Abstract
Objectives:
To investigate changes in serum lipid profile, levels of serum minerals associated with thyroid disorders, and to compare these with the serum lipid and mineral profiles in hypothyroid patients receiving thyroxine therapy.
Methods:
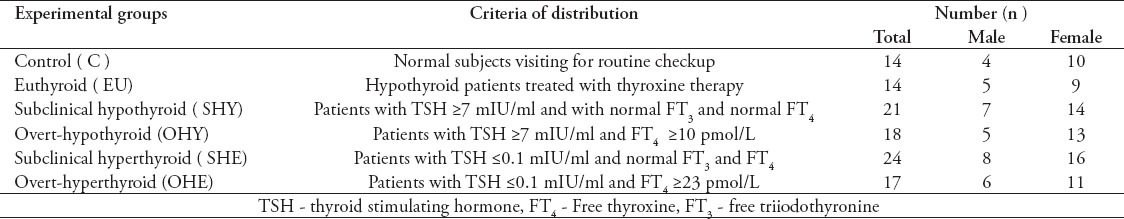
A cross-sectional study was conducted in King Khaled Hospital, Hail, Saudi Arabia. The patient database was searched for new patients with thyroid dysfunction between January 2011 and June 2012. They were classified into 5 groups: 1) subclinical-hypothyroid (SHY), 2) overt-hypothyroid (OHY), 3) subclinical-hyperthyroid (SHE), 4) overt-hyperthyroid (OHE), 5) patients under thyroxine therapy (EU), and normal controls.
Results:
The OHY group showed impaired renal function; whereas, the kidney function of the SHE, OHE, and EU groups was normal. The OHY and OHE groups exhibited elevated serum glucose. The OHY group showed elevated serum cholesterol, triglyceride, and low-density lipoprotein cholesterol, and decreased high-density lipoprotein cholesterol. Serum lipids were reduced in the OHE group, and no different in the EU group compared with controls. The serum calcium and phosphate were reduced in the OHY group, whereas, in the OHE group, the phosphate was increased while magnesium and potassium were reduced.
Conclusion:
Hypothyroidism caused impaired renal function, glucose intolerance, hyperlipidemia, and reduction in serum phosphate. Hyperthyroidism caused a reduction in serum lipids, magnesium, and potassium. Thyroxine therapy normalized the deranged lipids and minerals, but not glucose. Results indicate that thyroid function tests should be considered when diagnosing those metabolic disorders.
Normal thyroid function plays an important role in regulation of cellular activity, and influences basal metabolic rate and general body metabolism.1 Thus, thyroid dysfunction is often associated with dyslipidemia and disturbed mineral metabolism. Hypothyroidism is known to cause hypercholesterolemia, elevated low-density lipoprotein (LDL), and hypertriglyceridemia.2 It was reported that high circulating thyroid stimulating hormone (TSH) levels were associated with abnormally elevated serum lipids,3 and triggered increased oxidation of the LDL particles.4 Moreover, the increased cardiovascular risk in thyroid dysfunction was related to the deranged lipid profile, endothelial dysfunction, metabolic, hormonal, and hemodynamic changes and coagulation disturbances.5 Hence, hypothyroid patients are considered to be at high risk of cardiovascular diseases.6 Interestingly, some studies have revealed normalized lipid profiles in hypothyroid patients when treated with thyroxine replacement therapy.7 On the other hand, thyroid hormones are known to stimulate the bone turnover.8 Thyrotoxicosis was reported to be associated with decreased bone mineral density,9 increased risk of osteoporotic fractures,10 and disturbed serum calcium and phosphate levels.11 Following a literature search, studies investigating thyroid disorders and their consequences on lipid and mineral metabolism in the Saudi population are scarce. Therefore, this study was planned to investigate the changes in serum lipid profile, and changes in the levels of serum calcium, phosphate, and magnesium associated with thyroid disorders in the Hail Region of Saudi Arabia. The results were also compared with the lipid and mineral profiles in hypothyroid patients treated with thyroxine substitution therapy.
Methods
This cross-sectional study was carried out in King Khaled hospital, Hail, Saudi Arabia. The study was conducted in accordance with the principles of the Helsinki Declaration and written permission was obtained from the Research Ethical Authority in the Deanship of the Scientific Research in the University of Hail. The patient`s electronic database was searched for newly diagnosed patients with thyroid disorders who visited the outpatient clinic of Endocrinology and Diabetes between January 2011 and June 2012. Inclusion criteria included all newly diagnosed patients, males and females aged 12-97 years, and hypothyroid patients receiving thyroxine replacement therapy. Exclusion criteria included patients with abnormal liver function, chronic kidney disease, or overt diabetes mellitus. The number of patients files selected with complete chemistry results were 94 (M=31, F=63). The patients had an average age of 38±14 years (range 12-97 years). Based on their thyroid function results, the patients were classified into 5 groups with matching age and gender. The serum test results of normal fasting subjects visiting the hospital for routine medical check-up were recorded and used as controls. The groups were distributed as described in Table 1. The missing chemistry results in the control group, including the thyroid function tests, lipids, and mineral profiles were assayed in our laboratory using the residual serum samples and commercial kits. The measurements of free triiodothyronine (FT3), free thyroxine (FT4), and thyroid stimulating hormone (TSH) were carried out using an Autoanalyzer (ELecsys 2010, Cobas E 411, Mannheim Germany). The other biochemical tests: serum total cholesterol, triglyceride, high-density lipoprotein (HDL)-cholesterol, LDL-cholesterol, glucose, blood urea nitrogen, uric acid, total calcium, phosphate, magnesium, sodium and potassium were carried out by Dimension RxL-Max, Germany. The very low-density lipoprotein (VLDL)-cholesterol was calculated by dividing the concentration of serum triglyceride value by 5.
Table 1.
The distribution of experimental groups used in the study according to their thyroid function data.
Statistical analysis
Results are expressed as means ± SD. The differences between means were computed by one-way analysis of variance (ANOVA) using the Statistical Package for Social Sciences version 10.0 (SPSS Inc, Chicago, IL, USA). The significance of differences between the means was carried out by unpaired Student`s t-test. P values <0.05 were considered significant. The regression analysis between TSH, as independent parameter, and the lipids, minerals, and creatinine values, as dependent parameters, was carried out by Spearman`s regression analysis. P-values <0.05 were considered significant.
Results
In the present study, subclinical hypothyroidism was defined as those patients with TSH values ≥7.00 mIU/L with normal FT3 and FT4 values. The data indicated that from the reviewed files, 64% of the total number of patients with thyroid disorder were females, whereas 36% were males. As shown in Figure 1, in the euthyroid group (under exogenous thyroxine therapy) their FT3 and FT4 was not statistically different from the controls, whereas, the TSH value was significantly lower (p<0.05).
Figure 1.
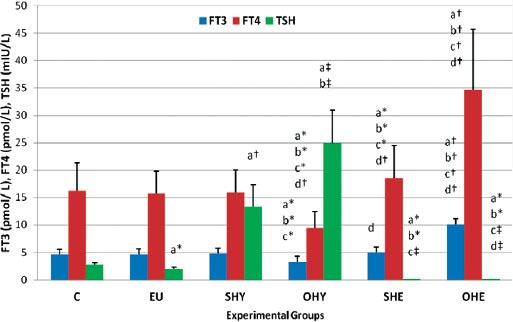
The thyroid function parameters [free triiodothyrpnine (FT3), free thyroxine (FT4) and thyroid stimulating hormone (TSH)] in subclinical-hypothyroid (SHY), overt-hypothyroid (OHY), subclinical-hyperthyroid (SHE), overt-hyperthyroid (OHE), hypothyroid under thyroxine replacement therapy - euthyroid (EU) and normal control (C) groups. Column and vertical bar represent mean ± SD. *p<0.05, †p<0.01, ‡p<0.001. asignificantly different from C, bsignificantly different from EU, csignificantly different from SHY, dsignificantly different from OHY, esignificantly different from SHE.
Table 2 summarizes the values of certain kidney function parameters, uric acid, and serum glucose concentrations. The serum creatinine concentration was not altered in the EU and SHY groups, whereas, in the OHY it was significantly elevated by 21.8%, compared with the control group. However, the serum creatinine concentrations in the SHE and OHE groups were significantly lower than that of SHY, OHY, and control groups. A highly significant positive correlation was evident between TSH and creatinine (r=0.72, p<0.0001). A similar trend was also exhibited by both serum BUN and uric acid. The serum BUN (p<0.05) and uric acid (p<0.001) showed significantly higher levels in the OHY group, and showed significantly lower levels in the OHE group, compared with the control group. On the other hand, the fasting serum glucose level was significantly higher in the OHE by 46.5%, and by 19.45% in the OHY group compared with the control group. Whereas, it was slightly but significantly (p<0.05) elevated in the other groups including the EU group compared with the control group. A slight but significant correlation was also shown between TSH and glucose (r=0.30, p=0.052).
Table 2.
Certain kidney function parameters, uric acid and fasting serum glucose concentration in subclinical hypothyroid (SHY), overt-hypothyroid (OHY), subclinical-hyperthyroid (SHE), overt-hyperthyroid (OHE), hypothyroid under thyroxine replacement therapy - euthyroid (EU), and normal control (C) groups.
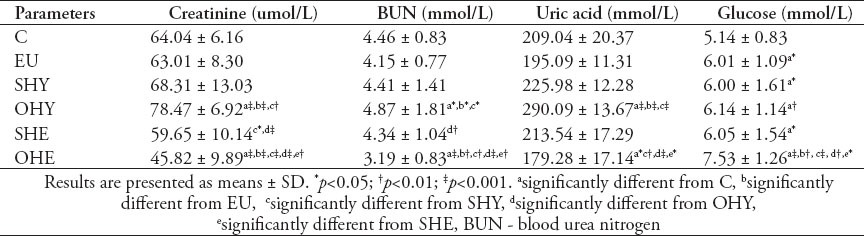
Table 3 depicts the serum lipids and lipoprotein profiles in the various groups. The serum total cholesterol level exhibited a significant elevation by 12.8%, in the OHY group, and a significant reduction by 14.2% in the OHE group compared with control. There was a significant positive correlation between TSH and cholesterol (r=0.50, p<0.001). On the other hand, the serum triglyceride levels were significantly elevated in the SHY group by 24%, and the OHY group by 30.8%, whereas it was significantly reduced in the OHE group by 30%, compared with controls. There was a significant positive correlation between TSH and TG (r=0.65, p<0.001).
Table 3.
Serum lipids and lipoprotein profiles in in subclinical hypothyroid (SHY); overt-hypothyroid (OHY), subclinical-hyperthyroid (SHE), overt-hyperthyroid (OHE), hypothyroid under thyroxine replacement therapy - Euthyroid (EU) and normal control (C) groups.
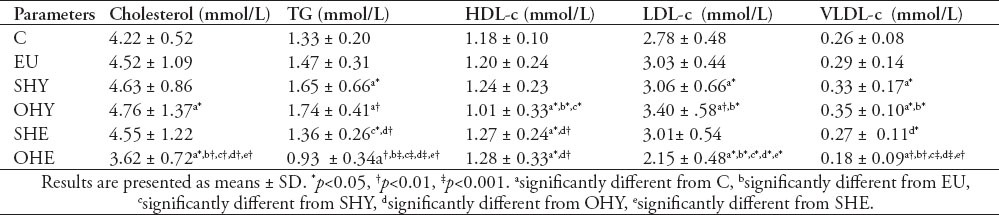
The level of serum cholesterol associated with HDL was significantly lower in the OHY group by 14.4%, and higher in the OHE group by 8.4%. Inversely however, the LDL-c was significantly higher in the OHY group by 22.3%, and lower in the OHE groups by 22.6%. Similarly, the level of TG-rich lipoprotein, VLDL, was higher in the OHY group by 34.6%, and lower in the OHE groups by 30.7% compared with controls.
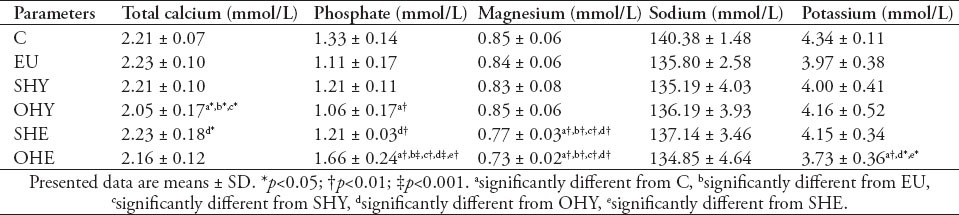
Table 4 summarizes the effects of thyroid disorders on serum mineral profile. The serum total calcium level was not altered in the EU and SHY groups; however, it showed a significant (p<0.05) depression in the OHY group compared with control. There was a significant correlation between TSH and total calcium (r=0.26, p=0.015). However, the serum phosphate showed a significant reduction in the OHY group by 20.3%, and exhibited a significant increase in the OHE patients by 24.8%. There was a significant negative correlation between TSH and phosphate (r=-0.56, p<0.001). Serum magnesium, on the other hand, was significantly reduced in the OHE group. A highly significant positive correlation was also evident between TSH and magnesium (r=0.86, p=0.000). However, the correlation between magnesium and phosphate was strongly significant in the SHE and OHE groups (r=0.68, p<0.0001), whereas, it showed a positive significant correlation, but at a lower extent, in the SHY and OHY groups (r=0.3, p=0.049). Serum sodium levels were not changed in any of the groups compared with the control group. However, the serum potassium level showed a significant (p<0.01) decrease in the OHE group, but no significant correlation was evident between TSH and potassium.
Table 4.
Serum mineral levels in subclinical hypothyroid (SHY), overt-hypothyroid (OHY), subclinical-hyperthyroid (SHE), overt-hyperthyroid (OHE), hypothyroid under thyroxine replacement therapy - euthyroid (EU) and normal control (C) groups.
Discussion
It was shown that in subclinical hypothyroidism, patients with the TSH values >7 mIU/L (normal range: 0.4-6.4 mIU/L) had less possibility to develop into overt hypothyroidism.12 Therefore, in the present study, the cut-off TSH value for subclinical hypothyroidism was ≥7 mIU/L. The thyroxine replacement therapy is the adopted treatment for hypothyroidism with close monitoring of the thyroid function. In the present study, we investigated the possible changes in the lipid and mineral metabolism in patients with thyroid disorders and the possible ameliorations in their profiles following normalization of the hormone levels in the treated hypothyroid patients. The slight depression in the TSH values of the euthyroid group with normal FT3 and FT4 may indicate the temporary effect of the feedback inhibitory control of the exogenous thyroxine therapy on the hypothalamus and pituitary gland secretions. The association of disturbed kidney function with hypothyroidism has been reported in human and animals. Some authors demonstrated severe impairment of the kidney function in overt hypothyroid patients.13 In experimental animals, some have reported that amiodarone-induced hypothyroidism in the rat was associated with a rapid decrease of renal function, which was reversible upon amiodarone-withdrawal.14 In the present results, the improvement of kidney function by thyroxine treatment was evidenced by the normal serum creatinine and BUN levels in the thyroxine-treated euthyroid group. It was also reported that hypothyroidism can aggravate the already impaired kidney function, which can be reversed by thyroxine therapy.15 Interestingly, the data showed lower serum creatinine levels in the overt-hyperthyroid group. That was probably attributed to the enhanced rate of renal hemodynamic in the hyperthyroid patients that would in turn increase the rate of urea and creatinine filtration by the kidney. The present results also exhibited a significant rise in the serum glucose level in the overt hyperthyroid group, and a slight increase in that of the hypothyroid group. Some authors have indicated that hyper- and hypothyroidism are associated with insulin resistance, which has been reported to be the major cause of impaired glucose metabolism in type 2 diabetes mellitus associated with thyroid disorders. The most probable mechanism underlying the etiology of diabetes in thyroid dysfunction was suggested to be the perturbed genetic expression along with impaired glucose utilization in muscles, overproduction of hepatic glucose, and enhanced absorption of splanchnic glucose.16 Some investigators observed a rise in the fasting glucagon-like peptide-1, and the serum glucose level in the overt-hyperthyroid patients.17 These reports explain the impaired glucose tolerance observed in the hyperthyroid group and the slight rise in the serum glucose of the hypothyroid patients. The present data also showed significant increases in the serum total cholesterol accompanied with a rise in the LDL-c and triglyceride levels in the overt-hypothyroid group, whereas, their levels were significantly reduced in the overt-hyperthyroid subjects. This was in congruence with the results reported by some investigators.
The association of hypercholesterolemia with overt-hypothyroidism is consistently reported,2 whereas hypercholesterolemia in subclinical hypothyroidism is debatable. Some researchers found normal cholesterol levels,18 whereas, others showed elevated19 serum cholesterol levels in subclinical-hypothyroidism. In the present data, the subclinical-hypothyroid group showed a trend of increase, but this was not statistically significant (p>0.05). Thyroid hormones are known to regulate the plasma cholesterol level by increasing the expression of LDL-receptors, and enhancing the cellular uptake of LDL-c from circulation.20 This was suggested to be carried out by a T3-mediated gene activation carried out by direct binding of T3 to a specific thyroid hormone responsive element.21 Moreover, T3 has been shown to be involved in protecting LDL particles from oxidation.22 Thyroid hormones are known to increase the lipoprotein-lipase activity, an enzyme responsible for clearance of VLDL and chylomicron from circulation.23 Thus, in hypothyroidism the clearance of chylomicron remnant and intermediate density lipoprotein from circulation was delayed.24 These lipoprotein remnants are taken up by macrophages in the arterial walls to produce foam cells involved in the formation of atherosclerosis.
The present study also revealed a significant reduction in HDL-c in the hypothyroid group. The HDL particles are known to have numerous atheroprotective functions, including facilitation of reverse cholesterol transport, improvement of endothelial function, protection of LDL from oxidation, limitation of hemostasis, and retardation of inflammatory activity related to the vascular wall.25 The reduction in HDL-c causes an increase in the LDL-c/HDL-c ratio, which is an important prognostic marker for cardiovascular disease. Interestingly, the present data revealed a significant reduction in the total cholesterol levels of the overt-hyperthyroid group accompanied with a significant reduction in the LDL-c, and increase in the HDL-c levels. This was in accordance with some reported findings. It was shown that levels of total cholesterol, LDL-c, ApoB, and Lp (a) tend to decrease in patients with clinical and subclinical hyperthyroidism.26 This was suggested to be due to increased LDL receptor gene expression resulting in enhanced LDL receptor-mediated cellular uptake of LDL particles.26,27 A reduced serum cholesterol level was also demonstrated in experimental hyperthyroid mice.28 The mechanism underlying the cholesterol reduction in hyperthyroid mice was suggested to be reduction in the hepatic expression of ATP-binding cassette transporter-1. The authors indicated that the sterol content of bile, liver, and feces were markedly increased, accompanied by up-regulation of hepatic cholesterol 7-alpha-hydroxylase, and ATP-binding cassette transporter, which is known to promote biliary sterol secretion.28 In the present results, the serum TG exhibited significant elevations in the sub- and overt-hypothyroid groups and significant reductions in the hyperthyroid groups. This is also in agreement with the findings of investigators who observed increased serum TG in overt and subclinical hypothyroid patients with reduced TG in the sub- and overt hyperthyroid groups.29 The mechanism underlying this was suggested to be that hypothyroidism increases, whereas hyperthyroidism decreases the hepatic VLDL-TG secretion rate compared with the euthyroid state.29 Moreover, the reduction in serum TG in the hyperthyroid patients was attributed to an increase in the activity of lipoprotein lipase.24 They also observed that hypothyroid patients had abnormally low levels of post-heparin hepatic triglyceride lipase activity, which may explain the hypertriglyceridemia observed in these hypothyroid patients.
Beside dyslipidemia, the alterations in serum calcium, phosphate, and magnesium levels are considered as cardiovascular risk factors.30 The present results revealed reductions in the serum phosphate and calcium levels in the overt-hypothyroid group with an increase in the serum phosphate of the hyperthyroid group. These findings were similar to the results of the authors who found that the mean serum calcium and phosphorus levels were significantly higher in hyperthyroid patients and significantly lower in the hypothyroids compared with controls.31 Previous studies investigating serum calcium and phosphorous in thyroid disorders have revealed conflicting results. Some authors have reported normal levels,32 while others have reported decreased serum calcium and phosphorous in hypothyroidism33 with increased or decreased levels in the hyperthyroidism.34,35 This controversy in findings indicates the complexity of the hormonal and cellular mechanisms involved in regulation of calcium and phosphate metabolism at the intestinal and renal tubular levels, which may be disturbed in thyroid dysfunction. In experimental animals, the T3-treated mice showed elevation in the serum phosphate concentration, but not calcium with a concomitant decrease in plasma calcitriol levels.36 It is also believed that thyroid hormones stimulate bone resorption increasing the serum calcium and phosphorous levels and suppressing the secretion of parathyroid hormone.37 Moreover, the treatment with physiological and pharmacological doses of T3 was shown to stimulate the renal reabsorption of inorganic phosphate resulting in an increase in serum phosphate concentrations.31 This effect was suggested to be mediated by an increase in the amount of Na/Pi co-transporter in the brush border of proximal tubules.38 From these reports, one can conclude that since thyroid hormones can suppress parathyroid hormone and calcitriol, which have opposing effects on phosphate regulation, if any of the hormones are over-suppressed, this would shift the phosphate balance towards excretion or reabsorption resulting in an increase or decrease of serum phosphate level accordingly.
Magnesium, on the other hand, is an important cation that ameliorates atherosclerosis and hypertension, promotes coronary vasodilatation, and unloading of the heart causing an increase in its efficiency.39 Therefore, low-serum magnesium appears often to be associated with arrhythmias, coronary vasospasm, and high blood pressure.39 The present data showed a significant decrease in the serum magnesium of the hyperthyroid group. This was in congruence with the results reported in humans,40 and in experimental animals.41 They suggested that the mechanism underlying the reduction of magnesium in hyperthyroidism to be due to the increased renal hemodynamic. However, magnesium of the hypothyroid group did not exhibit any significant change. This was different from the findings of authors who reported increased42 or decreased43 serum magnesium in hypothyroid patients. Similarly, the serum potassium was reduced in the hyperthyroid group, but not significantly altered in the hypothyroids. Some reports have indicated an association of hypokalemia with thyrotoxicosis.44 Thus, in contrast to the metabolism of calcium and phosphate, the homeostasis of magnesium and potassium seems to be overwhelmed by the rate of renal hemodynamics.
Limitations of the study
First, the sample size was relatively small. Second, anthropometric and blood pressure data for the patients were not obtained. We recommend that future studies are conducted at a larger scale in different regions of Saudi Arabia for comparison.
In conclusion, hypothyroidism caused impaired renal function and glucose intolerance. Moreover, hypothyroidism was associated with hypercholesterolemia, hypertriglyceridemia with increased LDL, and decreased HDL. In contrast, the hyperthyroidism caused a reduction in total cholesterol, triglyceride, and LDL. Hypothyroidism was associated with a reduction in serum calcium and phosphate levels, whereas, the hyperthyroidism caused a reduction in serum magnesium and potassium levels. All disturbed parameters, except glucose, returned to normal in the hypothyroid patients following their treatment with thyroxine substitution therapy. The present data indicated that the derangement in lipid and mineral metabolism in patients with thyroid disorders from Hail region were comparable with the internationally reported values. The authors suggest that physicians consider requesting thyroid function tests when diagnosing the etiology of dyslipidemia, serum mineral disturbances, glucose intolerance, and unexplained impairment in renal function.
Acknowledgment
The author would like to thank Mr. Ali S. Al-Malaq and Mr. Salih K. Al-Shammari for their assistance in data collection.
Footnotes
Disclosure.
Related Articles.
Alherabi AZ, Marglani OA, Gazzaz MJ, Abbas MM. Colon cancer metastasis to the thyroid gland. Saudi Med J 2014; 35: 868-871.
Aldebasi YH, Mohieldein AH, Almansour YS, Almutairi BL. Dyslipidemia and lipid peroxidation of Saudi type 2 diabetics with proliferative retinopathy. Saudi Med J 2013; 34: 616-622.
Abdel-Aal NM, Ahmad AT, Froelicher ES, Batieha AM, Hamza MM, Ajlouni KM. Prevalence of dyslipidemia in patients with type 2 diabetes in Jordan. Saudi Med J 2008; 29: 1423-1428.
References
- 1.Dillmann WH. Mechanism of action of thyroid hormones. Med Clin North Am. 1985;69:849–861. doi: 10.1016/s0025-7125(16)30993-2. [DOI] [PubMed] [Google Scholar]
- 2.Risal P, Maharjan BR, Koju R, Makaju RK, Gautam M. Variation of total serum cholesterol among the patient with thyroid dysfunction. Kathmandu Univ. Med J. 2010;8:265–268. doi: 10.3126/kumj.v8i2.3573. [DOI] [PubMed] [Google Scholar]
- 3.Duntas LH, Brenta G. The effect of thyroid disorders on lipid levels and metabolism. Med Clin North Am. 2012;96:269–281. doi: 10.1016/j.mcna.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 4.Ittermann T, Baumeister SE, Völzke H, Wasner C, Schminke U, Wallaschofski H, et al. Are serum TSH levels associated with oxidized low-density lipoprotein? Results from the Study of Health in Pomerania. Clin Endocrinol (Oxf) 2012;76:526–532. doi: 10.1111/j.1365-2265.2011.04186.x. [DOI] [PubMed] [Google Scholar]
- 5.Neves C, Alves M, Medina JL, Delgado JL. Thyroid diseases, dyslipidemia and cardiovascular pathology. Rev Port Cardiol. 2008;27:1211–1236. [PubMed] [Google Scholar]
- 6.Turhan S, Sezer S, Erden G, Guctekin A, Ucar F, Ginis Z, et al. Plasma homocysteine concentrations and serum lipid profile as atherosclerotic risk factors in subclinical hypothyroidism. Ann Saudi Med. 2008;28:96–101. doi: 10.5144/0256-4947.2008.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teixeira PF, Reuters VS, Ferreira MM, Almeida CP, Reis FA, Buescu A, et al. Lipid profile in different degrees of hypothyroidism and effects of levothyroxine replacement in mild thyroid failure. Transl Res. 2008;151:224–231. doi: 10.1016/j.trsl.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Baqi L, Payer J, Killinger Z, Hruzikova P, Cierny D, Susienkova K, et al. hyrotropin versus thyroid hormone in regulating bone density and turnover in premenopausal women. Endocr Regul. 2010;44:57–63. doi: 10.4149/endo_2010_02_57. [DOI] [PubMed] [Google Scholar]
- 9.Vestergaard P, Mosekilde L. Hyperthyroidism, bone mineral, and fracture risk-a meta-analysis. Thyroid. 2003;13:585–593. doi: 10.1089/105072503322238854. [DOI] [PubMed] [Google Scholar]
- 10.Vestergaard P, Mosekilde L. Fractures in patients with hyperthyroidism and hypothyroidism: a nationwide follow-up study in 16,249 patients. Thyroid. 2002;12:411–419. doi: 10.1089/105072502760043503. [DOI] [PubMed] [Google Scholar]
- 11.Falhammar H, Thorén M, Calissendorff J. Thyrotoxic periodic paralysis: clinical and molecular aspects. Endocrine. 2013;43:274–284. doi: 10.1007/s12020-012-9777-x. [DOI] [PubMed] [Google Scholar]
- 12.Fatourechi V. Subclinical hypothyroidism: An update for primary care physicians. Mayo Clin Proc. 2009;84:65–71. doi: 10.4065/84.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saini V, Yadav A, Arora MK, Arora S, Singh R, Bhattacharjee J. Correlation of creatinine with TSH levels in overt hypothyroidism. A requirement for monitoring of renal function in hypothyroid patients? Clin Biochem. 2012;45:212–214. doi: 10.1016/j.clinbiochem.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Luciani R, Falcone C, Principe F, Punzo G, Menè P. Acute renal failure due to amiodarone-induced hypothyroidism. Clin Nephrol. 2009;72:79–80. doi: 10.5414/cnp72079. [DOI] [PubMed] [Google Scholar]
- 15.Makino Y, Fujii T, Kuroda S, Inenaga T, Kawano Y, Takishita S. Exacerbation of renal failure due to hypothyroidism in a patient with ischemic nephropathy. Nephron. 2000;4:267–269. doi: 10.1159/000045587. [DOI] [PubMed] [Google Scholar]
- 16.Wang C. The Relationship between Type 2 Diabetes Mellitus and Related Thyroid Diseases. J Diabetes Res 2013. 2013 doi: 10.1155/2013/390534. 390534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng JP, Yue HN, Ma SG, Jin Y, Xu W, Bai F. Fasting glucagon-like peptide-1 in patients with overt hyperthyroidism and euthyroid congenital hypothyroidism. J Pediatr Endocrinol Metab. 2013;6:1–5. doi: 10.1515/jpem-2013-0135. [DOI] [PubMed] [Google Scholar]
- 18.Imaizumi M, Akahoshi M, Ichimaru S, Nakashima E, Hida A, Soda M, et al. Risk for ischemic heart disease and all-cause mortality in subclinical hypothyrodism. J Clin Endocrinol Metab. 2004;89:3365–3370. doi: 10.1210/jc.2003-031089. [DOI] [PubMed] [Google Scholar]
- 19.Lee WY, Suh JY, Rhee EJ, Park JS, Sung KC, Kim SW. Plasma CRP, apolipoprotein A-1, apolipoprotein B and Lpa levels according to thyroid function status. Arch Med Res. 2004;35:540–545. doi: 10.1016/j.arcmed.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Rizos CV, Elisaf MS, Liberopoulos EN. Effects of thyroid dysfunction on lipid profile. Open Cardiovasc Med J. 2011;5:76–84. doi: 10.2174/1874192401105010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin DJ, Osborne TF. Thyroid hormone regulation and cholesterol metabolism are connected through Sterol Regulatory Element-Binding Protein-2 (SREBP-2) J Biol Chem. 2003;278:34114–34118. doi: 10.1074/jbc.M305417200. [DOI] [PubMed] [Google Scholar]
- 22.Faure P, Oziol L, Artur Y, Chomard P. Thyroid hormone (T3) and its acetic derivative (TA3) protect low-density lipoproteins from oxidation by different mechanisms. Biochimie. 2004;86:411–418. doi: 10.1016/j.biochi.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Santamarina-Fojo S, Gonzalez-Navarro H, Freeman L, Wagner E, Nong Z. Hepatic lipase, lipoprotein metabolism, and atherogenesis. Arterioscler Thromb Vasc Biol. 2004;24:1750–1754. doi: 10.1161/01.ATV.0000140818.00570.2d. [DOI] [PubMed] [Google Scholar]
- 24.Ito M, Takamatsu J, Matsuo T, Kameoka K, Kubota S, Fukata S, et al. Serum concentrations of remnant-like particles in hypothyroid patients before and after thyroxine replacement. Clin Endocrinol (Oxf) 2003;58:621–626. doi: 10.1046/j.1365-2265.2003.01762.x. [DOI] [PubMed] [Google Scholar]
- 25.Gotto AM., Jr Low high-density lipoprotein cholesterol as a risk factor in coronary heart disease: A working group report. Circulation. 2001;103:2213–2218. doi: 10.1161/01.cir.103.17.2213. [DOI] [PubMed] [Google Scholar]
- 26.Duntas LH. Thyroid disease and lipids. Thyroid. 2002;12:287–293. doi: 10.1089/10507250252949405. [DOI] [PubMed] [Google Scholar]
- 27.Kung AW, Pang RW, Lauder I, Lam KS, Janus ED. Changes in serum lipoprotein(a) and lipids during treatment of hyperthyroidism. Clin Chem. 1995;41:226–231. [PubMed] [Google Scholar]
- 28.Tancevski I, Wehinger A, Demetz E, Eller P, Duwensee K, Huber J, et al. Reduced plasma high-density lipoprotein cholesterol in hyperthyroid mice coincides with decreased hepatic adenosine 5’-triphosphate-binding cassette transporter 1 expression. Endocrinology. 2008;149:3708–3712. doi: 10.1210/en.2007-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fabbrini E, Magkos F, Patterson BW, Mittendorfer B, Klein S. Subclinical hypothyroidism and hyperthyroidism have opposite effects on hepatic very-low-density lipoprotein-triglyceride kinetics. J Clin Endocrinol Metab. 2012;93:E414–E418. doi: 10.1210/jc.2011-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lutsey PL, Alonso A, Michos ED, Loehr LR, Astor BC, Coresh J, et al. Serum magnesium, phosphorus, and calcium are associated with risk of incident heart failure: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. 2014;100:756–764. doi: 10.3945/ajcn.114.085167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shivaleela MB, Poornima RT, Jayaprakash DS. Serum calcium and phosphorus levels in thyroid dysfunction. Indian Journal of Fundamental and Applied Life Sciences. 2012;2:179–183. [Google Scholar]
- 32.Sabuncu T, Aksoy N, Arikan E, Ugur B, Tasan E, Hatemi H. Early changes in parameters of bone and mineral metabolism during therapy for hyperthyroidism and hypothyroidism. Endocrine Research. 2001;27:201–213. doi: 10.1081/erc-100107181. [DOI] [PubMed] [Google Scholar]
- 33.Malamos B, Sfikakis P, Pandos P. The renal handling of phosphate in thyroid disease. J Endocrinol. 1969;45:269–273. doi: 10.1677/joe.0.0450269. [DOI] [PubMed] [Google Scholar]
- 34.Mosekilde L, Christensen MS. Decreased parathyroid function in hyperthyroidism : interrelationship between serum parathyroid hormone, calcium phodphorous metabolism and thyroid function. Acta Endocrinologica. 1977;84:566–575. doi: 10.1530/acta.0.0840566. [DOI] [PubMed] [Google Scholar]
- 35.Al-Tonsi AA, Abdel-Gayoum AA, Saad M. The secondary dyslipidemia and deranged serum phosphate concentration in thyroid disorders. Exp Mol Pathol. 2004;76:182–187. doi: 10.1016/j.yexmp.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 36.Shiguro M, Yamamoto H, Masuda M, et al. Thyroid hormones regulate phosphate homoeostasis through transcriptional control of the renal type IIa sodium-dependent phosphate cotransporter (Npt2a) gene. Biochem J. 2010;427:161–169. doi: 10.1042/BJ20090671. [DOI] [PubMed] [Google Scholar]
- 37.Majima T, Komatsu Y, Doi K, Takagi C, Shigemoto M, Fukao A, et al. Negative correlation between bone mineral density and TSH receptor antibodies in male patients with untreated Graves’ disease. Osteoporos Int. 2006;17:1103–1110. doi: 10.1007/s00198-006-0091-4. [DOI] [PubMed] [Google Scholar]
- 38.Sarasa M, Raldúa D, Aramayona J, Morales R, Biber J, Murer H, et al. Role of Thyroid Hormone in Regulation of Renal Phosphate Transport in Young and aged rats. Endocrinology. 1999;140:1544–1551. doi: 10.1210/endo.140.4.6658. [DOI] [PubMed] [Google Scholar]
- 39.Barbour RL, Altura BM, Reiner SD. Influence of Mg 2+on cardiac performance, intracellular free Mg 2+and pH in perfused hearts as assessed with 31P-NMR spectroscopy. Magnes Trace Elem. 1992;10:99–116. [PubMed] [Google Scholar]
- 40.Jone JE, Desper PC, Shane SR, Flink EB. Magnesium Metabolism in Hyperthyroidism and Hypothyroidism. J Clin Invest. 1966;45:891–900. doi: 10.1172/JCI105404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simsek G, Andican G, Ünal E, Hatemi H, Yigit G, Candan G. Calcium, magnesium, and zinc status in experimental hyperthyroidis. Biol Trace Elem Res. 1997;57:131–137. doi: 10.1007/BF02778196. [DOI] [PubMed] [Google Scholar]
- 42.Al-Hakeim HK. Serum levels of lipids, calcium and magnesium in women with hypothyroidism and cardiovascular diseases. J Lab Physicians. 2009;1:49–52. doi: 10.4103/0974-2727.59698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aldasouqi S, Bokhari SA, Khan PM, Al-Zahrani AS. Thyrotoxic periodic paralysis in a Saudi patient complicated by life-threatening arrhythmia. Saudi Med J. 2009;30:564–568. [PubMed] [Google Scholar]
- 44.Al-Jubouri MA, Inkster GD, Nee PA, Andrews FJ. Thyrotoxicosis presenting as hypokalaemic paralysis and hyperlactataemia in an oriental man. Ann Clin Biochem. 2006;43:323–325. doi: 10.1258/000456306777695681. [DOI] [PubMed] [Google Scholar]


