality, HF, and diabetes incidence, β-blockers proved better for angina prevention. Diuretics were also better than β-blockers in reducing all-cause mortality, MI, stroke, and HF. Betα-blockers were also inferior to ACEIs, CCB and β-blockers for all-cause mortality, MI, and stroke. Despite the fact that some of these findings were not based on very strong evidence, the authors, based on these comparisons, suggested against using β-blockers or α-blockers as first-line drugs for hypertension. However, one caution here is that all β-blocker trials included in this analysis were carried out on atenolol, which may not be representative of all β-blockers, especially the third generation. Some strongly supported the argument that the cardio-protection in hypertensive patients is achieved by lowering the BP, regardless of how it is achieved.29
Many current guidelines allow any of the major antihypertensive drug classes to be considered for initiation, or maintenance of antihypertensive therapy in a given patient provided there is no contraindication for a particular class of drug, and the therapy is individualized.26,30 The US National Health And Nutrition Examination Survey (NHANES) of 2010 showed a 57% increase in the use of β-blockers from 2001-2002 to 2009-2010 period compared with 23% increase in the use of diuretics, 31% with ACEIs, and 100% with ARBs.31 One must keep in mind that ARBs were introduced into clinical practice in the mid-1990's and had a low baseline use in 2001-2002, while β-blockers use was already high. However, guidelines from the UK's National Institute for Health and Care Excellence (NICE) does not recommend β-blockers as preferred initial therapy for hypertension,32 and the JNC 8 did not include β-blockers in their recommendations for the initial antihypertensive therapy.33 In a global survey of the use of antihypertensive drugs, the International Society of Hypertension-affiliated representative societies of 31 countries reported using ACEIs, ARBs, CCBs and diuretics; β-blockers were only preferred for patients with IHD.34
Variations within β-blockers
All β-blockers are not equal as they are a heterogeneous group of drugs with variations in selectivity for β-adrenoceptors and additional qualities, such as lipophilicity, inverse agonist and intrinsic sympathomimetic activity, membrane stabilizing property, and α-receptors blocking activity. The third generation β-blocker, nebivolol has additional NO-mediated vasodilating and antioxidant properties. However, some of the effects of β-blockers are similar qualitatively as a drug class effect, and some vary due to individual properties of a drug. Representative members of different generation of β-blockers with their specific activities are mentioned in Table 1.
Table 1.
Representative beta-blockers of different generations with specific properties.
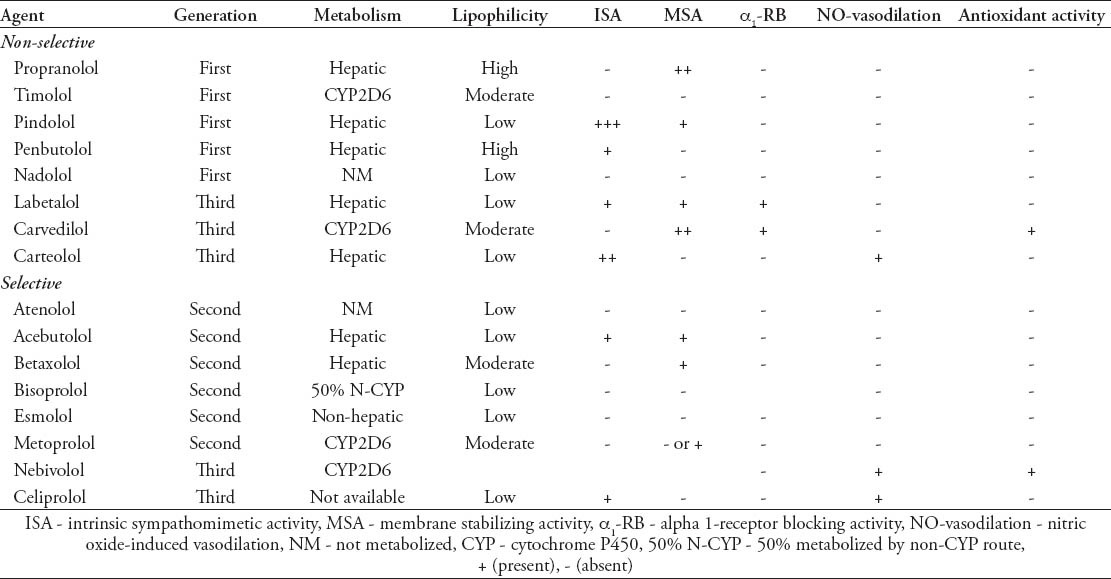
Cardiovascular events incidence (MI, HF, and stroke) was not statistically different in patients who used second generation atenolol, or metoprolol for controlling their hypertension over a median observation period of 5.2 years.35 Atenolol use is also associated with reduction in high density lipoprotein levels which correlates with SBP response.36 Similarly, both nebivolol (third generation) and metoprolol (second generation) reduced HR, brachial BP, and mean arterial pressure (MAP), but the central aortic BP, pulse pressure (PP), and left ventricular septal wall thickness were significantly reduced only in the nebivolol treatment group after one year of treatment.37 Both carvedilol and propranolol do not differ significantly in reducing hepatic venous pressure gradient in portal hypertension, though they differ in many properties, and belong to the third (carvedilol) and first (propranolol) generation.38 Similarly, both atenolol and metoprolol produce similar reductions in BP and do not affect vascular endothelial function, but atenolol increases the peripheral augmentation index.39 When metoprolol or atenolol was added to an existing treatment regimen of low dose hydrochlorothiazide, metoprolol was more effective in sustained reduction of 24-hour and early morning BP than atenolol.40
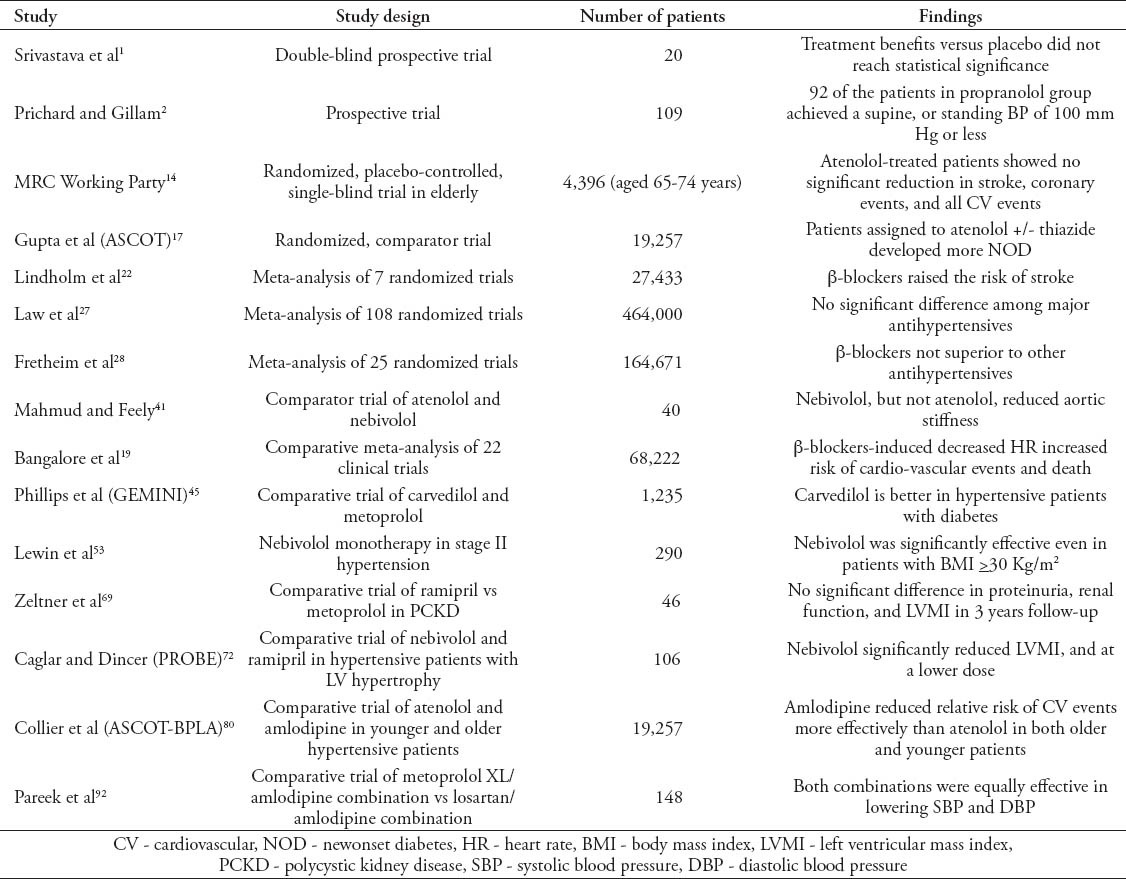
Nebivolol is significantly more effective in lowering aortic PP than atenolol, though both are equally effective in lowering brachial BP and aortic stiffness in treatment-naïve patients with isolated SBP hypertension.41 However, in a comparative study of nebivolol with metoprolol, for relief of symptoms of intermittent claudication in peripheral artery disease (PAD), and mild to moderate hypertension, both treatments significantly improved claudication distance without improving quality of life.42 Vascular insulin sensitivity is blunted by metoprolol but preserved by carvedilol in patients with type 2 diabetes,43 and carvedilol treatment for 6 months significantly increased coronary flow reserve, and endothelium function, and lowered the left ventricular mass index compared with metoprolol treatment of hypertensive patients with left ventricular hypertrophy.44 Moreover, in the Glycemic Effects in Diabetes Mellitus: Carvedilol-Metoprolol Comparison in Hypertension (GEMINI) trial,45 both carvedilol and metoprolol reduced BP significantly, but carvedilol showed better metabolic effects in all races and both genders. Metoprolol significantly increased hemoglobin A(1c) in all groups, except in the non-white/non-black group, and carvedilol was better in both white and female subgroups.45 Results of some important clinical trials and comparative studies and meta-analyses are briefly presented in Table 2.
Table 2.
Selected studies on beta-blockers for use in the management of hypertension.
Third generation β-blockers
Third generation β-blockers, such as carvedilol, Labetolol and nebivolol, have unique properties ascribed to each one of them. Labetolol is a non-selective β-blocker with additional α1 receptors blocking activity and no significant effect on HR and cardiac output.46 It also has specific indication for pheochromocytoma before surgery, and pregnancy-induced hypertension (preeclampsia), though recent observations indicate more hospitalization of infants during infancy for respiratory distress syndrome, sepsis and seizures whose mothers were treated with labetalol during pregnancy, compared with those treated with methyldopa.47 Carvedilol is also a non-selective β-blocker with α1-receptor blocking activity, and is indicated for left ventricular dysfunction following MI, chronic primary hypertension, and mild to severe chronic HF, due to its limited effects on HR, and cardiac contractility.48
Nebivolol is the newer and highly selective β-blocker with vasodilating property due to stimulation of endothelial nitric oxide synthase, and the resultant NO release from endothelium. It also directly reacts with free radicals, scavenging reactive oxygen species (ROS), and thus reducing oxidative stress.49 Nebivolol significantly lowers sitting SBP and DBP in mild to moderate hypertension, even in patients with African heritage,50 in Hispanics,51 and with a response rate ranging from 66-68.9% at doses of 5-20 mg once daily.52 Nebivolol monotherapy significantly lowered both SBP and DBP, and a higher percentage (30.6%) of individuals achieved target BP (<140/90 mm Hg) compared with placebo in a group of patients where 63.9% had body mass index (BMI) of >30 Kg/m2, and 35.2% patients were black, and 37.3% Hispanics. However, in this study nebivolol was not effective in reducing SBP in black patients.53
In a retrospective analysis, monotherapy with nebivolol was effective for patients of all age groups with stage I to II hypertension as it significantly lowered DBP at various dosages (5-20 mg/day) but the SBP in a population older than 62 years was only significantly lowered at 20 mg dose because this age group tends to have higher baseline SBP values.54 Nebivolol monotherapy is as effective as combination therapy with a diuretic, CCB, or other antihypertensive and with a favorable adverse effects profile with more patients responding to monotherapy than in combination with a diuretic.55 Evening dosing of nebivolol significantly lowers daytime, nighttime, and 24 hour BP, and the pre-waking SBP, called the morning BP surge.56 Even in patients with pre-hypertension, nebivolol significantly reduces central aortic systolic, diastolic, and MAP, and significantly increases urinary nitrite/nitrite excretion, an indication of increased NO production.57
Beta-blockers versus diuretics
Diuretics have been an integral part of antihypertensive therapy and their effectiveness is still without doubt, but with negative effects on patient's metabolic profile.58 Both thiazide diuretics and β-blockers increase diabetes risk, but their combined use is frankly diabetogenic.59 Messerli et al60 raised the question if β-blockers were useful as first-line antihypertensive therapy in the elderly, and they later reported that β-blockers use with diuretics, in fact, resulted in a worse outcome than the use of diuretics alone.61 Patients treated with diuretics, or in combination with other antihypertensive drugs, especially CCBs have significantly lower variation of 24-hour SBP than those treated with other antihypertensives.62 Thiazide diuretics reduce the risk of stroke more than β-blockers and ACEIs,63 but are associated with increased insulin-resistance, and the risk of gout.64,65 Hydrochlorothiazide (HCTZ) also increases hepatic triglycerides level.66 Chlorthalidone has lately been reported to be more effective than HCTZ in preventing cardiovascular events in hypertensive patients.67
Beta-blockers versus renin-angiotensin inhibitors
The control rate of SBP and DBP in mild-to-moderately hypertensive middle-aged and elderly patients was significantly higher after a 12-week treatment with zofenopril than with atenolol.68 After a 3-year follow-up, both ramipril and metoprolol significantly decreased MAP, and showed no significantly different effects on renal function, albuminuria, and left ventricular mass index in patients with autosomal dominant polycystic kidney disease.69 Atenolol also increases triglycerides levels, but not as much insulin sensitivity as ramipril or candesartan, while significantly reducing more SBP than ramipril.70 In a study to observe any gender difference of antihypertensive effect of various drug classes, atenolol had a better BP-lowering effect and target BP was achieved more in Chinese women than in men, and women also experienced more adverse effects with sustained release nifedipine and captopril than men.71
In the PROBE trial,72 both nebivolol and ramipril significantly decreased left ventricular mass and mass index in hypertensive patients with left ventricular hypertrophy. However, the effect of nebivolol was significantly better than ramipril.72 In combination with lisinopril, nebivolol significantly lowers DBP in stage II diastolic hypertension compared with placebo, nebivolol, or lisinopril alone.73 Nebivolol was however, equally effective in reducing central systolic and DBP, peripheral PP, and the augmentation index, as quinapril and aliskiren in treatment-naïve patients with stage I-II hypertension.74 Treatment with atenolol or perindopril/indapamide combination for one year, showed less reduction in 24 hour SBP, and pulse pressure in the atenolol group, but the ambulatory arterial stiffness index and aortic pulse wave velocity were similar in both treated groups.75 Perindopril and metoprolol-treatment for 6 months also showed no significantly different effects on aortic elasticity in patients with pre-hypertension.76 Metoprolol and valsartan also showed comparable effects on endothelial function and carotid artery elasticity, and reducing BP in mildly hypertensive patients.77 However, metoprolol was more effective in reducing 24-hour MAP without affecting artery stiffness than candesartan after the repair of aorta coarctation in hypertensive patients.78 Nebivolol is equally effective as valsartan in hypertensive patients with obstructive sleep apnea, but reduces HR significantly more than valsartan, which could be beneficial for certain patients.79
Beta-blockers versus calcium channel blockers
In the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA),80 compared with atenolol, the amlodipine treatment of hypertension reduced the relative risk of cardiovascular events (non-fatal MI, fatal CHD) by 17% in patients older than 65 years, and 15% in those younger than 65 years. The events were higher in older patients and thus benefit was more significant in these patients.80 The amlodipine-treated group also showed lower within-individual visit-to-visit, and 24-hour ambulatory blood pressure monitoring (ABPM) variability in SBP which also decreased, whereas variability in the atenolol-treated group increased over time. The lower variability in the amlodipine group was partly credited for the reduced risk of stroke in this group.81 The same ASCOT trial also showed a significant reduction in total cardiovascular events and procedures in a subgroup of patients with diabetes mellitus,82 and a significantly lower carotid SBP, a significant independent predictor of left ventricular mass index, in amlodipine-treated group than in atenolol group, despite no significant differences in brachial pressure.83 In a multicenter randomized comparative effectiveness trial (CLUE) of patients with SBP of 180 mm Hg or more during hypertensive emergency, nicardipine, and labetalol were compared regarding their ability to lower BP to a target level within 30 minutes without causing any end-organ hypo-perfusion. Both treatments lowered BP but patients treated with nicardipine reached the physician-specified SBP target range within 30 minutes than those treated with labetalol; HR was consistently lower in the labetalol group.84
One of the arguments for use of β-blockers in hypertension has been the higher baseline HR. However, in the ASCOT trial,80 HR was significantly reduced more by atenolol than by amlodipine, but still the total cardiovascular events and procedures were reduced significantly more in amlodipine-treated hypertensive patients, an indication that higher baseline HR was not an impediment for amlodipine's beneficial effects, and β-blockers use is not justified simply due to higher baseline HR in hypertensive patients uncomplicated by IHD.85 The INVEST trial86 also showed prevention of cardiovascular events by verapamil-SR equivalent to atenolol, but with a better subjective feeling of well-being.
Beta-blocker combinations
Multidrug treatment is required in many patients with uncontrolled hypertension. However, the order of initiation and addition was an interesting observation of Johnson et al.87 They started 2 groups of uncomplicated hypertension patients on either HCTZ or atenolol monotherapy, and later added the ‘other’ drug to the regimen. The group initially started with HCTZ, and then added atenolol showed a greater BP-lowering response than when the order was switched, indicating the importance of the order, in which therapy was initiated with HCTZ and atenolol. In various dose combinations, atenolol with amlodipine was significantly more effective in lowering SBP and DBP, and more patients achieved target BP than patients treated with monotherapy with either drug.88 Atenolol with aliskiren was also more effective in lowering SBP and DBP than aliskiren alone, and patients with high baseline plasma renin activity (PRA) registered a significant drop in PRA in both atenolol/aliskiren, and aliskiren groups than in atenolol-treated stage I-II hypertensive patients.89
The Combination Therapy of Hypertension to Prevent Cardiovascular Event (COPE) Trial90 evaluated combinations of calcium channel blocker benidipine with an ARB, a β-blocker, or a thiazide diuretic in hypertensive patients to achieve target BP and prevent cardiovascular events. All combinations were similarly effective but in a sub-analysis the incidence of the primary cardiovascular end point in patients older than 65 years was higher than those younger than 65 years. The hazard ratios for fatal and non-fatal stroke, and for new-onset diabetes in older patients on β-blocker combination were higher than with a thiazide and an ARB.90 Combining atenolol with nitrendipine significantly increases body weight and fasting blood glucose level in overweight and obese hypertensive patients, which needs to be controlled with metformin.91 A fixed dose combination of metoprolol extended release with amlodipine was as effective, and well tolerated as a combination of losartan and amlodipine in reducing both SBP and DBP.92 However, combining carvedilol extended release with lisinopril was not superior to monotherapy with the either drug, except in high dose combinations, despite producing additional reduction in 24-hour mean DBP.93 Adding nebivolol to resistant stage I-II hypertensive patients undergoing antihypertensive therapy significantly improves the response and control rate.94
Carvedilol in combination with lisinopril significantly improved endothelial function in hypertensive obese patients compared with a combination of hydrochlorothiazide and lisinopril, though oxidative stress was not significantly affected by either treatment.95 In diabetic hypertensive patients receiving a renin-angiotensin blocker, addition of carvedilol results in a significant reduction in triglycerides, total cholesterol, and non-HDL cholesterol levels, whereas addition of metoprolol caused an increase in both triglycerides and non-HDL cholesterol levels, and a decrease in LDL and HDL cholesterol levels.96 Addition of carvedilol but not metoprolol to high-risk diabetic African-American patients, who had persistent microalbuminuria despite receiving ACEI treatment, improves endothelial function and reduced microalbuminuria.97
A study judging the effectiveness of various antihypertensive drug classes showed that the average reduction (mm Hg, with 95% CI) in SBP achieved over a 24 hour period in descending order was: 10.3 (9.9-10.8) for ARBs; 9.2 (8.6-9.9) for β-blockers; 8.5 (7.9-9.0) for ACEIs; 8.8 (8.3-9.2) for CCBs; and 8.8 (8.3-9.4) for diuretics. The percentage of patients reporting adverse effects attributable to treatment in descending order was: 9.9 for diuretics; 8.3 for CCBs; 7.5 for β-blockers; 3.9 for ACEIs; and 0 for ARBs.98 The annual drug cost using standard doses was estimated to be the highest for ARBs, followed by ACEIs, CCBs, β-blockers, and diuretics.99 A similar conclusion was reached after a meta-analysis of randomized controlled clinical trials by the Blood Pressure Lowering Treatment Trialists’ Collaboration,100 who stated that there is little evidence from these overviews to support the preferential choice of particular drug classes for the prevention of cardiovascular events in chronic kidney disease.
In conclusion, it is our view that β-blockers may no longer be the undisputed leader, however they still hold a special place in the treatment of cardiovascular diseases, including hypertension due to their cost-effectiveness, and a reasonable adverse effects profile. While there are differences of opinion regarding their preference based on meta-analyses of clinical trials, there is still no unequivocal evidence against their use in all forms of cardiovascular diseases. Beta-blockers are still regarded useful for patients with IHD but more important is the individualization of therapy. Third generation β-blockers have many advantages over the first and second generation β-blockers, due to their unique properties and better effects on metabolic profile, and should be preferred whenever possible. Nevertheless, more comparative clinical trials involving third generation β-blockers and other classes of antihypertensive agents would be required to have a better understanding regarding the current role of β-blockers in the treatment of cardiovascular diseases.
Footnotes
Disclosure.
References
- 1.Srivastava SC, Dewar HA, Newell DJ. Double-blind trial of propranolol (Inderal) in angina of effort. Br Med J. 1964;2:724–725. doi: 10.1136/bmj.2.5411.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prichard BNC, Gillam PMS. Treatment of hypertension with propranolol. Br Med J. 1969;1:7–16. doi: 10.1136/bmj.1.5635.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsolakas TC, Davies JP, Oram S. Propranolol in attempted maintenance of sinus rhythm after electrical defibrillation. Lancet. 1964;2:1064. doi: 10.1016/s0140-6736(64)91020-7. [DOI] [PubMed] [Google Scholar]
- 4.Ahlquist RP. A study of the adrenotropic receptors. Am J Physiol. 1948;153:586–600. doi: 10.1152/ajplegacy.1948.153.3.586. [DOI] [PubMed] [Google Scholar]
- 5.Black J. Reflections on drug research. Br J Pharmacol. 2010;161:1204–1216. doi: 10.1111/j.1476-5381.2010.01019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bristow MR, Hershberger RE, Port JD, Gilbert EM, Sandoval A, Rasmussen R, et al. Beta-adrenergic pathways in nonfailing and failing human ventricular myocardium. Circulation. 1990;82:112–125. [PubMed] [Google Scholar]
- 7.Brodde OE, Leineweber K. Autonomic receptor systems in the failing and aging human heart: similarities and differences. Eur J Pharmacol. 2004;500:167–176. doi: 10.1016/j.ejphar.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 8.Morimoto A, Hasegawa H, Cheng HJ, Little WC, Cheng CP. Endogenous beta3- adreno-receptor activation contributes to left ventricular and cardiomyocyte dysfunction in heart failure. Am J Physiol Heart Circ Physiol. 2004;286:2425–2433. doi: 10.1152/ajpheart.01045.2003. [DOI] [PubMed] [Google Scholar]
- 9.Bhadada SV, Patel BM, Mehta AA, Goyal RK. β(3) receptors: role in cardiometabolic disorders. Ther Adv Endocrinol Metab. 2011;2:65–79. doi: 10.1177/2042018810390259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pott C, Brixius K, Bloch W, Ziskoven C, Napp A, Schwinger RH. Beta3-adrenergic stimulation in the human heart: signal transduction, functional implications and therapeutic perspectives. Pharmazie. 2006;61:255–260. [PubMed] [Google Scholar]
- 11.Belge C, Hammond J, Dubois-Deruy E, Manoury B, Hamelet J, Beauloye C, et al. Enhanced expression of b3-adrenoceptors in cardiac myocytes attenuates neurohormone-induced hypertrophic remodeling through nitric oxide synthase. Circulation. 2014;129:451–462. doi: 10.1161/CIRCULATIONAHA.113.004940. [DOI] [PubMed] [Google Scholar]
- 12.McAlister FA, Wiebe N, Ezekowitz JA, Leung AA, Armstrong PW. Meta-analysis: beta-blocker dose, heart rate reduction, and death in patients with heart failure. Ann Intern Med. 2009;150:784–794. doi: 10.7326/0003-4819-150-11-200906020-00006. [DOI] [PubMed] [Google Scholar]
- 13.Elliott WJ, Childers WK. Shouldb blockers no longer be considered first-line therapy for the treatment of essential hypertension without comorbidities? Curr Cardiol Rep. 2011;13:507–516. doi: 10.1007/s11886-011-0216-z. [DOI] [PubMed] [Google Scholar]
- 14.MRC Working Party. Medical Research Council trial of treatment of hypertension in older adults: principal results. Br Med J. 1992;304:405–412. doi: 10.1136/bmj.304.6824.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prandin MG, Cicero AF, Veronesi M, Cosentino E, Dormi A, Strocchi E, et al. Persistence on treatment and blood pressure control with different first-line antihypertensive treatments: a prospective evaluation. Clin Exp Hypertens. 2007;29:553–562. doi: 10.1080/10641960701744061. [DOI] [PubMed] [Google Scholar]
- 16.Gibbons RJ, Chatterjee K, Daley J, Douglas JS, Fihn SD, Gardin JM, et al. ACC/AHA/ACP-ASIM guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients With Chronic Stable Angina) J Am Coll Cardiol. 1999;33:2092–2197. doi: 10.1016/s0735-1097(99)00150-3. [DOI] [PubMed] [Google Scholar]
- 17.Gupta AK, Dahlof B, Dobson J, Sever PS, Wedel H, Poulter NR, et al. Determinants of new-onset diabetes among 19,257 hypertensive patients randomized in the Anglo-Scandinavian Cardiac Outcomes Trial--Blood Pressure Lowering Arm and the relative influence of antihypertensive medication. Diabetes Care. 2008;31:982–988. doi: 10.2337/dc07-1768. [DOI] [PubMed] [Google Scholar]
- 18.Courand PY, Lantelme P. Significance, prognostic value and management of heart rate in hypertension. Arch Cardiovasc Dis. 2014;107:48–57. doi: 10.1016/j.acvd.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Bangalore S, Sawhney S, Messerli FH. Relation of beta-blocker-induced heart rate lowering and cardioprotection in hypertension. J Am Coll Cardiol. 2008;52:1482–1489. doi: 10.1016/j.jacc.2008.06.048. [DOI] [PubMed] [Google Scholar]
- 20.Salles GF, Cardoso CR, Fonseca LL, Fiszman R, Muxfeldt ES. Prognostic significance of baseline heart rate and its interaction with beta-blocker use in resistant hypertension: a cohort study. Am J Hypertens. 2013;26:218–226. doi: 10.1093/ajh/hps004. [DOI] [PubMed] [Google Scholar]
- 21.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure;National Heart, Lung, and Blood Institute;National High Blood Pressure Education Program Coordinating Committee 7th Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 22.Lindholm LH, Carlberg B, Samuelsson O. Should beta blockers remain first choice in the treatment of primary hypertension? A meta-analysis. Lancet. 2005;366:1545–1553. doi: 10.1016/S0140-6736(05)67573-3. [DOI] [PubMed] [Google Scholar]
- 23.Webb AJ, Fischer U, Rothwell PM. Effects of β-blocker selectivity on blood pressure variability and stroke: a systematic review. Neurology. 2011;77:731–737. doi: 10.1212/WNL.0b013e31822b007a. [DOI] [PubMed] [Google Scholar]
- 24.Cayley WE. Are beta blockers effective first-line treatments for hypertension? Am Fam Physician. 2007;76:1306–1308. [PubMed] [Google Scholar]
- 25.Wiysonge CS, Opie LH. b-Blockers as initial therapy for hypertension. JAMA. 2013;310:1851–1852. doi: 10.1001/jama.2013.277510. [DOI] [PubMed] [Google Scholar]
- 26.Fagard R. Reappraisal of the European guidelines on hypertension management: the European Society of Hypertension Task Force document: a short review. Pol Arch Med Wewn. 2010;120:31–35. [PubMed] [Google Scholar]
- 27.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fretheim A, Odgaard-Jensen J, Brørs O, Madsen S, Njølstad I, Norheim OF, et al. Comparative effectiveness of antihypertensive medication for primary prevention of cardiovascular disease: systematic review and multiple treatments meta-analysis. BMC Medicine. 2012;10:33. doi: 10.1186/1741-7015-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Czernichow S, Zanchetti A, Turnbull F, Barzi F, Ninomiya T, Kengne AP, et al. The effects of blood pressure reduction and of different blood pressure-lowering regimens on major cardiovascular events according to baseline blood pressure: meta-analysis of randomized trials. J Hypertens. 2011;29:4–16. doi: 10.1097/HJH.0b013e32834000be. [DOI] [PubMed] [Google Scholar]
- 30.Khan NA, Hemmelgarn B, Herman RJ, Bell CM, Mahon JL, Leiter LA, et al. The 2009 Canadian Hypertension Education Program recommendations for the management of hypertension: Part 2--therapy. Can J Cardiol. 2009;25:287–298. doi: 10.1016/s0828-282x(09)70492-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu Q, Burt VL, Dillon CF, Yoon S. Trends in antihypertensive medication use and blood pressure control among United States adults with hypertension: the National Health and Nutrition Examination Survey, 2001 to 2010. Circulation. 2012;126:2105–2114. doi: 10.1161/CIRCULATIONAHA.112.096156. [DOI] [PubMed] [Google Scholar]
- 32.National Institute for Health and Clinical Excellence. NICE guidelines [CG127] Hypertension: Clinical management of primary hypertension in adults. 2011. Available from: http://www.nice.org.uk/guidance/cg127 .
- 33.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 Evidence-Based Guideline for the Management of High Blood Pressure in Adults Report From the Panel Members Appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 34.Chalmers J, Arima H, Harrap S, Touyz RM, Park JB. Global survey of current practice in management of hypertension as reported by societies affiliated with the International Society of Hypertension. J Hypertens. 2013;31:1043–1048. doi: 10.1097/HJH.0b013e32835f7eef. [DOI] [PubMed] [Google Scholar]
- 35.Parker ED, Margolis KL, Trower NK, Magid DJ, Tavel HM, Shetterly SM, et al. Comparative effectiveness of 2 β-blockers in hypertensive patients. Arch Intern Med. 2012;172:1406–1412. doi: 10.1001/archinternmed.2012.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith SM, Gong Y, Turner ST, Cooper-DeHoff RM, Beitelshees AL, Chapman AB, et al. Blood pressure responses and metabolic effects of hydrochlorothiazide and atenolol. Am J Hypertens. 2012;25:359–365. doi: 10.1038/ajh.2011.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kampus P, Serg M, Kals J, Zagura M, Muda P, Karu K, et al. Differential effects of nebivolol and metoprolol on central aortic pressure and left ventricular wall thickness. Hypertension. 2011;57:1122–1128. doi: 10.1161/HYPERTENSIONAHA.110.155507. [DOI] [PubMed] [Google Scholar]
- 38.Hobolth L, Møller S, Grønbæk H, Roelsgaard K, Bendtsen F, Feldager Hansen E. Carvedilol or propranolol in portal hypertension? A randomized comparison. Scand J Gastroenterol. 2012;47:467–474. doi: 10.3109/00365521.2012.666673. [DOI] [PubMed] [Google Scholar]
- 39.Heffernan KS, Suryadevara R, Patvardhan EA, Mooney P, Karas RH, Kuvin JT. Vascular Function Study Group: Effect of atenolol vs metoprolol succinate on vascular function in patients with hypertension. Clin Cardiol. 2011;34:39–44. doi: 10.1002/clc.20841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarafidis P, Bogojevic Z, Basta E, Kirstner E, Bakris G. Comparative efficacy of two different beta-blockers on 24-hour blood pressure control. J Clin Hypertens (Greenwich) 2008;10:112–118. doi: 10.1111/j.1751-7176.2008.08021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahmud A, Feely J. Beta-blockers reduce aortic stiffness in hypertension but nebivolol, not atenolol, reduces wave reflection. Am J Hypertens. 2008;21:663–667. doi: 10.1038/ajh.2008.156. [DOI] [PubMed] [Google Scholar]
- 42.Espinola-Klein C, Weisser G, Jagodzinski A, Savvidis S, Warnholtz A, Ostad MA, et al. β-Blockers in patients with intermittent claudication and arterial hypertension: results from the nebivolol or metoprolol in arterial occlusive disease trial. Hypertension. 2011;58:148–154. doi: 10.1161/HYPERTENSIONAHA.110.169169. [DOI] [PubMed] [Google Scholar]
- 43.Kveiborg B, Hermann TS, Major-Pedersen A, Christiansen B, Rask-Madsen C, Raunsø J, et al. Metoprolol compared to carvedilol deteriorates insulin-stimulated endothelial function in patients with type 2 diabetes - a randomized study. Cardiovasc Diabetol. 2010;9:21. doi: 10.1186/1475-2840-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiaozhen H, Yun Z, Mei Z, Yu S. Effect of carvedilol on coronary flow reserve in patients with hypertensive left-ventricular hypertrophy. Blood Press. 2010;19:40–47. doi: 10.3109/08037050903450492. [DOI] [PubMed] [Google Scholar]
- 45.Phillips RA, Fonseca V, Katholi RE, McGill JB, Messerli FH, Bell DS, et al. Demographic analyses of the effects of carvedilol vs metoprolol on glycemic control and insulin sensitivity in patients with type 2 diabetes and hypertension in the Glycemic Effects in Diabetes Mellitus: Carvedilol-Metoprolol Comparison in Hypertensives (GEMINI) study. J Cardiometab Syndr. 2008;3:211–217. doi: 10.1111/j.1559-4572.2008.00017.x. [DOI] [PubMed] [Google Scholar]
- 46.Chatterjee K. Potential use of third-generation beta-blockers in heart failure. J Cardiovasc Pharmacol. 1989;14(Suppl 7):S22–S27. [PubMed] [Google Scholar]
- 47.Xie RH, Guo Y, Krewski D, Mattison D, Walker MC, Nerenberg K, et al. Association between labetolol use for hypertension in pregnancy and adverse infant outcomes. Eur J Obstet Gynecol Reprod Biol. 2014;175:124–128. doi: 10.1016/j.ejogrb.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 48.Eggertsen R, Sivertsson R, Andrén L, Hansson L. Haemodynamic effects of carvedilol, a new beta-adrenoceptor blocker and precapillary vasodilator in essential hypertension. J Hypertens. 1984;2:529–534. doi: 10.1097/00004872-198410000-00013. [DOI] [PubMed] [Google Scholar]
- 49.Wehland M, Grosse J, Simonsen U, Infanger M, Bauer J, Grimm D. The effects of newer beta-adrenoceptor antagonists on vascular function in cardiovascular disease. Curr Vasc Pharmacol. 2012;10:378–390. doi: 10.2174/157016112799959323. [DOI] [PubMed] [Google Scholar]
- 50.Saunders E, Smith WB, DeSalvo KB, Sullivan WA. The efficacy and tolerability of nebivolol in hypertensive African American patients. J Clin Hypertens (Greenwich) 2007;9:866–875. doi: 10.1111/j.1524-6175.2007.07548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Punzi H, Lewin A, Lukić T, Goodin T, Wei C. Efficacy and safety of nebivolol in Hispanics with stage I-II hypertension: a randomized placebo-controlled trial. Ther Adv Cardiovasc Dis. 2010;4:349–357. doi: 10.1177/1753944710387629. [DOI] [PubMed] [Google Scholar]
- 52.Greathouse M. Nebivolol efficacy and safety in patients with stage I-II hypertension. Clin Cardiol. 2010;33:20–27. doi: 10.1002/clc.20508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lewin A, Punzi H, Luo X, Stapff M. Nebivolol monotherapy for patients with systolic stage II hypertension: results of a randomized, placebo-controlled trial. Clin Ther. 2013;35:142–152. doi: 10.1016/j.clinthera.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 54.Germino FW, Lin Y, Pejović V, Bowen L. Efficacy and tolerability of nebivolol: does age matter? A retrospective analysis of three randomized, placebo-controlled trials in stage I-II hypertension. Ther Adv Cardiovasc Dis. 2012;6:185–199. doi: 10.1177/1753944712459593. [DOI] [PubMed] [Google Scholar]
- 55.Papademetriou V. Comparison of Nebivolol monotherapy versus Nebivolol in combination with other antihypertensive therapies for the treatment of hypertension. Am J Cardiol. 2009;103:273–278. doi: 10.1016/j.amjcard.2008.08.063. [DOI] [PubMed] [Google Scholar]
- 56.Acelajado MC, Pisoni R, Dudenbostel T, Oparil S, Calhoun DA, Glasser SP. Both morning and evening dosing of nebivolol reduces trough mean blood pressure surge in hypertensive patients. J Am Soc Hypertens. 2012;6:66–72. doi: 10.1016/j.jash.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davis JT, Pasha DN, Khandrika S, Fung MM, Milic M, O’Connor DT. Central hemodynamics in prehypertension: effect of the β-adrenergic antagonist nebivolol. J Clin Hypertens (Greenwich) 2013;15:69–74. doi: 10.1111/jch.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tamargo J, Segura J, Ruilope LM. Diuretics in the treatment of hypertension. Part 1: thiazide and thiazide-like diuretics. Expert Opin Pharmacother. 2014;15:527–547. doi: 10.1517/14656566.2014.879118. [DOI] [PubMed] [Google Scholar]
- 59.Cooper-DeHoff RM, Bird ST, Nichols GA, Delaney JA, Winterstein AG. Antihypertensive drug class interactions and risk for incident diabetes: a nested case-control study. J Am Heart Assoc. 2013;2:e000125. doi: 10.1161/JAHA.113.000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Messerli FH, Grossman E, Goldbourt U. Are beta-blockers efficacious as first-line therapy for hypertension in the elderly? A systematic review. J Am Med Assoc. 1998;279:1903–1907. doi: 10.1001/jama.279.23.1903. [DOI] [PubMed] [Google Scholar]
- 61.Messerli FH, Grossman E. Beta-blockers and diuretics: to use or not to use. Am J Hypertens. 1999;12:157–163. doi: 10.1016/s0895-7061(99)00220-4. [DOI] [PubMed] [Google Scholar]
- 62.Levi-Marpillat N, Macquin-Mavier I, Tropeano AI, Parati G, Maison P. Antihypertensive drug classes have different effects on short-term blood pressure variability in essential hypertension. Hypertens Res. 2014;37:585–590. doi: 10.1038/hr.2014.33. [DOI] [PubMed] [Google Scholar]
- 63.Roush GC, Kaur R, Ernst ME. Diuretics: a review and update. J Cardiovasc Pharmacol Ther. 2014;19:5–13. doi: 10.1177/1074248413497257. [DOI] [PubMed] [Google Scholar]
- 64.Buscemi S, Nicolucci A, Lucisano G, Galvano F, Grosso G, Massenti FM, et al. Impact of chronic diuretic treatment on glucose homeostasis. Diabetol Metab Syndr. 2013;5:80. doi: 10.1186/1758-5996-5-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bruderer S, Bodmer M, Jick SS, Meier CR. Use of diuretics and risk of incident gout: a population-based case-control study. Arthritis Rheumatol. 2014;66:185–196. doi: 10.1002/art.38203. [DOI] [PubMed] [Google Scholar]
- 66.Price AL, Lingvay I, Szczepaniak EW, Wiebel J, Victor RG, Szczepaniak LS. The metabolic cost of lowering blood pressure with hydrochlorothiazide. Diabetol Metab Syndr. 2013;5:35. doi: 10.1186/1758-5996-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burnier M, Vuignier Y, Wuerzner G. State-of-the-art treatment of hypertension: established and new drugs. Eur Heart J. 2014;35:557–562. doi: 10.1093/eurheartj/eht465. [DOI] [PubMed] [Google Scholar]
- 68.Nilsson P. Antihypertensive efficacy of zofenopril compared with atenolol in patients with mild to moderate hypertension. Blood Press Suppl. 2007;2:25–30. doi: 10.1080/08038020701561745. [DOI] [PubMed] [Google Scholar]
- 69.Zeltner R, Poliak R, Stiasny B, Schmieder RE, Schulze BD. Renal and cardiac effects of antihypertensive treatment with ramipril vs metoprolol in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2008;23:573–579. doi: 10.1093/ndt/gfm731. [DOI] [PubMed] [Google Scholar]
- 70.Koh KK, Quon MJ, Han SH, Lee Y, Kim SJ, Koh Y, et al. Distinct vascular and metabolic effects of different classes of anti-hypertensive drugs. Int J Cardiol. 2010;140:73–81. doi: 10.1016/j.ijcard.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fan X, Han Y, Sun K, Wang Y, Xin Y, Bai Y, et al. Sex differences in blood pressure response to antihypertensive therapy in Chinese patients with hypertension. Ann Pharmacother. 2008;42:1772–1781. doi: 10.1345/aph.1L036. [DOI] [PubMed] [Google Scholar]
- 72.Cağlar N, Dincer I. Comparison between nebivolol and ramipril in patients with hypertension and left ventricular hypertrophy: a randomized open blinded end-point (PROBE) trial. Eur Rev Med Pharmacol Sci. 2011;15:1359–1368. [PubMed] [Google Scholar]
- 73.Weber MA, Basile J, Stapff M, Khan B, Zhou D. Blood pressure effects of combined b-blocker and angiotensin-converting enzyme inhibitor therapy compared with the individual agents: a placebo-controlled study with nebivolol and lisinopril. J Clin Hypertens (Greenwich) 2012;14:588–592. doi: 10.1111/j.1751-7176.2012.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koumaras C, Tziomalos K, Stavrinou E, Katsiki N, Athyros VG, Mikhailidis DP, et al. Effects of renin-angiotensin-aldosterone system inhibitors and beta-blockers on markers of arterial stiffness. J Am Soc Hypertens. 2014;8:74–82. doi: 10.1016/j.jash.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 75.Jin Y, Thijs L, Richart T, Li Y, Dolan E, Wang JG, et al. Responses of the ambulatory arterial stiffness index and other measures of arterial function to antihypertensive drugs. Hypertens Res. 2011;34:489–495. doi: 10.1038/hr.2010.256. [DOI] [PubMed] [Google Scholar]
- 76.Celik T, Iyisoy A, Acikel C, Yuksel C, Celik M, Yaman H, et al. The comparative effects of metoprolol and perindopril on aortic elasticity in young patients with prehypertension. Blood Press Monit. 2008;13:169–176. doi: 10.1097/MBP.0b013e3282fed786. [DOI] [PubMed] [Google Scholar]
- 77.Kosch M, Levers A, Lang D, Bartels V, Rahn KH, Pavenstädt H, et al. Randomized, double-blind study of valsartan versus metoprolol on arterial distensibility and endothelial function in essential hypertension. Nephrol Dial Transplant. 2008;23:2280–2285. doi: 10.1093/ndt/gfm936. [DOI] [PubMed] [Google Scholar]
- 78.Moltzer E, Mattace Raso FU, Karamermer Y, Boersma E, Webb GD, Simoons ML, et al. Comparison of Candesartan versus Metoprolol for treatment of systemic hypertension after repaired aortic coarctation. Am J Cardiol. 2010;105:217–222. doi: 10.1016/j.amjcard.2009.08.674. [DOI] [PubMed] [Google Scholar]
- 79.Heitmann J, Greulich T, Reinke C, Koehler U, Vogelmeier C, Becker HF, et al. Comparison of the effects of nebivolol and valsartan on BP reduction and sleep apnoea activity in patients with essential hypertension and OSA. Curr Med Res Opin. 2010;26:1925–1932. doi: 10.1185/03007995.2010.497326. [DOI] [PubMed] [Google Scholar]
- 80.Collier DJ, Poulter NR, Dahlöf B, Sever PS, Wedel H, Buch J, et al. Impact of amlodipine-based therapy among older and younger patients in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA) J Hypertens. 2011;29:583–591. doi: 10.1097/HJH.0b013e328342c845. [DOI] [PubMed] [Google Scholar]
- 81.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, et al. Effects of beta blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol. 2010;9:469–480. doi: 10.1016/S1474-4422(10)70066-1. [DOI] [PubMed] [Google Scholar]
- 82.Ostergren J, Poulter NR, Sever PS, Dahlöf B, Wedel H, Beevers G, et al. The Anglo-Scandinavian Cardiac Outcomes Trial: blood pressure-lowering limb: effects in patients with type II diabetes. J Hypertens. 2008;26:2103–2111. doi: 10.1097/HJH.0b013e328310e0d9. [DOI] [PubMed] [Google Scholar]
- 83.Manisty CH, Zambanini A, Parker KH, Davies JE, Francis DP, Mayet J, et al. Differences in the magnitude of wave reflection account for differential effects of amlodipine- versus atenolol-based regimens on central blood pressure: an Anglo-Scandinavian Cardiac Outcome Trial substudy. Hypertension. 2009;54:724–730. doi: 10.1161/HYPERTENSIONAHA.108.125740. [DOI] [PubMed] [Google Scholar]
- 84.Peacock WF, Varon J, Baumann BM, Borczuk P, Cannon CM, Chandra A, et al. CLUE: a randomized comparative effectiveness trial of IV nicardipine versus labetalol use in the emergency department. Crit Care. 2011;15:157. doi: 10.1186/cc10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Poulter NR, Dobson JE, Sever PS, Dahlöf B, Wedel H, Campbell NR. ASCOT Investigators: Baseline heart rate, antihypertensive treatment, and prevention of cardiovascular outcomes in ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial) J Am Coll Cardiol. 2009;54:1154–1161. doi: 10.1016/j.jacc.2009.04.087. [DOI] [PubMed] [Google Scholar]
- 86.Bangalore S, Messerli FH, Cohen JD, Bacher PH, Sleight P, Mancia G, et al. Verapamil-sustained release-based treatment strategy is equivalent to atenolol-based treatment strategy at reducing cardiovascular events in patients with prior myocardial infarction: an INternational VErapamil SR-Trandolapril (INVEST) substudy. Am Heart J. 2008;156:241–247. doi: 10.1016/j.ahj.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 87.Johnson JA, Gong Y, Bailey KR, Cooper-DeHoff RM, Chapman AB, Turner ST, et al. Hydrochlorothiazide and atenolol combination antihypertensive therapy: effects of drug initiation order. Clin Pharmacol Ther. 2009;86:533–539. doi: 10.1038/clpt.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pareek A, Salkar H, Mulay P, Desai S, Chandurkar N, Redkar N. A randomized, comparative, multicentric evaluation of atenolol/amlodipine combination with atenolol alone in essential hypertensive patients. Am J Ther. 2010;17:46–52. doi: 10.1097/MJT.0b013e3181a9db74. [DOI] [PubMed] [Google Scholar]
- 89.Dietz R, Dechend R, Yu CM, Bheda M, Ford J, Prescott MF, et al. Effects of the direct renin inhibitor aliskiren and atenolol alone or in combination in patients with hypertension. J Renin Angiotensin Aldosterone Syst. 2008;9:163–175. doi: 10.1177/1470320308096411. [DOI] [PubMed] [Google Scholar]
- 90.Matsuzaki M, Ogihara T, Umemoto S, Rakugi H, Matsuoka H, Shimada K, et al. Combination Therapy of Hypertension to Prevent Cardiovascular Events Trial Group Collaborators (1031). Prevention of cardiovascular events with calcium channel blocker-based combination therapies in patients with hypertension: a randomized controlled trial. J Hypertens. 2011;29:1649–1659. doi: 10.1097/HJH.0b013e328348345d. [DOI] [PubMed] [Google Scholar]
- 91.Zhang JL, Zheng X, Zou DJ, Qiu JL, Zhao XX, Qin YW. Effect of metformin on weight gain during antihypertensive treatment with a beta-blocker in Chinese patients. Am J Hypertens. 2009;22:884–890. doi: 10.1038/ajh.2009.93. [DOI] [PubMed] [Google Scholar]
- 92.Pareek A, Chandurkar NB, Sharma R, Tiwari D, Gupta BS. Efficacy and tolerability of a fixed-dose combination of metoprolol extended release/amlodipine in patients with mild-to-moderate hypertension: a randomized, parallel-group, multicentre comparison with losartan plus amlodipine. Clin Drug Investig. 2010;30:123–131. doi: 10.2165/11531770-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 93.Bakris GL, Iyengar M, Lukas MA, Ordronneau P, Weber MA. Effect of combining extended-release carvedilol and lisinopril in hypertension: results of the COSMOS study. J Clin Hypertens (Greenwich) 2010;12:678–686. doi: 10.1111/j.1751-7176.2010.00341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Neutel JM, Smith DH, Gradman AH. Adding nebivolol to ongoing antihypertensive therapy improves blood pressure and response rates in patients with uncontrolled stage I-II hypertension. J Hum Hypertens. 2010;24:64–73. doi: 10.1038/jhh.2009.33. [DOI] [PubMed] [Google Scholar]
- 95.Kelly AS, Gonzalez-Campoy JM, Rudser KD, Katz H, Metzig AM, Thalin M, et al. Carvedilol-lisinopril combination therapy and endothelial function in obese individuals with hypertension. J Clin Hypertens (Greenwich) 2012;14:85–91. doi: 10.1111/j.1751-7176.2011.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bell DS, Bakris GL, McGill JB. Comparison of carvedilol and metoprolol on serum lipid concentration in diabetic hypertensive patients. Diabetes Obes Metab. 2009;11:234–238. doi: 10.1111/j.1463-1326.2008.00927.x. [DOI] [PubMed] [Google Scholar]
- 97.Jawa A, Nachimuthu S, Pendergrass M, Asnani S, Fonseca V. Beta-blockers have a beneficial effect upon endothelial function and microalbuminuria in African-American subjects with diabetes and hypertension. J Diabetes Complications. 2008;22:303–308. doi: 10.1016/j.jdiacomp.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Law M, Wald N, Morris J. Lowering blood pressure to prevent myocardial infarction and stroke: a new preventive strategy. Health Technol Assess. 2003;7:1–94. doi: 10.3310/hta7310. [DOI] [PubMed] [Google Scholar]
- 99.Degli-Esposti L, Di Martino M, Saragoni S, Sgreccia A, Capone A, Buda S, et al. Pharmacoeconomics of antihypertensive drug treatment: an analysis of how long patients remain on various antihypertensive therapies. J Clin Hypertens (Greenwich) 2004;6:76–84. doi: 10.1111/j.1524-6175.2004.03044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ninomiya T, Perkovic V, Turnbull F, Neal B, Barzi F, et al. Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure lowering and major cardiovascular events in people with and without chronic kidney disease: meta-analysis of randomised controlled trials. BMJ. 2013;347:5680. doi: 10.1136/bmj.f5680. [DOI] [PMC free article] [PubMed] [Google Scholar]


