Abstract
Objectives:
To collect data on all detectable histologic and immune alterations from the kidneys of 55 autopsy cases.
Methods:
This prospective study was carried out at the Department of Pathology, Medical Faculty, Trakya University, Edirne, Turkey. Fifty-five cases were subjected to the study among 248 autopsies that were performed in 2011 and 2012. All kidney samples were evaluated under a light microscope and fresh tissue samples were used for immunofluorescence microscopy. Immunohistochemically kappa (κ) and lambda (λ) antibodies were applied to the tissue sections. The glomerular, tubulo-interstitial, and vascular alterations, as well as immune depositions were noted.
Results:
The microscopic morphology was close to normal histology in only 23 cases, and 23 cases had glomerular alterations. Nineteen cases had at least one immune deposition. There was immunoglobulin A deposition in 13 cases, and 9 cases showed positivity for both κ and λ immunohistochemically, and there was no clonal positivity.
Conclusion:
The most striking outcome of our study is the high rate of immune depositions. There was also a significant number of glomerular and non-glomerular renal alterations.
End stage kidney disease (ESKD) is one of the leading health problems worldwide with high morbidity, and mortality rates.1,2 Biopsy proven data shows that glomerulonephritis (GN) is the most important cause of chronic renal insufficiency, in which primary GN comprises the biggest proportion, especially in Western and Eastern Europe.3,4 Vascular diseases (VDs) and tubulointerstitial diseases (TIDs) seem to be less frequent diseases leading to chronic renal insufficiency. However, biopsy policies and biopsy indications are changing from country to country, and in daily practice kidneys prone to VDs and TIDs are rarely biopsied in most regions. Regarding the daily clinical practice, although they are not proven by biopsies, VDs, and TIDs seem to be the leading cause of chronic renal insufficiency worldwide, where the former is a more important reason when compared with the latter. Among acute lesions developing renal insufficiency, tubular injury takes place after crescentic GN and necrotizing vasculitis.4,5 Incidence and prevalence studies of chronic renal diseases are mostly based on native renal biopsies, which usually depends on single center studies along with a retrospective review of the diagnostic criteria obtained from the files of patient registries.6-12 There are rarely regional multicenter or national studies, in which the diagnostic data are obtained from the national registry system for renal biopsies.1-5,13,14 These types of studies are of importance as they investigate the incidence of the categorized renal diseases to determine the leading causes of ESKD. However, it should be kept in mind that they are providing data only from a symptomatic population. Population based studies are needed to obtain the real incidence of renal lesions in an asymptomatic population. Although there are some retrospective studies on kidneys of patients that underwent nephrectomy due to kidney masses,15 it is not assumable that the data of those studies can reflect the real incidence of alterations in otherwise normal kidneys of the population. Thus, autopsy studies play an important role in achieving the closest information towards this target. There are a few autopsy studies related to renal diseases in the literature. Two of those studies16,17 aimed to investigate GN due to hepatitis C virus infection retrospectively in autopsy cases with prior known hepatitis C virus infection, thus, they did not provide any data regarding other possible causes of kidney diseases related to primary or secondary GN, VDs, or TIDs. The Hisayama study18 was a large retrospective autopsy study with 839 subjects, and the risk factors for glomerular sclerosis and vascular changes were sought without assistance of immunofluorescence or immunohistochemistry and lacked detailed data for possible reasons of glomerular sclerosis, except for the reasons of glomerular sclerosis based on vascular disorders of the kidneys. In a prospective autopsy kidney study,19 which was conducted on a West African urban community, the authors investigated the glomerular numbers and volumes, as well as renal pathology in 81 subjects. In this study,19 the histopathological changes were assessed with histochemical methods without guidance of immunofluorescence microscopy. In Turkey, there is very limited information regarding the epidemiology of kidney diseases, and it is mainly based on a few single center studies, concerned with retrospective evaluation of the data obtained from previous renal biopsies.20-22 Very recently a national registry system was created in Turkey for medical kidney biopsies, but we still need time to receive the first reliable data from this system. In the present study, we aimed to collect data obtained from the kidneys of 55 autopsy cases, regarding all detectable histologic and immune alterations. Likewise, we aimed to provide a regional data regarding kidney lesions in an asymptomatic population.
Methods
This prospective study was carried out at the Department of Pathology, Medical Faculty, Trakya University, Edirne, Turkey. The study was granted approval by the Ethics Committee of Clinical Research of Trakya University. Although there is 28 years of autopsy archive of tissue blocks and slides in this laboratory, we structured a prospective study as we aimed to use fresh frozen tissue samples for immunofluorescence microscopy. The kidneys of forensic autopsies performed in the years 2011 and 2012 were subjected to our study. After applying exclusion criteria, 55 cases were included in the study among the 248 autopsies that were performed in the 2-year period. The kidneys of 85 autopsies were not suitable for the study as their tissues were severely damaged due to fire burn or electrical burn, or because of the decay of the corpses due to long term submersion in water or nature before recognition. We also excluded 78 cases with known chronic systemic diseases, chronic organ failure, or chronic drug usage during the last 5 years of their lives. Eighteen cases who had died in the intensive care unit as a result of DIC, septic, or hypovolemic shock was also excluded from the study because of probable tubular damage in the kidneys. Finally, the frozen tissues of 12 cases showed severe freezing artefact, thus, they were also excluded from the study.
The kidneys of the remaining well-preserved 55 autopsies were included in the study. The age range of the autopsies was between 16 and 84 years, and the mean age was 48.8 years. Forty-three of the 55 autopsies were male, while 12 of the selected autopsies were female. All 55 autopsy cases had suspicious sudden death. According to the final autopsy reports, the subjects died due to myocardial infarction (MI [14 cases]), traffic accident (multiple trauma [11 cases]), drowning (6 cases), carbon monoxide (CO) intoxication (5 cases), methyl alcohol intoxication (2 cases), subarachnoid hemorrhage (4 cases), hemorrhage from upper gastrointestinal (GI) tract (one case), pulmonary embolism (3 cases), and gunshot wounds (6 cases), and 3 cases committed suicide by hanging. None of those 55 cases were admitted to the hospital before their death, thus it was not possible to obtain urine and blood samples for biochemical analysis, and it was not possible to perform a physical examination. According to the information obtained from their relatives, none of them had a prior known kidney disease. After bilateral resection, all kidneys were inspected macroscopically before and after their dissection, as described in the autopsy guidelines. Fresh tissue samples, representing cortico-medullary region, with dimensions of 1×1×0.2 cm from the right kidneys of each case were frozen in a -80ºC deep freezer for immunofluorescence microscopy procedure. Subsequently, all kidneys were fixed for 12 hours in 10% neutral buffered formaldehyde, and the formalin fixed tissues were sampled, each sample including the cortico-medullary region. Four micrometer thick sections were obtained from the paraffin embedded tissue samples, and were histochemically stained with hematoxylin and eosin, periodic acid-Schiff reagent (PAS), silver methanamine, and Masson's trichrome. Kongo red amyloid stain was applied to 8 µm thick sections of each kidney sample, and were evaluated under a polarized light microscope (Olympus BX51, Olympus Co., Shinjuku-ku, Tokyo, Japan). Two sections of 5 µm thickness from each paraffin block were obtained, and placed on a positively charged slide for performing immunohistochemical staining for kappa (κ) and lambda (λ) rabbit polyclonal light chain antibodies (Dako Inc., Glostrup, Denmark). All sections were examined under a light microscope (Olympus BX51, Olympus Co., Shinjuku-ku, Tokyo, Japan) by the same experienced pathologist in a blinded manner to the subjects. Immunofluorescence techniques were performed to the 5 µm thick sections of the fresh frozen tissue samples using fluorescence-labelled rabbit anti-human immunoglobulin (Ig)G, IgM, IgA, complement (C)3, fibrinogen, and C1q antibodies (Polyclonal, rabbit, Dako Inc., Glostrup, Denmark), and were evaluated under a light microscope (Nikon E200, Nikon Instruments, NY, USA) with an attached immunofluorescence device. The glomeruli, tubulo-interstitial region, and vascular structures were evaluated microscopically. Mesengial proliferation (with, or without increase in the matrix), and basement membrane thickening was scored from 1+ to 3+ (mild, moderate, severe) semi quantitatively. Global glomerular sclerosis was designated as absent, focal (<50%), or diffuse (≥50%). The immunofluorescence staining intensity of each antibody was scored from 1+ to 3+ semi quantitatively, and localized as membranous, or mesengial with a granular, or linear staining pattern. Tubular atrophy and interstitial inflammation (with, or without fibrosis) were designated as absent, focal, or diffuse. Arterial intimal thickening and arteriolar subintimal hyaline depositions were scored from 1+ to 3+ semi quantitatively. Other rare lesions were also noted separately.
Results
In 23 cases, the microscopic morphology was close to normal histology without any identifiable lesions, and without any immune depositions (Figure 1A). Sixteen cases were male, and 7 cases were female, and the mean age of those cases without any identifiable lesion was 36.6 years. On the other hand, all other 32 cases had one or more than one alterations in their kidneys. Five of those 32 cases were female, and 27 cases were male, while the mean age was calculated as 52.3 years.
Figure 1.

An image showing: A) normal histology of a kidney (PAS x 100); B) a glomerulus showing global fibrosis and a normal glomerulus (Masson's trichrome x 100); C) diffuse mesengial increase (silver methanamine x 50); D) focal fibrosis without inflammation and focal tubular atrophy next to a normal glomerulus (PAS x 100); E) moderate interstitial mononuclear inflammation, fibrosis, tubular atrophy, and a globally sclerotic glomerulus around a glomerulus with mild increase in the mesengial matrix (PAS x 50); F) moderate intimal thickening in a large caliber artery (PAS x 100); G) arteriolar subintimal hyaline deposition (PAS x 200), and H) tubular adenoma (HE x 50)
Glomerular alterations
Twenty-two cases had glomerular alterations (Table 1). There was no segmental sclerosis in any of the cases. Eleven cases had focal global sclerosis (excluding the 4 cases, which had focal pyelonephritic alterations, and one case with chronic pyelonephritis with a natural result of focal, or diffuse global glomerular sclerosis), and 5 of those cases were among the IgA positive cases (Figure 1B). Two cases with focal global sclerosis did not have any immune deposition. There were 12 cases with mesengial increase (Figure 1C), and 8 of those cases had moderate or severe proliferation in the mesengium. Only one case had mild basement membrane thickening, all other cases had normal basement membranes. There was no amyloid deposition, and there was no nodular glomerular sclerosis.
Table 1.
The glomerular alterations and immune depositions among the autopsied kidneys.

Tubulo-interstitial and vascular alterations
There were tubulo-interstitial and/or vascular alterations in a total of 23 cases (Table 2). One of those cases had chronic pyelonephritis with diffuse interstitial fibrosis, tubular atrophy, and diffuse global sclerosis. Tubulo-interstitial and/or vascular alterations accompanied glomerular alterations in 14 cases. Usually, focal interstitial inflammation accompanied focal interstitial fibrosis, but rarely there was focal fibrosis without inflammation (Figure 1D). Focal interstitial inflammation accompanied to 5 of the cases with focal tubular atrophy (Figure 1E). One case who died because of upper GI bleeding and who had focal global glomerular sclerosis, focal tubular atrophy, focal interstitial inflammation, and fibrosis had acute tubular necrosis, which also showed mesengial granular positivity for fibrinogen. There was severe intimal thickening in large caliber arteries in 4 cases (65, 60, 83, and 30 years old), while 8 cases (mean age 61 years old) had moderate intimal thickening (Figure 1F). Two cases with severe, and 3 cases with moderate intimal thickening died because of MI. Concerning the arteriolar changes, severe subintimal hyaline deposition was observed in one case (65 years old) (Figure 1G), and moderate subintimal hyaline deposition was detected in 2 cases (60 and 49 years old). Tubular adenoma was detected in 2 cases (Figure 1H) and one of them had double foci of tubular adenoma. Except for the 3 cases who had died because of MI and who also had intimal arterial thickening, it was not possible to state a correlation between the causes of death and the kidney lesions.
Table 2.
The tubulo-interstitial and vascular alterations among the autopsied kidneys.

Immunofluorescence findings
Nineteen cases had one or more than one immune deposition (Tables 1 & 3). There was mesengial granular IgA deposition in 13 cases, where 2 cases had 1+ depositions, and all the rest had 2+ or 3+ depositions (Figure 2A). Nine of those IgA positive cases had mesengial proliferation, while one case with mesengial proliferation, and without IgA deposition showed only membranous/mesengial granular C1q positivity. The remaining 3 cases with mesengial proliferation did not have any other immune deposition. Among the IgA positive cases, except for one case, all cases were male, and the mean age was 47.4 years old. Three of the IgA positive cases belonged to the same family (father [40 years old], 2 sons [18 and 16 years old], all lost their lives due to CO intoxication), and all of them had mesengial proliferation. Two of the IgA positive cases had severe vascular lesions (65 and 60 years old male), and 2 cases had foci of chronic pyelonephritis. Regarding all IgA positive cases; in 4 cases mesengial granular fibrinogen positivity, in 2 cases mesengial granular C3 (Figure 2B) positivity, and in one case mesengial granular C3 and membranous granular IgG positivity accompanied IgA positivity. The last case with triple positivity did not show mesengial proliferation. The IgG positivity was not observed in any other case. Four cases showed granular membranous IgM positivity, and among those 4 cases, one case had membranous granular C3 deposition, and 2 cases had membranous granular fibrinogen depositions. One case showed only mesengial granular fibrinogen deposition without any other immune deposition. Nine of the 55 cases showed similar positivity for both κ and λ antibodies in 2 different locations. Five kidneys showed positivity for both antibodies in the tubular epithelium with a granular pattern (Figures 2C & 2D), while 3 cases showed positivity in the tubular luminal proteinous material, and one case showed positivity in both locations. Seven of all κ and λ positive cases also showed positivity for IgA. Of those kappa and lambda positive cases, one case was positive for C1q and the other was positive for IgM. None of the 9 cases showed clonal positivity for either κ or λ antibodies, and none of them were positive in the glomerular tuft.
Table 3.
The case distribution of immune depositions and staining intensities among the autopsied kidneys.

Figure 2.
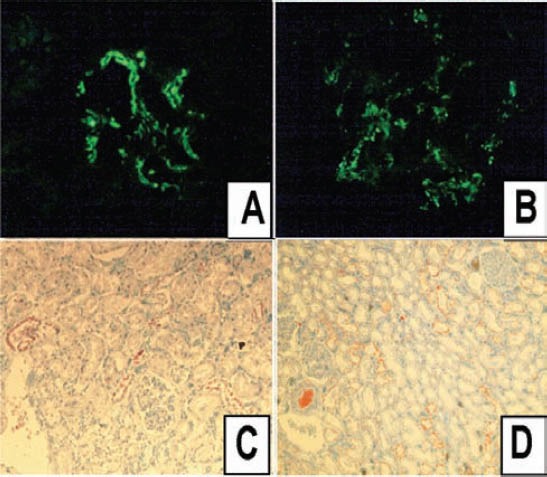
An image showing: A) 3+ IgA deposition in the mesengium (immunofluorescence x 100); B) 2+ C3 deposition in the mesengium (immunofluorescence x 200); C) focal positivity for kappa antibody in the tubular epithelium (immunohistochemistry x 50); and D) more widespread positivity for lambda antibody in the tubular epithelium (immunohistochemistry x 50).
Discussion
The distribution of kidney pathologies shows diversity from country to country, and even from region to region depending on age, gender, and the environmental, nutritional, and genetic factors, and socioeconomic status of the population.2,10,12,13 Also the terminology, which can play an important role on the conflicting results from different studies can differ from country to country, and from study to study. For example, while in some studies, IgA nephropathy (IgAN) is included in mesengio-proliferative glomerulonephritis (MesGN) in some other studies IgAN is considered as a different entity, and still in some studies there is no MesGN category.1,3-5,13,14
According to the biopsy proven data given by the Turkish Nephrology Association in 1997, the most frequent reason for chronic renal insufficiency is chronic GN (23%), which is followed by diabetic nephropathy (13.6%), and vascular reasons (9.6%). Chronic pyelonephritis was rarer when compared with other reasons (6.6%). A single center study21 with the largest number of biopsy review in Turkey, listed the diagnosis of native renal biopsies, and the first 7 reasons were lupus nephritis (12.2%), IgAN (11.9%), crescentic GN (11.5%), amiloid nepropathy (10.6%), membranous glomerulonephritis (MGN) (9%), MesGN (8.8%), and focal segmental glomerulosclerosis (8.1%). Two different single center studies showed MGN as the leading diagnosis for native kidney biopsies while IgAN was third in rank.20,22
Our data did not reflect the incidence of diagnosis in native renal biopsies; however, it can be a reflection of kidney lesions of an asymptomatic population in a regional area. The most striking result of our study was the existence of mesengial increase in 12 cases, and in 9 of these cases IgA positivity accompanied mesengial proliferation. Also there were 4 more IgA positive cases with no distinct mesengial proliferation.
The IgAN was first described by Berger and Hinglais in 1968, and since then it is known to be the most common type of primary glomerulopathy worldwide.23-26 On literature review, there were studies supporting this declaration,1,5,13 while there are contrary results in several studies from all over the world, and also from Turkey.3,4,14,20-22,27 This discrepancy can be due to the different conditions in different study centers, and also it can be due to the biopsy criteria of the medical centers, and the frequency of checkups in different populations as the presentation of IgAN can change from asymptomatic disease to macroscopic hematuria.24
The rate of formation, clearance, and elimination of the immune complexes seem to be the most important factors leading to immune depositions in the tissues. In animal models, it was found that poor quality antibodies, which are not precipitating are prone to develop chronic disease. The existence of complement, as well as the type of the existing complement in the immune complexes, the local increase in vascular permeability, the state of immune deficiency, and the size of the immune complexes are some of the factors affecting deposition of immune complexes in glomeruli.28 Formation of IgA immune complexes is the inevitable response of the body towards the food antigen entering the body via the gastrointestinal tract, and the clearance of this antigen is achieved by the liver. There is a specific suggestion for IgA, claiming that if their clearance is impaired after their formation in a mucosal barrier as a response to an antigen entering through a mucosal surface, they can be deposited in the tissues as “bystanders.”28 This suggestion supports our results, and suggests that IgA deposition in tissues does not need to be symptomatic, and also does not necessarily lead to a clinical presentation due to the accumulation of IgA in the tissues. This suggestion also leads to a conclusion that every glomerular IgA deposited in the mesengium should not necessarily be diagnosed as IgAN, unless there are clinical signs and symptoms concerning IgAN. Formation of crescents, and the ratio and type of crescents in an IgAN is accepted to be the most important prognostic coefficient of IgAN. In a series of 265 biopsy proven IgAN cases from 4 continents, 45% of the patients developed glomerular crescents.23 There was no crescent formation in any of the cases in our study.
In our study IgG, IgM, and C1q depositions did not accompany deposition of IgA except for a single case that showed triple deposition of IgA, IgG, and C3 with mesengial widening, but without mesengial proliferation. It is claimed that if there is an accompaniment of IgG deposition in an IgAN, there should be microscopic, or macroscopic hematuria.28 Our case probably had hematuria before death, but unfortunately it was impossible for us to detect it retrospectively. There is a single case showing only 3+ C1q deposition, without any other accompanying antibodies, and with mesengial proliferation and focal glomerular sclerosis. This case can be regarded as C1q nephropathy, however there is no known supporting clinical data regarding this specific diagnosis.
Regarding the alterations outside the glomeruli, the most severe lesions belonged to the arteries and small caliber vessels. The tubular, interstitial, and vascular lesions had a tendency to increase with age. The seriousness of the vascular lesions showed a parallel increase in the cases who had lost their lives due to MI. Thus, the vascular problems of the kidneys were probably systemic.
The most important limitation of our study is the relatively small number of autopsy cases that were suitable for our study, and we think that prospective autopsy studies with larger series can give more detailed data regarding possible lesions and alterations in the kidneys of an asymptomatic population. Although our study provided enough satisfactory data without ultra-structural findings, lack of electron microscopy seems to be another deficit of the study. Another limitation arises from the high percentage of male subjects among the autopsy population, and regarding the regional population, this discrepancy may lead to divergent data.
In conclusion, the most striking outcome of our study is the high rate of positivity for IgA antibody, as well as, C3 and fibrinogen. There were also a significant number of glomerular and non-glomerular renal alterations. Those findings can show that in our region nearly 55% of the population is prone to upcoming kidney diseases. On the other hand, our data supports the idea that although a large percentage of the regional population can probably have morphologic and immune alterations in their kidneys, only a small percentage of them shows clinical signs and symptoms, which will lead to referral to a hospital. Future studies with larger autopsy series from different regions are needed to estimate the probable renal lesions in an otherwise asymptomatic population.
Footnotes
Disclosure.
Related Articles.
Kari JA, Roebuck DJ, Tullus K. Renal artery stenosis in association with congenital anomalies of the kidney and urinary tract. Saudi Med J 2014; 35: 1264-1266.
Al-Qahtani HH. Arterioenteric fistula on a kidney graft site. A rare cause of massive lower gastrointestinal bleeding. Saudi Med J 2014; 35: 495-498.
Hussein AM, Badawoud MH, Noaman MH. The effects of diethylstilbestrol administration on rat kidney. Ultrastructural study. Saudi Med J 2013; 34: 1114-1124.
References
- 1.Wu YQ, Wang Z, Xu HF, Jin XM, Zhou HZ. Frequency of primary glomerular disease in northeastern China. Braz J Med Biol Res. 2011;44:810–813. doi: 10.1590/s0100-879x2011007500089. [DOI] [PubMed] [Google Scholar]
- 2.Zaza G, Bernich P, Lupo A. ‘Triveneto’ Register of Renal Biopsies (TVRRB). Incidence of primary glomerulonephritis in a large North-Eastern Italian area: a 13-year renal biopsy study. Nephrol Dial Transplant. 2013;28:367–372. doi: 10.1093/ndt/gfs437. [DOI] [PubMed] [Google Scholar]
- 3.Covic A, Schiller A, Volovat C, Gluhovschi G, Gusbeth-Tatomir P, Petrica L, et al. Epidemiology of renal disease in Romania: a 10 year review of two regional renal biopsy databases. Nephrol Dial Transplant. 2006;21:419–424. doi: 10.1093/ndt/gfi207. [DOI] [PubMed] [Google Scholar]
- 4.Horvatic I, Tisljar M, Bulimbasic S, Bozic B, Galesic Ljubanovic D, Galesic K. Epidemiologic data of adult native biopsy-proven renal diseases in Croatia. Int Urol Nephrol. 2013;45:1577–1587. doi: 10.1007/s11255-013-0397-z. [DOI] [PubMed] [Google Scholar]
- 5.Schena FP. Survey of the Italian Registry of Renal Biopsies. Frequency of the renal diseases for 7 consecutive years. The Italian Group of Renal Immunopathology. Nephrol Dial Transplant. 1997;12:418–426. doi: 10.1093/ndt/12.3.418. [DOI] [PubMed] [Google Scholar]
- 6.Habib MA, Badruddoza SM. Pattern of glomerular diseases among adults in Rajshahi, the Northern Region of Bangladesh. Saudi J Kidney Dis Transpl. 2012;23:876–880. doi: 10.4103/1319-2442.98195. [DOI] [PubMed] [Google Scholar]
- 7.Ibrahim S, Fayed A, Fadda S, Belal D. A five-year analysis of the incidence of glomerulonephritis at Cairo University Hospital-Egypt. Saudi J Kidney Dis Transpl. 2012;23:866–870. doi: 10.4103/1319-2442.98191. [DOI] [PubMed] [Google Scholar]
- 8.Monfared A, Khosravi M, Lebadi M, Mosavian Roshan Zamir SA, Hoda S, Habibzadeh SM, et al. Distribution of renal histopathology in Guilan: a single-center report. Iran J Kidney Dis. 2012;6:173–177. [PubMed] [Google Scholar]
- 9.Rabbani MA, Memon GM, Ahmad B, Memon S, Tahir SA, Tahir S. Percutaneous renal biopsy results: a retrospective analysis of 511 consecutive cases. Saudi J Kidney Dis Transpl. 2012;23:614–618. [PubMed] [Google Scholar]
- 10.Al Riyami D, Al Shaaili K, Al Bulushi Y, Al Dhahli A, Date A. The spectrum of glomerular diseases on renal biopsy: data from a single tertiary center in oman. Oman Med J. 2013;28:213–215. doi: 10.5001/omj.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saleemi AN, Nabi Z, Ali S, Salloom A, El Nasri A, Albaqumi M. Spectrum of biopsy-proven glomerular disease in Al Qassim region: a single centre experience. Saudi J Kidney Dis Transpl. 2012;23:1084–1087. doi: 10.4103/1319-2442.100963. [DOI] [PubMed] [Google Scholar]
- 12.Jeganathan J, Kumar S, Khalid M, Maroli C. Pattern of glomerular diseases in a tertiary care center in South India: a prospective study. Saudi J Kidney Dis Transpl. 2013;24:168–171. doi: 10.4103/1319-2442.106363. [DOI] [PubMed] [Google Scholar]
- 13.Kurnatowska I, Jędrzejka D, Małyska A, Wągrowska-Danilewicz M, Danilewicz M, Nowicki M. Trends in the incidence of biopsy-proven glomerular diseases in the adult population in central Poland in the years 1990-2010. Kidney Blood Press Res. 2012;35:254–258. doi: 10.1159/000334418. [DOI] [PubMed] [Google Scholar]
- 14.Rychlík I, Jancová E, Tesar V, Kolsky A, Lácha J, Stejskal J, et al. The Czech registry of renal biopsies Occurrence of renal diseases in the years 1994-2000. Nephrol Dial Transplant. 2004;19:3040–3049. doi: 10.1093/ndt/gfh521. [DOI] [PubMed] [Google Scholar]
- 15.Henriksen KJ, Meehan SM, Chang A. Non-neoplastic renal diseases are often unrecognized in adult tumor nephrectomy specimens: a review of 246 cases. Am J Surg Pathol. 2007;31:1703–1708. doi: 10.1097/PAS.0b013e31804ca63e. [DOI] [PubMed] [Google Scholar]
- 16.Gopalani A, Ahuja TS. Prevalence of glomerulopathies in autopsies of patients infected with the hepatitis C virus. Am J Med Sci. 2001;322:57–60. doi: 10.1097/00000441-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Arase Y, Ikeda K, Murashima N, Chayama K, Tsubota A, Koida I, et al. Glomerulonephritis in autopsy cases with hepatitis C virus infection. Intern Med. 1998;37:836–840. doi: 10.2169/internalmedicine.37.836. [DOI] [PubMed] [Google Scholar]
- 18.Kubo M, Kiyohara Y, Kato I, Tanizaki Y, Katafuchi R, Hirakata H, et al. Risk factors for renal glomerular and vascular changes in an autopsy-based population survey: the Hisayama study. Kidney Int. 2003;63:1508–1515. doi: 10.1046/j.1523-1755.2003.00886.x. [DOI] [PubMed] [Google Scholar]
- 19.McNamara BJ, Diouf B, Hughson MD, Douglas-Denton RN, Hoy WE, Bertram JF. Renal pathology, glomerular number and volume in a West African urban community. Nephrol Dial Transplant. 2008;23:2576–2585. doi: 10.1093/ndt/gfn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Usta M, Gül CB, Yildiz A, Kilicarslan I, Ersoy A. İkinci basamak bir hastanede renal biyopsi deneyimi. Uludag Uni Tip Fak Derg. 2011;37:113–115. Turkish. [Google Scholar]
- 21.Ecder SA, Kilicarslan I, Ecder, Türkmen A, Özağarı A, Uysal V, et al. Beşyüz onüç böbrek biyopsisinin klinikopatolojik açıdan değerlendirilmesi. Istanbul Universitesi Tip Fakultesi Dergisi. 2005;68:43–45. Turkish. [Google Scholar]
- 22.Pişkinpaşa S, Dede F, Akoglu H, Doğru F, Coşkun Yenigün E, Öztürk R, et al. Clinicopathological evolution of the kidney biopsies: Our center's experience. Turk Neph Dial Transpl. 2012;21:167–172. [Google Scholar]
- 23.Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76:534–545. doi: 10.1038/ki.2009.243. [DOI] [PubMed] [Google Scholar]
- 24.Welander A, Sundelin B, Fored M, Ludvigsson JF. Increased risk of IgA nephropathy among individuals with celiac disease. J Clin Gastroenterol. 2013;47:678–683. doi: 10.1097/MCG.0b013e318284792e. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Wang C, Tang Y, Peng H, Ye ZC, Li CC, et al. Serum immunoglobulin A/C3 ratio predicts progression of immunoglobulin A nephropathy. Nephrology (Carlton) 2013;18:125–131. doi: 10.1111/nep.12010. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto R, Imai E. A novel classification for IgA nephropathy. Kidney Int. 2009;76:477–480. doi: 10.1038/ki.2009.206. [DOI] [PubMed] [Google Scholar]
- 27.Mittal N, Joshi K, Rane S, Nada R, Sakhuja V. Primary IgA nephropathy in north India: is it different? Postgrad Med J. 2012;88:15–20. doi: 10.1136/postgradmedj-2011-130077. [DOI] [PubMed] [Google Scholar]
- 28.Levinsky RJ. Role of circulating immune complexes in renal diseases. J Clin Pathol. 1981;34:1214–1222. doi: 10.1136/jcp.34.11.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]


