Abstract
Objectives:
To determine whether site-specific mouth rinsing with oral disinfectants can improve oral odor beyond the traditional panoral mouth disinfection with mouth rinses by targeting specifically oral malodor implicated anaerobic bacteria
Methods:
Twenty healthy fasting subjects volunteered for a blinded prospective, descriptive correlational crossover cross-section clinical trial conducted during the month of Ramadan between July and August 2013 in Albaha province in Saudi Arabia involving the application of Listerine® Cool Mint® mouth rinse by either the traditional panoral rinsing method, or a site-specific disinfection method targeting the subgingival and supragingival plaque and the posterior third of the tongue dorsum, while avoiding the remaining locations within the oral cavity. The viable anaerobic and aerobic bacterial counts, volatile sulfur compounds (VSCs) levels, organoleptic assessment of oral odor, and the tongue-coating index were compared at baseline, one, 5, and 9 hours after the treatment.
Results:
The site-specific disinfection method reduced the VSCs and anaerobic bacterial loads while keeping the aerobic bacterial numbers higher than the traditional panoral rinsing method.
Conclusion:
Site-specific disinfection can more effectively maintain a healthy oral cavity by predominantly disinfecting the niches of anaerobic bacteria within the oral cavity.
The oral cavity, in health and disease, is a highly diverse topographical and biological environment containing many surfaces, fissures, and sulci harboring bacterial biofilms.1 Dental caries, periodontitis, and halitosis are among the most common infectious diseases known to humans.2,3 It is estimated that 85-90% of halitosis originates from the oral cavity.4 Gram-negative anaerobic bacteria, such as Porphyromonas, Prevotella, Actinobacillus, and Fusobacterium are implicated in producing foul volatile sulphur compounds (VSCs), predominately hydrogen sulfide (H2S), and methyl mercaptan (CH3SH) - produced by putrefaction of proteins - which cause halitosis. A correlation has been established a long time ago between halitosis and the shift in the oral microbiota from predominately Gram-positive aerobes to Gram-negative anaerobes.5 It is now hypothesized that the tongue dorsum is an important site for anaerobic putrefaction, and an origin for malodor.5,6 Moreover, tongue dorsum malodor and the amount of coating present on it were found to be proportional to periodontal probing depths, and correlated with the existence of surface fissures on the tongue dorsum.5,6 There is a correlation between VSCs and periodontal disease since it was shown that periodontal disease could increase the presence of these aforementioned anaerobic bacteria in the subgingival plaque and tongue dorsum.5,6 Oral bacteria show site-specific or preferential colonization of different surfaces, fissures, and sulci in the oral cavity.1 That is, different oral locations contain noticeably different bacterial communities.7 Malodor-producing anaerobic bacteria have been found to accumulate in anaerobic environments, such as, subgingival and supragingival plaque material, and the coating of the crypts and fissures of the posterior dorsum of the tongue. Nonetheless, oral bacteria include not only pathogenic bacteria, but also commensal bacteria,1 and strains that have been identified, isolated, and re-administered as health-promoting oral probiotic bacteria.8 Moreover, oral probiotics have been shown to deliver noteworthy success in the prevention of dental caries, plaque formation, streptococcal pharyngitis and oral malodor.8 Furthermore, molecular techniques failed to detect the anti-halitosis probiotic Streptococcus salivarius strain k12 on teeth surfaces and sulci.9 The literature contains many reports on the effects of mouth rinses,10 and chewing gums11 on oral malodor reduction. All of these methods, however, include a non-selective chemical antimicrobial regimen, which we hypothesize will reduce the non-pathogenic commensal, and potentially essential probiotic-like bacteria more than the Gram-negative anaerobic bacteria accumulating within plaques, crypts, and fissures.5 This study sought to investigate whether halitosis treatment could be improved by the selective targeting of Gram-negative anaerobic pathogens though the site-specific application of antimicrobial mouth rinses on the subgingival and supragingival plaque, and on the fissures and crypts of the posterior third of the tongue dorsum, while sparing the natural microbiota located on the remaining oral locations. The study design is based on the evaluation of aerobic-to-anaerobic salivary bacterial count ratios, and amount of VSCs after the use of selective site-specific mouth disinfection and traditional mouth-rinsing with Listerine® Cool Mint® mouth rinse in a crossover clinical experiment.
Methods
This is a blind, crossover clinical trail conducted between July and August 2013 in Albaha province, Saudi Arabia. The local Research Committee Institutional Review Board, Applied Medical Sciences, Albaha University provided ethical approval for the research, and all volunteers provided consent after receiving a comprehensive explanation of the procedures involved. The study adhered to the principles of the Helsinki Declaration. A PubMed (Medline) and EMBASE online search was conducted using the search words: ‘oral bacteria’, ‘halitosis bacteria’, ‘tongue bacteria’, ‘oral probiotic’, ‘oral malodor and bacteria’, ‘periodontitis and VSC-producing bacteria’ and ‘dental caries bacteria’. The identified articles using the search queries mentioned above were retrieved and studied. Lastly, all relevant articles were downloaded, read, and cited as appropriate within the introduction and discussion sections. Twenty medically healthy subjects from Albaha, Saudi Arabia volunteered to participate in this research during the Islamic holy month of Ramadan, the month of fasting. They all reported no antibiotic usage for the last 3 months, and no antiseptic mouth rinse use one week prior to the commencement of the study. Subjects were all dentate healthy Saudi males aged 17-65 years who adhered to the ritual of fasting. No subjects had received any professional periodontal treatments (prophylactic scaling, root planning, and periodontal surgery), or professional advice during the previous year. Prior to the inception of the study each subject was provided with an informed consent form. The subjects were selected based on these criteria described from a total of 43 subjects that responded to an online request on the e-learning blog: www.alqumber.wordpress.com. The onset of the study was the first day of the month of Ramadan (July 2013). All subjects observed fasting from dawn (4 AM) until dusk (7 PM), and ate and drank only during the night throughout the 8-day study period. Participants have 2 main meals per day; one at 7 PM after sunset, and the next just before 4 AM. The experiment started 6 hours after starting the fast by obtaining a saliva sample, an organoleptic assessment of mouth air, Winkel tongue-coating index (WTCI), and VSCs measurement using a Halimeter® (Interscan Corporation, Chatsworth, CA, USA). Each subject used Listerine® Cool Mint® mouth rinse (Johnson and Johnson, Skillman, New Jersey, USA) in the traditional method (TM) according to the manufacturer's instructions for the first half of the study period (4 days). Briefly, 20 ml of the mouth rinse was used full strength to rinse the whole mouth for 20 seconds. For the site-specific method (SSM), the volunteers were asked to apply a thick layer of Colgate® toothpaste on the anterior two-thirds of the tongue dorsum, the palate, and the medial surfaces of the cheeks. Then, they applied one ml of Listerine® Cool Mint® mouth rinse on each last molar, and immediately brushed (with a clean soft Colgate® Extra Clean tooth brush) all their teeth surfaces (including the upper teeth). Afterwards, an additional one ml of Listerine® Cool Mint® mouth rinse was applied on the posterior third of the tongue dorsum. Then, the posterior dorsum was brushed with Listerine® Cool Mint® mouth rinse with the same toothbrush for 10 seconds, and the toothbrush was rinsed with water. This brushing and rinsing cycle was repeated 3 times for the posterior dorsum before the whole tongue and teeth were washed with water, and brushing to remove all the Listerine® Cool Mint® mouth rinse and toothpaste. This protocol was carried out under supervision during the second half of the study period (the second 4 days of the 8-day study). An additional saliva sample, an organoleptic assessment of mouth air, WTCI, and VSCs measurement were carried out at baseline, and after one, 5, and 9 hours. The evaluation period ended before breaking the fast at 7 PM, and after completion of the 9-hour assessment. Thus, the intervention and the entire assessment period were carried out during fasting (complete abstinence from all ingestible materials, for example, food, liquids, and smoking).
Oral malodor assessment was carried out using a 0-5 scale after mouth closure for one minute by a trained examiner located at a distance of 10 cm from the participant's mouth. Afterwards, the coating of the tongue was assessed by the WTCI, dividing the tongue into 6 parts, and scoring every part from 0 to 2. Mouth levels of VSC were determined in parts per billion (ppb), using a portable detector, Halimeter® (Interscan Corporation, Chatsworth, CA, USA) after one minute of mouth closure. Two successive measurements were carried out, and the mean value used. After the end of the study, each subject was asked which treatment protocol they favor, and whether they aim to continue to use any of the 2 protocols.
Saliva was obtained by asking the subjects to spit into a test tube without any stimulation. Specimens were processed immediately by initially, vortexing for 30 seconds, then serially diluting the sample in normal saline and inoculation in 2 series of 5% sheep blood agar plates (Oxoid, Basingstoke, England). One series was incubated aerobically at 37°C for 24 hours, and the second series anaerobically for 48 hours. Then the plates showing 30-300 colonies were used to calculate colony-forming units (CFU) per ml of saliva. The CFU/ml results were averaged and log transformed. The VSCs presented as averages. The analysis of variance (ANOVA test) was used to estimate whether differences are significant. The changes between the baseline and post-treatment in the different group were measured using paired t-test. The Statistical Package for Social Sciences version 14 program (SPSS Inc., Chicago, IL, USA) was used for all statistical analysis. All comparisons were declared significant at p<0.05.
Results
At baseline, the organoleptic values ranged from 1.4-1.9 (standard deviation [SD] = 0.5-0.8) while WTCI scores were more heterogeneous, ranging from 2-10. There was no significant difference in the organoleptic values between the 2 groups. The SSM group showed lower WTCI scores at baseline (4.7, SD=1.4), whereas in the TM group the mean WTCI was 6.2 (SD=1.4). The mean values were significantly different (p=0.05). No correlation was found between WTCI and VSC. However, VSC correlated with organoleptic scores (r=0.45, p=0.05). The mean VSCs levels are present in Figure 1. The mean log-CFU/ml of aerobic bacteria in saliva at baseline did not show differences between the 2 groups, but at one, 5, and 9 hours post-treatment higher aerobic log-CFU/ml was observed in the SSM group than in the TM group (Figure 2). The mean log-CFU/ml of aerobic bacteria in saliva increased from the one hour post-treatment levels, and reached 5.87 and 6.3 for the TM group (at 5 hours), and 6.75 and 7.12 for the SSM group (at 9 hours) post-treatment, and the differences were significant (p=0.034). The log-CFU/ml of anaerobic bacteria in saliva at baseline was almost the same (7.25 for the TM and 7.20 for the SSM groups). Figure 3 illustrates the changes observed in the means of log-CFU/ml of anaerobic bacteria post-treatment. A sharper reduction was detected with the SSM. All volunteers indicated that the SSM method provided them with a better feeling and more comfort than the TM method. They also indicated they are keen to continue the SSM method after completion of the study. Finally, the ratio of aerobic/anaerobic bacterial count levels remains above parity (>1) in the SSM group, but was always below parity (<1) in the TM group throughout the 9-hour assessment period (Figure 4).
Figure 1.
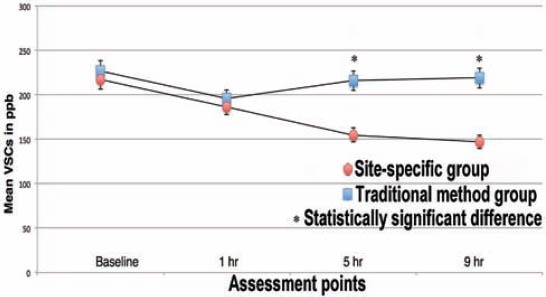
Mean volatile sulfur compounds (VSCs) in parts per million (ppb) for each group at every assessment point.
Figure 2.
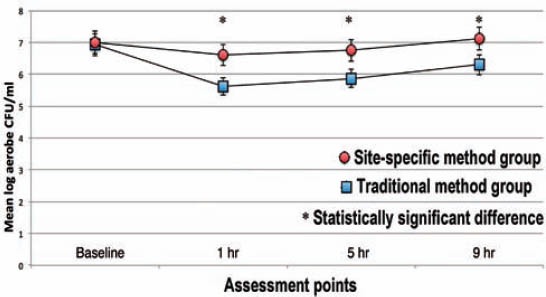
Mean log colony forming units per milliliter (CFU/ml) of aerobic bacteria for each group at every assessment point.
Figure 3.
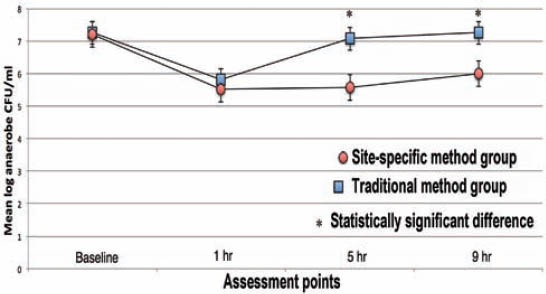
Mean log colony forming units per milliliter (CFU/ml) of anaerobic bacteria for each group at every assessment point.
Figure 4.
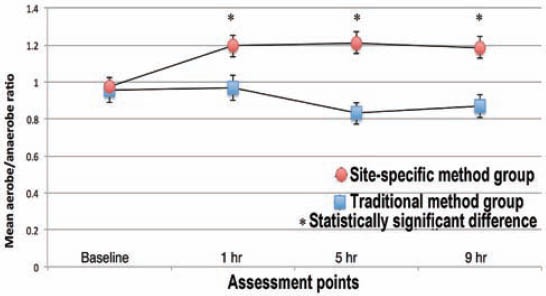
Mean aerobes/anerobes ratio for each group at every assessment point.
Discussion
It is estimated that one third of the general population suffers from halitosis, which causes social and psychological problems.2 The established correlation between oral malodor and the shift in the oral microbiota from predominately Gram-positive to Gram-negative anaerobes,5 plus the usefulness of Gram-positive commensal bacteria in promoting oral health8 expose a serious weakness in the currently prescribed protocol for antimicrobial mouth rinses, that is, their unspecific antimicrobial nature. Given Gram-positive oral probiotics, which include strains sourced from the oral cavity itself, have been shown to be beneficial in the medical management of tooth decay, plaque formation, streptococcal pharyngitis, and oral malodor,12 it is clear that the traditional mouth rinsing practice is a proverbial double-edged sword. On one hand, mouth rinses kill anaerobic pathogenic bacteria; on the other, they kill the commensal Gram-positive beneficial bacteria. This fact can be used to explain 2 important phenomena. First, that tongue cleaning was shown to give lower malodor reduction than periodontal treatment in patients with periodontitis,13 and second, the observed short-term effect of mouth rinses on VSCs and aerobic/anaerobic ratios of bacteria.14 Thus, we aim to examine the hypothesis that a novel site-specific mouth rinsing protocol targeting mouth crypts, fissures, and subgingival and supragingival plaque can kill more anaerobes than aerobes.
The palatine and the anterior tongue dorsal mucosae and the inside lining of the cheeks are not known to support the buildup of food debris, or anaerobic bacteria. In addition, there are no recommendations for brushing these surfaces with toothbrushes or other mouth scraping instruments. These surfaces are smooth, unlike the sub-and supragingival plaque and the posterior tongue dorsum, which support anaerobic bacteria.1 Thus, we sought to investigate wether targeting the reservoirs of anaerobic bacteria with antiseptic mouth rinsing, while sparing other oral ecosystems may have a longer-term reduction effect on mouth air VSC values, and stabilize the aerobic/anaerobic bacterial ratios at a higher level, regardless of any observed short-term effects. Therefore, this study was designed to compare a site-specific mouth disinfection protocol to the traditional panoral rinsing procedure. While this study used fasting healthy volunteers not suffering from halitosis, it still showed that the baseline VSC values were sufficiently elevated to test the hypothesis. The fasting ritual practiced ensured that the subjects did not smoke, or ingest any liquids or food throughout the experimentation period that may affect the results. The covering of untargeted surfaces with toothpaste aims to inhibit the effects of the mouth rinse if any of it was to spill over these surfaces.15
The study design, which took place in the holy month of Ramadan, when people change their sleeping habits so that they wake up at 9 AM, and start their working day at 10 AM, assessed the halitosis-related outcome variables in the morning after waking up. Then the 2 protocols, SSM and TM were evaluated in a crossover study in an 8-day period without mechanical plaque management. The study demonstrated that site-specific rinsing could reduce and maintain VSC levels lower then panoral rinsing for up to 9 hours. The site-specific method was also the most effective in reducing the number of viable anaerobic bacteria, and sustaining the aerobic bacterial levels near baseline values. Unlike in the panoral rinsing group, the ratio of aerobic/anaerobic levels remained above one for the site-specific disinfection group throughout the 9-hour assessment period.
More research can now address different disinfection methods to improve oral hygiene by limiting the growth of offensive bacteria while allowing probiotic bacteria to remain present, and effective in limiting oral diseases. The development of methods capable of site-specific disinfection of oral surfaces to improve oral hygiene is now warranted. In addition, in this study the bacterial communities were isolated metabolically into only 2 large groups, the aerobes and the anaerobes. More detailed (in depth) study of the different microbial groups (genera and species) would allow for a better understanding of the oral ecosystem and its relationship to health and disease. Moreover, even when this study adopted a crossover design to prevent limitations in the collection or interpretation of the results, nonetheless, the study did not isolate the effect of fasting, if any exist, on the mouth microbial ecology, and this needs to be addressed in future research. This study warrants more clinical trials aimed at testing the site-specific protocol on halitosis patients. If the results were similar to our findings, the prescribed methodology for mouth rinses would need to be adjusted accordingly. Then, this site-specific rinsing method should be prescribed for improving oral health outcomes in halitosis sufferers and the general population.
In conclusion, this study demonstrated the suitability of a site-specific oral disinfection protocol in reducing anaerobes and VSC levels while keeping the aerobes at near baseline values. In addition, the study indicated that the traditional panoral rinsing protocol can kill both the incriminated anaerobes and the welcomed aerobes, and cause the aerobe/anaerobe ratio to remain below one, and the VSC value to be reduced only temporarily. The better outcomes shown from the site-specific disinfection may be attributed to the killing of more anaerobes then aerobes, thus allowing for a more balanced oral microbial ecosystem in which malodor-producing anaerobes are preferentially reduced and allowed to be out-competed by the aerobes. More studies are now needed to confirm these findings and adjust the mouth disinfection protocol accordingly.
New Peer Reviewers.
Join our team of expert peer reviewers for the Saudi Medical Journal by sending an enquiry and summarized CV to info@smj.org.sa. Note that SMJ reviewers, whose reviews are returned on time and are judged satisfactory by the Editors, may receive 1 CME credit per review, with a maximum of 5 credits per year, from the Saudi Council for Health Specialties.
References
- 1.Preza D, Olsen I, Willumsen T, Grinde B, Paster BJ. Diversity and site-specificity of the oral microflora in the elderly. Eur J Clin Microbiol Infect Dis. 2009;28:1033–1040. doi: 10.1007/s10096-009-0743-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bornstein MM, Kislig K, Hoti BB, Seemann R, Lussi A. Prevalence of halitosis in the population of the city of Bern, Switzerland: a study comparing self-reported and clinical data. Eur J Oral Sci. 2009;117:261–267. doi: 10.1111/j.1600-0722.2009.00630.x. [DOI] [PubMed] [Google Scholar]
- 3.Kämppi A, Tanner T, Päkkilä J, Patinen P, Järvelin MR, Tjäderhane L, et al. Geographical distribution of dental caries prevalence and associated factors in young adults in Finland. Caries Res. 2013;47:346–354. doi: 10.1159/000346435. [DOI] [PubMed] [Google Scholar]
- 4.Scully C, Greenman J. Halitosis (breath odor) Periodontol 2000. 2008;48:66–75. doi: 10.1111/j.1600-0757.2008.00266.x. [DOI] [PubMed] [Google Scholar]
- 5.Amou T, Hinode D, Yoshioka M, Grenier D. Relationship between halitosis and periodontal disease - associated oral bacteria in tongue coatings. Int J Dent Hyg. 2014;12:145–151. doi: 10.1111/idh.12046. [DOI] [PubMed] [Google Scholar]
- 6.Yasukawa T, Ohmori M, Sato S. The relationship between physiologic halitosis and periodontopathic bacteria of the tongue and gingival sulcus. Odontology. 2010;98:44–51. doi: 10.1007/s10266-009-0114-7. [DOI] [PubMed] [Google Scholar]
- 7.Allaker RP, Waite RD, Hickling J, North M, McNab R, Bosma MP, et al. Topographic distribution of bacteria associated with oral malodour on the tongue. Arch Oral Biol. 2008;53(Suppl 1):S8–S12. doi: 10.1016/S0003-9969(08)70003-7. [DOI] [PubMed] [Google Scholar]
- 8.Saha S, Tomaro-Duchesneau C, Rodes L, Malhotra M, Tabrizian M, Prakash S. Investigation of probiotic bacteria as dental caries and periodontal disease biotherapeutics. Benef Microbes. 2014;5:447–460. doi: 10.3920/BM2014.0011. [DOI] [PubMed] [Google Scholar]
- 9.Horz HP, Meinelt A, Houben B, Conrads G. Distribution and persistence of probiotic Streptococcus salivarius K12 in the human oral cavity as determined by real-time quantitative polymerase chain reaction. Oral Microbiol Immunol. 2007;22:126–130. doi: 10.1111/j.1399-302X.2007.00334.x. [DOI] [PubMed] [Google Scholar]
- 10.Shinada K, Ueno M, Konishi C, Takehara S, Yokoyama S, Kawaguchi Y. A randomized double blind crossover placebo-controlled clinical trial to assess the effects of a mouthwash containing chlorine dioxide on oral malodor. Trials. 2008;9:71. doi: 10.1186/1745-6215-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keller MK, Bardow A, Jensdottir T, Lykkeaa J, Twetman S. Effect of chewing gums containing the probiotic bacterium Lactobacillus reuteri on oral malodour. Acta Odontol Scand. 2012;70:246–250. doi: 10.3109/00016357.2011.640281. [DOI] [PubMed] [Google Scholar]
- 12.Pradeep K, Kuttappa MA, Prasana KR. Probiotics and oral health: an update. SADJ. 2014;69:20–24. [PubMed] [Google Scholar]
- 13.Pham TA, Ueno M, Zaitsu T, Takehara S, Shinada K, Lam PH, et al. Clinical trial of oral malodor treatment in patients with periodontal diseases. J Periodontal Res. 2011;46:722–7229. doi: 10.1111/j.1600-0765.2011.01395.x. [DOI] [PubMed] [Google Scholar]
- 14.Roldán S, Herrera D, Santa-Cruz I, O’Connor A, González I, Sanz M. Comparative effects of different chlorhexidine mouth-rinse formulations on volatile sulphur compounds and salivary bacterial counts. J Clin Periodontol. 2004;31:1128–1134. doi: 10.1111/j.1600-051X.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- 15.Sheen S, Eisenburger M, Addy M. Effect of toothpaste on the plaque inhibitory properties of a cetylpyridinium chloride mouth rinse. J Clin Periodontol. 2003;30:255–260. doi: 10.1034/j.1600-051x.2003.300312.x. [DOI] [PubMed] [Google Scholar]


