Abstract
Objectives:
To evaluate the prevalence and severity of plaque-induced gingivitis among a Saudi adult population in Riyadh region.
Methods:
Three hundred and eighty-five eligible participants in this cross-sectional study were recruited from routine dental patients attending the oral diagnosis clinic at Al-Farabi College in Riyadh, Saudi Arabia from June 2013 to December 2013. A clinical examination was performed by 2 dentists to measure the gingival and plaque indices of Löe and Silness for each participant.
Results:
The prevalence of gingivitis was 100% among adult subjects aged between 18-40 years old. Moreover, the mean gingival index was 1.68±0.31, which indicates a moderate gingival inflammation. In fact, males showed more severe signs of gingival inflammation compared with females (p=0.001). In addition, the mean plaque index was 0.875±0.49, which indicates a good plaque status of the participants. Interestingly, the age was not related either to the gingival inflammation (p=0.13), or to the amount of plaque accumulation (p=0.17). However, males were more affected than females (p=0.005).
Conclusion:
The results of this study show that plaque accumulation is strongly associated with high prevalence of moderate to severe gingivitis among Saudi subjects.
Plaque-induced gingivitis is the most common form of periodontal disease,1 which is considered to be the second most common oral disease after dental caries, affecting more than 75% of the population worldwide.2,3 In 2000, the United States Surgeon General released a report calling interest to the ‘‘silent epidemic’’ of dental and oral diseases, mainly dental caries and periodontal diseases suffered by millions of people throughout the US.4 The prevalence of periodontal diseases varies in different studies and different countries as a result of variations in study populations, age of participants, and the procedure of defining and diagnosing this type of disease. In general, gingivitis begins in early childhood, and becomes more prevalent and severe with age.5,6 Epidemiological studies revealed that plaque-induced gingivitis is prevalent among all ages of dentate individuals.7-9 Plaque-induced gingivitis is characterized by the presence of inflammation confined to gingiva without extension into other tooth-supporting structures.10-12 Persistence of this type of inflammation is correlated with the presence of microbial dental plaque. As long as this microbial biofilm is present adjacent to the gingival tissues, the inflammation will not resolve.13 However, it has been shown to be reversible after removing these causative factors.14 The clinical features that can be used as characteristic of gingivitis could be one of the following signs: erythematic and sponginess; changes in contour; bleeding upon stimulation; and presence of calculus, or plaque without clinical attachment loss, or radiographic evidence of crestal bone loss.15 Clinically, the severity and signs of gingival inflammation can be expressed by means of gingival index (GI) of Löe and Silness.16 According to this index, gingival inflammation can be classified as mild, moderate, or severe. However, the presence of these signs of inflammation is considered the initial stage for the more severe and irreversible form of periodontal diseases.17-19 A patient's susceptibility to develop this type of disease also is highly variable and depends on the host response towards periodontal pathogens,17-19 which may be influenced by both acquired and genetic factors that can modify this susceptibility to infection.12,20 Prevention of dental plaque accumulation and early treatment of gingivitis reduces the risks associated with the development of a more severe, and destructive form of periodontal diseases.11,21 It is well known and documented that gingivitis develops after 10-21 days of accumulation of dental plaque,22 necessitating a daily effort to prevent plaque accumulation. Several studies revealed a significant correlation between reducing the incidence of gingivitis and regular plaque control measures.23-25 The aim of this study was to evaluate the prevalence and severity of plaque-induced gingivitis among a Saudi adult population in Riyadh region.
Methods
Study design and population
Three hundred and eighty-five eligible participants in this cross-sectional study were recruited from the routine dental patients who attended the oral diagnosis clinic at the dental hospital of Al-Farabi College in Riyadh, Saudi Arabia, from June 2013 to December 2013. The medical history of each subject was recorded at the time of examination in a special recording form. Subjects who were wearing fixed or removable prosthesis, or with orthodontic appliances, subjects under current periodontal treatment, tobacco smokers, female subjects who were pregnant or using oral contraceptives, or subjects with any other systemic conditions that are known to predispose, or exaggerate gingival inflammation were not included. In addition, any subject who was on antibiotics, antifungals, or antiseptic mouth wash for therapeutic reasons over the past 3 months was also excluded. A minimum of 20 permanent teeth had to be present for inclusion in the study. The study was ethically approved by the Institutional Review Board of Al-Farabi College. All subjects were asked to sign a consent form, and all procedures were undertaken in accordance with the principles of the Helsinki Declaration.
Measurements
Periodontal examination was performed by 2 dentists for all subjects in a dental chair, using a mouth mirror, and a calibrated periodontal probe. Full periodontal charting was made for all participants, and the data was recorded in a special form. Periodontal health was defined as the complete absence of gingivitis at any site, and gingivitis was defined as inflammation of the gingiva in at least one site with an absence of clinical attachment loss.11 Gingival health status was determined using the GI of Löe and Silness,16 and dental plaque status for all study subjects was determined using plaque index (PI) of Löe and Silness.13 To obtain GI and PI for the entire dentition, “Ramfjord” teeth were used.
In accordance with the GI score, the subject's gingival health was assigned as follows:16 no inflammation (<0.1); mild inflammation (0.1-1.0); moderate inflammation (1.1-1.9); and severe inflammation (2-3). For the PI score, the subject's plaque status was assigned as follows:13 excellent (<0.1); good (0.1-0.9); fair (1.0-1.9); and poor (2.0-3.0).
Statistical analysis
Data was entered into a personal computer and analyzed using the Statistical Package for Social Sciences (SPSS) software version 22 (IBM Corp, Armonk, NY, USA). Frequency distributions, and means and standard deviations were calculated. Chi square, t-and ANOVA tests were used for comparisons between groups. The level of significance was set at p<0.05. Forward stepwise multivariate logistic regression analysis was then used to control potential confounding variables, and confidence limits for potential independent predictors of age. Biologically relevant variables (age and gender), and variables that had 0.20 in the initial analyses were entered into a logistic regression model as independent variables. Regarding the stepwise analysis, 0.05 was the cut-off level for inclusion, and 0.10 was the cut-off level for excluding a variable in the analysis.
Results
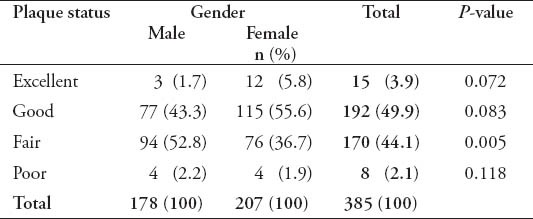
The study included 385 adult dentate subjects, 178 males (46.2%), and 207 females (53.8%), age ranging between 18 and 40 years with a mean age of 25.03 (±5.57) years. The mean age for male subjects was 26.72 (±5.31) years, which is significantly more than females (23.57±5.38 years) (p=0.000). Subjects were divided into 3 groups according to their age range as shown in Table 1. The results of the study showed that 100% of all participants had some form of gingival inflammation (GI score more than 0.1); the mean GI for study subjects was 1.68±0.31, which indicates a moderate gingival inflammation. Male subjects had more severe signs of gingival inflammation compared with females (p=0.001) as shown in Table 2. The mean GI for males was 1.72±0.28, and 1.64±0.335 for females. There were 111 subjects (28.8%) who had a GI score equals to 2, which indicates severe gingival inflammation and bleeding on probing. Bleeding on probing was significantly higher in male subjects (55.9%) compared with 44.1% in females (p=0.016). The mean PI for all subjects was 0.875±0.49, which indicates a good plaque status of the participants. Male subjects had more plaque accumulation compared with females (p=0.005) (Table 3). The mean PI for males was 0.994±0.49 and 0.772±0.47 for females. Moreover, gingival health status and amount of plaque accumulation were not related to the age of the study subjects (p=0.13).
Table 1.
Demographic features of the participants included in this study.
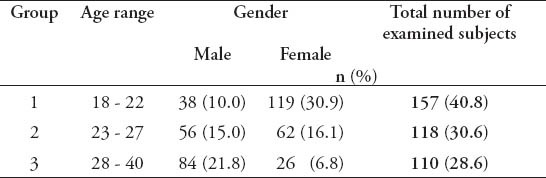
Table 2.
The gingival health status among study subjects included in this study.
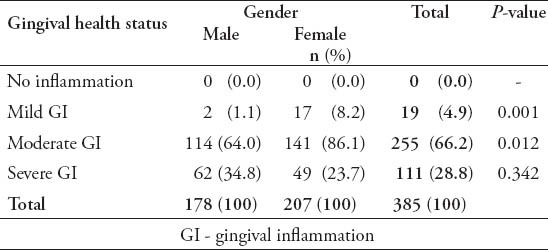
Table 3.
The plaque status of the participants included in this study.
Discussion
The prevalence of gingivitis in adulthood is difficult to be estimated worldwide as a result of the differences in study populations, genetics, and environmental factors. In addition, presence of different clinical methods for definition of gingivitis and absence of clear objective cut-off points between health and disease adds difficulty. Many previous epidemiological studies estimated that the prevalence of adult gingivitis varies from approximately 50-100% for dentate patients.5,26-30 In this study, the prevalence of gingivitis was 100% among the adult subjects aged between 18 and 40 years old.
Zhang et al29 reported gingival inflammation in 97.9% of Chinese adults aged between 18 and 90 years old. Li et al28 reported that gingival inflammation was present in 95.7% of American adults aged between 18 and 90 years. However in 2 previous studies, the criterion for defining the gingival inflammation was GI=0.5, or more. In other words, gingival inflammation should be present at 3 sites or more to be considered inflammation. In contrast, our criterion for defining gingival inflammation is an inflammation in at least one site, or GI>0.1, and this illustrates the higher prevalence of this disease in our study compared to other studies.
In the current study, the prevalence of subjects with bleeding gingiva was 28.8%, and it was higher in males compared with females. A higher prevalence of bleeding gingiva among males was reported in Australia in 2009.31 It is well-documented that the presence of plaque deposits is closely correlated with the presence of gingival inflammation,13,32,33 and this explains a significant association in the current study between plaque accumulation and gingival inflammation in male subjects. Our results are consistent with many previous studies that revealed a significant association of male gender with gingival diseases and plaque accumulations.27,28 This may be because men are less likely to visit the dentist,34 poor attitude towards health, and the better grooming behavior in females compared with males. In this study, age was not related to gingival inflammation and the amount of plaque accumulation, and this may be due to the narrow age range of participants between 18 and 40 years compared with other studies.27-29 In addition, strict exclusion criteria might have played an important role for this results. According to the World Health Organization, the age group 35-44 years was considered the key group because most populations revealed signs of oral diseases, and different forms of periodontal diseases at this stage.35 Moreover, the prevalence of periodontal diseases increases with age. Adults over 50 have the greatest risk for being affected.33 Zhang et al29 demonstrated that the group aged more than 59 years had significantly higher GI compared to other younger groups. However, our results are consistent with a previous study that shows no relationships between age and gingivitis.31
This study has limitations, due to its study design, and it was based on a single center. However, the rate of age and gender of our participants was close to that published by the Saudi National Office of Statistics.36 In addition, our oral diagnosis clinics provide primary care for Saudi patients.
In conclusion, this study showed that plaque accumulation is strongly associated with high prevalence of moderate to severe gingivitis among Saudi subjects. Further research is warranted to identify the factors that might contribute to this high prevalence of plaque-induced gingivitis. Community preventive programs should be assessed and re-implemented on a large and effective scale.
Footnotes
Disclosure.
Related Articles.
Al-Zahrani MS, Zawawi KH, Altaf FM. The effect of obesity and periodontitis on the expression of antimicrobial peptides in gingival tissues. Saudi Med J 2013; 34: 525-530.
Alzahrani AS, Bissada NF, Jurevic RJ, Narendran S, Nouneh IE, Al-Zahrani MS. Reduced systemic inflammatory mediators after treatment of chronic gingivitis. Saudi Med J 2013; 34: 415-419.
References
- 1.Califano JV Research, Science and Therapy Committee American Academy of Periodontology. Position paper: periodontal diseases of children and adolescents. J Periodontol. 2003;74:1696–1704. doi: 10.1902/jop.2003.74.11.1696. [DOI] [PubMed] [Google Scholar]
- 2.Petersen PE. The World Oral Health Report 2003: continuous improvement of oral health in the 21st century--the approach of the WHO Global Oral Health Programme. Community Dent Oral Epidemiol. 2003;31(Suppl 1):3–23. doi: 10.1046/j..2003.com122.x. [DOI] [PubMed] [Google Scholar]
- 3.Papapanou PN. Epidemiology of periodontal diseases: an update. J Int Acad Periodontol. 1999;1:110–116. [PubMed] [Google Scholar]
- 4.Oral health in America: a report of the Surgeon General. J Calif Dent Assoc. 2000;28:685–695. [PubMed] [Google Scholar]
- 5.Stamm JW. Epidemiology of gingivitis. J Clin Periodontol. 1986;13:360–370. doi: 10.1111/j.1600-051x.1986.tb01473.x. [DOI] [PubMed] [Google Scholar]
- 6.Russell AL. The prevalence of periodontal disease in different populations during the circumpubertal period. J Periodontol. 1971;42:508–512. doi: 10.1902/jop.1971.42.8.508. [DOI] [PubMed] [Google Scholar]
- 7.Washington (DC): Government Printing Office; 1965. National Center for Health Statistics. United States Public Health Service. Periodontal Disease in Adults, United States 1960-1962. [Google Scholar]
- 8.Kelly JE, Sanchez MJ. Periodontal disease and oral hygiene among children. United States. Vital Health Stat 11. 1972;117:1–28. [PubMed] [Google Scholar]
- 9.Oral Health of United States Adults; National Findings. Bethesda MD: NIH Publications; 1987. National Institute of Dental Research. United States Public Health Service. [Google Scholar]
- 10.Armitage GC. Clinical evaluation of periodontal diseases. Periodontol 2000. 1995;7:39–53. doi: 10.1111/j.1600-0757.1995.tb00035.x. [DOI] [PubMed] [Google Scholar]
- 11.Parameter on plaque-induced gingivitis. American Academy of Periodontology. J Periodontol. 2000;71(Suppl 5):851–852. doi: 10.1902/jop.2000.71.5-S.851. [DOI] [PubMed] [Google Scholar]
- 12.The pathogenesis of periodontal diseases. J Periodontol. 1999;70:457–470. doi: 10.1902/jop.1999.70.4.457. [DOI] [PubMed] [Google Scholar]
- 13.Silness J, Löe H. Periodontal Disease in Pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 14.Löe H. The Gingival Index, the Plaque Index and the Retention Index Systems. J Periodontol. 1967;38:610–616. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 15.Parameter on plaque-induced gingivitis. American Academy of Periodontology. J Periodontol. 2000;71:851–852. doi: 10.1902/jop.2000.71.5-S.851. [DOI] [PubMed] [Google Scholar]
- 16.Löe H, Silness J. Periodontal disease in pregnancy. I. prevalence and severity. Acta Odontol Scand. 1963;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 17.Kornman KS, Page RC, Tonetti MS. The host response to the microbial challenge in periodontitis: assembling the players. Periodontol 2000. 1997;14:33–53. doi: 10.1111/j.1600-0757.1997.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 18.Dennison DK, Van Dyke TE. The acute inflammatory response and the role of phagocytic cells in periodontal health and disease. Periodontol 2000. 1997;14:54–78. doi: 10.1111/j.1600-0757.1997.tb00192.x. [DOI] [PubMed] [Google Scholar]
- 19.Ishikawa I, Nakashima K, Koseki T, Nagasawa T, Watanabe H, Arakawa S, et al. Induction of the immune response to periodontopathic bacteria and its role in the pathogenesis of periodontitis. Periodontol 2000. 1997;14:79–111. doi: 10.1111/j.1600-0757.1997.tb00193.x. [DOI] [PubMed] [Google Scholar]
- 20.Mealey B. Diabetes and periodontal diseases. J Periodontol. 1999;70:935–949. doi: 10.1902/jop.1999.70.8.935. [DOI] [PubMed] [Google Scholar]
- 21.Baehni PC, Takeuchi Y. Anti-plaque agents in the prevention of biofilm-associated oral diseases. Oral Dis. 2003;9(Suppl 1):23–29. doi: 10.1034/j.1601-0825.9.s1.5.x. [DOI] [PubMed] [Google Scholar]
- 22.Löe H, Theilade E, Jensen Sb. Experimental gingivitis in man. J Periodontol. 1965;36:177–187. doi: 10.1902/jop.1965.36.3.177. [DOI] [PubMed] [Google Scholar]
- 23.Lang NP, Cumming BR, Löe H. Toothbrushing frequency as it relates to plaque development and gingival health. J Periodontol. 1973;44:396–405. doi: 10.1902/jop.1973.44.7.396. [DOI] [PubMed] [Google Scholar]
- 24.Jain Y. A comparison of the efficacy of powered and manual toothbrushes in controlling plaque and gingivitis: a clinical study. Clin Cosmet Investig Dent. 2013;5:3–9. doi: 10.2147/CCIDE.S40656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rode Sde M, Gimenez X, Montoya VC, Gómez M, Blanc SL, Medina M, et al. Daily biofilm control and oral health: consensus on the epidemiological challenge--Latin American Advisory Panel. Braz Oral Res. 2012;26(Suppl 1):S133–S143. doi: 10.1590/s1806-83242012000700020. [DOI] [PubMed] [Google Scholar]
- 26.Oliver RC, Brown LJ, Löe H. Periodontal diseases in the United States population. J Periodontol. 1998;69:269–278. doi: 10.1902/jop.1998.69.2.269. [DOI] [PubMed] [Google Scholar]
- 27.Ababneh KT, Abu Hwaij ZM, Khader YS. Prevalence and risk indicators of gingivitis and periodontitis in a multi-centre study in North Jordan: a cross sectional study. BMC Oral Health. 2012;12:1. doi: 10.1186/1472-6831-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Lee S, Hujoel P, Su M, Zhang W, Kim J, et al. Prevalence and severity of gingivitis in American adults. Am J Dent. 2010;23:9–13. [PubMed] [Google Scholar]
- 29.Zhang J, Xuan D, Fan W, Zhang X, Dibart S, De Vizio W, et al. Severity and prevalence of plaque-induced gingivitis in the Chinese population. Compend Contin Educ Dent. 2010;31:624–629. [PubMed] [Google Scholar]
- 30.Lembariti BS, Frencken JE, Pilot T. Prevalence and severity of periodontal conditions among adults in urban and rural Morogoro, Tanzania. Community Dent Oral Epidemiol. 1988;16:240–243. doi: 10.1111/j.1600-0528.1988.tb01763.x. [DOI] [PubMed] [Google Scholar]
- 31.Australian Research Centre for Population Oral Health, The University of Adelaide, South Australia. Periodontal diseases in the Australian adult population. Aust Dent J. 2009;54:390–393. doi: 10.1111/j.1834-7819.2009.01167.x. [DOI] [PubMed] [Google Scholar]
- 32.Burt B Research Science and Therapy Committee of the American Academy of Periodontology. Position paper: epidemiology of periodontal diseases. J Periodontol. 2005;76:1406–1419. doi: 10.1902/jop.2005.76.8.1406. [DOI] [PubMed] [Google Scholar]
- 33.Greenwell H Committee on Research, Science and Therapy. American Academy of Periodontology. Position paper: Guidelines for periodontal therapy. J Periodontol. 2001;72:1624–1628. doi: 10.1902/jop.2001.72.11.1624. [DOI] [PubMed] [Google Scholar]
- 34.Farsi JM. Dental visit patterns and periodontal treatment needs among Saudi students. East Mediterr Health J. 2010;16:801–806. [PubMed] [Google Scholar]
- 35.Basic Methods. 4th ed. Geneva (CH): World Health Organization; 1997. World Health Organization. Oral Health Surveys. [Google Scholar]
- 36.Saudi Population Census 2010 Saudi Central Department of Statistics and Information. [Accessed 20/08/2014]. Available from: www.cdsi.gov.sa .


