Abstract
Objectives:
To evaluate the clinical and biochemical characteristics of children with diabetic ketoacidosis (DKA).
Methods:
In this retrospective study conducted between June 2012 and November 2013 at the King Abdulaziz Medical City, Riyadh, Kingdom of Saudi Arabia, we evaluated pediatric DKA admissions from 1995-2008 (Phase 1). From the case files, we obtained information related to patients’ age, gender, weight, presenting complaints, serum biochemical profile, and management.
Results:
This study included 373 DKA admissions with a median age of 11 years (interquartile range [IQR]:8-13). The patients in the subgroup of age more than 10 years old had the highest proportion of admissions (n=250, 67%, p<0.000). The median duration of diagnosis of diabetes mellitus (DM) was 3 years (IQR:2-6). New-onset DM was 47%. Predominant precipitating cause was acute illness, mostly viral syndrome in 22% of all cases, and non-compliance to insulin regimen was in 79% of the diagnosed diabetic cases. Blood glucose, pH, anion gap, serum osmolality, serum potassium, and serum phosphate showed the highest change during the initial 6 hours of management, while trends of serum bicarbonate and blood urea nitrogen demonstrated a predominant change in the initial 12 hours.
Conclusion:
The notable findings in this study, such as, higher mean age of presentation, high rate of non-compliance to insulin as the cause of precipitation, and a high prevalence of abdominal pain at presentation should be followed up with further comparative studies.
Diabetes mellitus (DM) is an endocrine disease affecting millions of children worldwide.1-3 Diabetic ketoacidosis (DKA) is one of the serious complication of diabetes in the pediatric population,4 and its prevalence increases by an annual rate of 3% worldwide.3 It is associated with significant risk of life threatening complications.5-8 The criteria for diagnosis of DKA in children by the International Society for Pediatric and Adolescent Diabetes describes DKA as blood glucose >11 mmol/L, venous pH <7.3, or bicarbonate <15 mmol/L, and ketonemia with ketonuria.9 Previous studies1,2 have reported characteristics of DKA patients based on different geographical areas. Overall, there is a paucity of literature on this particular aspect. The aim of this study was to assess pediatric patients presenting with DKA regarding aspects of demographics, presentation, investigations, and management in the Kingdom of Saudi Arabia (KSA).
Methods
This study was conducted between June 2012 to November 2013 at the King Abdulaziz Medical City (KAMC), Riyadh, KSA, a 1500 bed medical university hospital. This study was approved by the Institutional Review Board in 2012 to study pediatric (0-14 years) admissions due to DKA from 1995-2013, and was conducted observing the principles of Helsinki declaration. This study was divided in 2 phases, and we are reporting Phase1. The DKA admissions between 1995-2008 was identified. Phase II is designed to compare phase I patients and covers the time frame from year 2009-2013. Inclusion criteria were patients admitted due to DKA, and were less than 14 years old. We identified the DKA admissions (n=394) from the computerized data system. To be accurate in enrollment, we verified that admission criteria was consistent with DKA definition by the International Society for Pediatric and Adolescent Diabetes.6,7 We then verified the completeness of the charts. Exclusion criteria were patients with incomplete documentation of DKA episode despite the system labelling them as DKA. Twenty-one patients were excluded, and the remaining 373 were the study subjects, and we searched the medical record, and extracted data related to the information regarding demographics, clinical presentation, investigations, and management. All the reported results had proper documentation. Those findings that had unclear documentations were neither included in the data, nor in the analysis, and were reported as missing. The major variables included age, gender, location of admission, co-morbidities, various presenting symptoms, non-compliance to health team instructions, duration of stay in the pediatric emergency room, Glasgow coma score (GCS), weight, acid base status, serum chemistry, hemoglobin A1c (HbA1c), immune panel, insulin therapy, and fluid/electrolyte management. Literature was searched through PubMed. We used mesh headings of DM, DKA, pediatrics, admissions, history, presentation, diagnosis, biochemical profile, investigations, and management. Non-compliance was assessed based on the history of non-adherence to prescribed insulin regimen. Self-administered insulin was assessed based on the mode of dosing if being taken by the patients themselves.
We recorded the data on Microsoft Excel version 2007. The data were then exported to IBM Statistical Package for Social Sciences statistics version 20 (IBM Inc., Armonk, New York, USA) for further analysis. The continuous variables were evaluated for the distributions via Kolmogorov-Smirnov test, and are reported as means or medians. Further, we evaluated means via one-sample non-parametric tests using one-sample chi-square test, or one-sample binomial test. The significant level for results was set at p<0.05.
Results
We identified 373 admissions as per inclusion criteria from 1995-2008. A total of 269 patients had 373 admissions. Sixty-seven patients had more than one admission-related-DKA. In Table 1, we present the demographics of the study subjects. Only subjects with complete documentation are included and reported. The study subjects had a median age of 11 years (interquartile range [IQR]: 8-13). When dividing age into sub-categories of less than 5 years (n=41, 11%), 5-10 years (n=82, 22%), and 11-14 years (250/67%), we found a clinically significant difference among these categories (p<0.005). Regarding the gender ratio, there were slightly more female patients than males (n=207, 55.5% versus n=166, 44.5%). We found that the median duration of diagnosis of DM was 3 years (IQR: 2-6). At presentation, almost half of the DKA patients (n=157/334, 47%) were new-onset DM. Moreover, approximately one out of 5 (n=78/354, 22%) presented with an acute illness mostly viral upper respiratory tract infections, or viral gastroenteritis as the precipitating factor. On the contrary, non-compliance to insulin regimen was found in approximately 4 out of 5 (n=81/102, 79.4%).
Table 1.
Clinical outcomes of subjects in a study on pediatric diabetic ketoacidosis in Riyadh, Saudi Arabia (N=373).
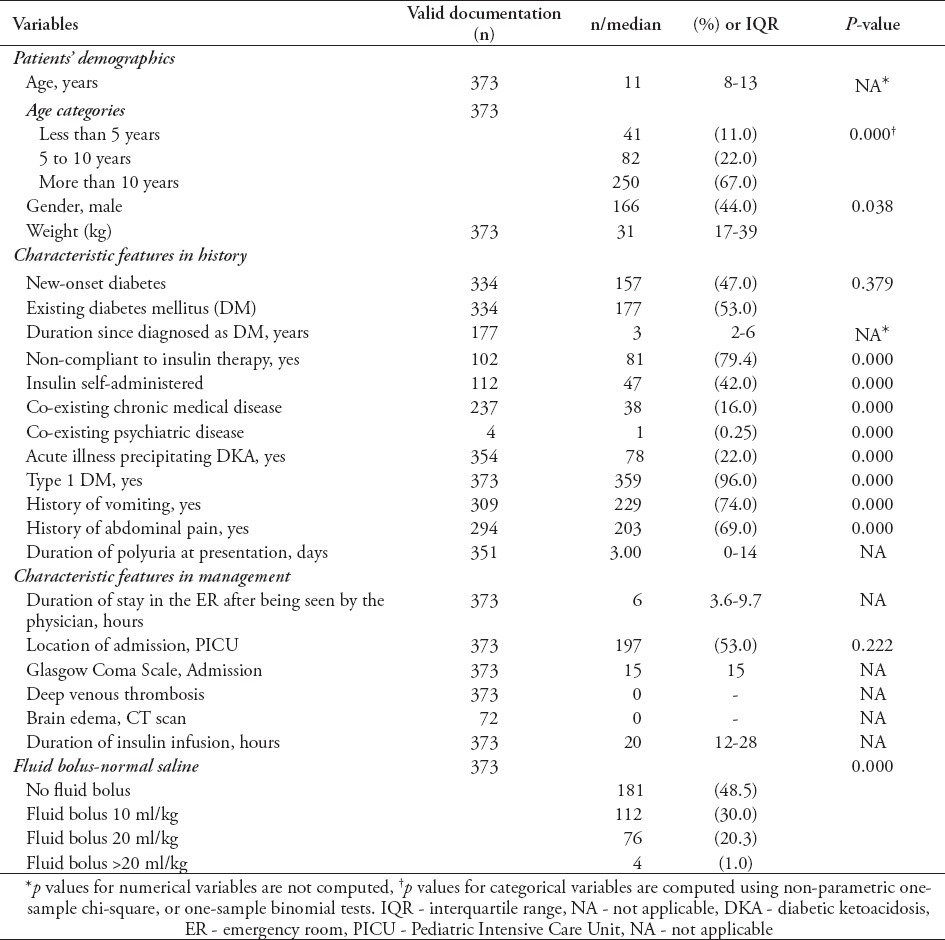
With regard to presenting complaints, we found that most patients had a history of vomiting of at least one day duration (n=229/309, 74%) and approximately two-thirds of the patients had abdominal pain (n=203/294, 69%). Patients who had co-morbidity with another chronic disease was 16%, and only one patient was found to have co-existing psychiatric disease. More patients were admitted at the Pediatric Intensive Care Unit (PICU) than the wards (197/53% versus 176/47%). Regarding fluid management in the emergency room, almost half of the patients did not receive any fluid bolus (181/48% versus 188/52%) while subjects receiving 10 ml/kg of normal saline were the second in frequency (112/373, 30%). In our study, the median duration of insulin infusion was 20 hours (IQR: 12-28).
In Table 2 we present clinical outcomes in DKA study subjects presenting with new-onset DM versus diagnosed DM. The study subjects had mean ages of 9 years and 11 years belonging to new-onset DM and diagnosed DM. There was a significant difference in age sub-groups. Similarly both groups showed significant difference regarding the presence of other acute and chronic conditions. The average duration of polyuria was 13 days in patients with new-onset DM as compared with 2 days in diagnosed DM, while the number of patients admitted at the PICU were almost the same.
Table 2.
Clinical outcomes of study subjects in terms of new-onset versus diagnosed diabetes mellitus (DM) in a study on pediatric diabetic ketoacidosis (DKA) in Riyadh, Saudi Arabia (N=373).
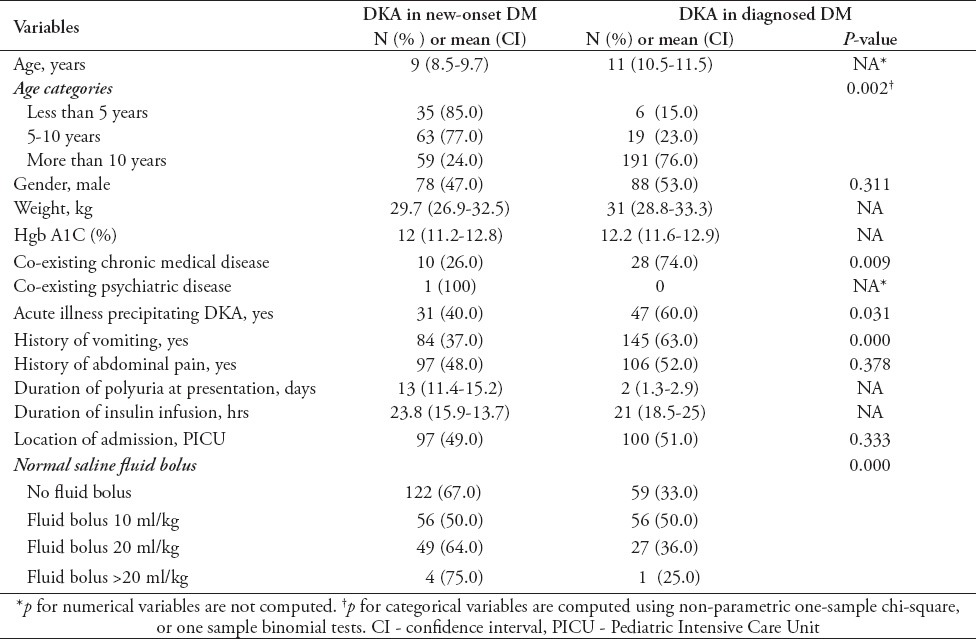
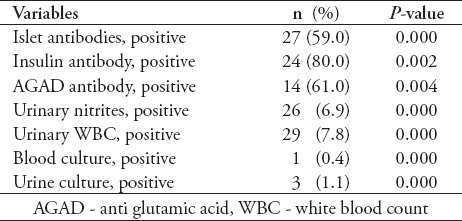
Table 3 shows the 12 hour trend related to acid base status and serum chemistry results along with other significant laboratory results. The tests were conducted in the hospital laboratory, which is of international standards and accredited by Joint Commission of accreditation. In this study, we found consistent improvement in acid base status and serum chemistry. Blood glucose, pH, anion gap, serum osmolality, serum potassium and serum phosphate showed the highest change during the first 6 hours. On the contrary, trends of serum bicarbonate and blood urea nitrogen were more linearly related to the time scale of 12 hours. Regarding white blood cell count, there was a bimodal response. In Table 4, we present immunologic profile including the islet cell antibodies (ICA), insulin antibodies (IA), and anti glutamic acid decarboxylase antibodies. Basic microbiology profile shows a low yield in tests including urinary nitrites, urinary white blood cells, blood cultures and urine cultures.
Table 3.
Laboratory outcomes of the subjects in a study on pediatric diabetic ketoacidosis in Riyadh, Saudi Arabia.
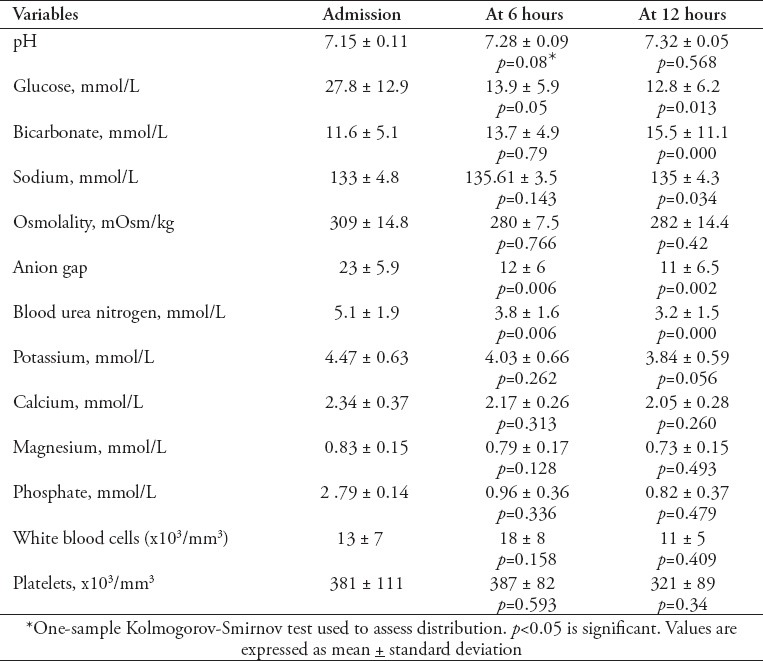
Table 4.
Immunological, microbiological and endocrine outcomes in a study on pediatric diabetic ketoacidosis in Riyadh, Saudi Arabia (N=373).
Discussion
We found 373 admissions in 14 years of study period. Predominant age group in our study regarding the number of admission was group age 10-14 (250/373, p 0.000). Forty seven percent of our study subjects had new-onset DM consistent with previously reported prevalence from 15 to 67.5,10 Again, we found that the number of new-onset cases were highest in patients 10-14 years (89/157, p=0.001) while this had been reported to be highest in younger age groups.11 It may be associated with the fact that in our study, the proportion of admissions of age group of 10-14 years is also the highest.
This study found that non-compliance to insulin regimen in diagnosed diabetics was high (79%). In a recent study, Randall et al12 reported that insulin discontinuation was the leading precipitating cause in 68% of patients; other causes were new-onset diabetes (10%), infection (15%), medical illness (4%), and undetermined causes (3%). The exact reason for non-compliance is not known. Some plausible reasons may be related to non-adherence to health team instructions, and lack of proper follow up. Further, we checked whether non-compliance rate was different in various age groups, and found that there was only a minor difference that was clinically insignificant (p=0.194). Implying these findings with the fact that DKA, being a preventable disease, we suggest that strategies, such as family counselling, patients’ education, and improved follow up may help to overcome non-compliance to insulin therapy, and to improve patient care, and reduce the cost of health care.
Regarding presenting complaints, we found a substantial number of patients presenting with abdominal pain (69%). Umpierrez and Freire13 while evaluating 189 adult patients with DKA reported abdominal pain to be present in 46% of cases. Our study is different in terms of patient population with emphasis on pediatric population. Clinicians keeping this fact in consideration can avoid unnecessary investigations. We suggest a more larger scale studies particular in this aspect.
The degree of severity of DKA at presentation in our study (mean PH: 7.15+0.11) was lower as compared with other reported studies.14 The possible reason may be due to an early access to hospital care for the study population. Cerebral edema had been reported to occur in 0.3-1% of children with DKA, and has a mortality rate of 21-24%,15,16 while in our study there is no episode of cerebral edema. This may be attributed to the milder presentation along with strict adherence to International Society for Pediatric and Adolescent Diabetes (ISPAD) DKA management protocol. Likewise, there is no mortality in our study.
Many studies have reported prevalence of ICA, IA, and anti glutamic acid decarboxylase antibodies (GAD-Ab).17-19 We found ICA positive in 59%, IA positive in 80%, and anti GAD-Ab positive in 46% of admissions (Table 4). We report these results to provide the data related to our patient population, and to look for future studies for wider comparison.
With regard to identification of infection in DKA, our study found results consistent with previous studies, and showed a low positive yield regarding urinary nitrite (6.9%, p=0.000), blood cultures (0.4%, p=0.000), and urine cultures (1.1%, p=0.000)(Table 4).20,21 Flood and Chiang21 studied 247 pediatric admissions for DKA for the presence of infections. They found a low yield of positive blood culture results in children who had bacterial infections (3.2%) and positive urine cultures (1.2%). Our findings reiterate the importance of existing recommendations regarding using clinical judgments in ordering these tests.
We found that upon presentation, the patients who did not require any fluid bolus were approximately half of the total (181/373, 48%) differentiating it from other reports.19 This fact may be attributed to the milder presentation.
This study has limitations. This is a single center study, therefore, caution should be exercised in generalizing the findings to the whole KSA. Despite efforts to overcome selection bias and information bias, there are chances that these processes would have affected the review of the files. Collecting data from the medical records was tried to be as precise as possible, however, we found many missing or incomplete entries of variables and outcomes.
In conclusion, this study found notable findings in many characteristics, such as, higher mean age of presentation (age group: 10-14 years), high rate of non-compliance to insulin as the cause of precipitation (67% of DKA episodes in diagnosed diabetics), and a high prevalence of abdominal pain (69%) at presentation. Trends of correction of metabolic profile in the time frame of 0-6 hours and 6-12 hours showed blood glucose, PH, anion gap, serum osmolality, serum potassium, and serum phosphate changes to be most prominent during the initial 6 hours of management, while trends of serum bicarbonate and blood urea nitrogen demonstrated predominant change in the initial 12 hours of management. We consider that these findings should open the venue for further comparative studies. In the future all the characteristics should be individually explored and thoroughly compared to the previous literature from other global geographic areas. Overall, provisions of awareness to clinicians regarding these findings shall help improve care of pediatric DKA patients.
Footnotes
References
- 1.DIAMOND Project Group. Incidence and trends of childhood Type 1 diabetes worldwide 1990-1999. Diabet Med. 2006;23:857–866. doi: 10.1111/j.1464-5491.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- 2.EURODIAB ACE, Study Group. Variation and trends in incidence of childhood diabetes in Europe. Lancet. 2000;355:873–876. [PubMed] [Google Scholar]
- 3.Newhook LA, Curtis J, Hagerty D, Grant M, Paterson AD, Crummel C, et al. High incidence of childhood type 1 diabetes in the Avalon Peninsula, Newfoundland, Canada. Diabetes Care. 2004;27:885–888. doi: 10.2337/diacare.27.4.885. [DOI] [PubMed] [Google Scholar]
- 4.Rewers A, Chase HP, Mackenzie T, Walravens P, Roback M, Rewers M, et al. Predictors of acute complications in children with type 1 diabetes. JAMA. 2002;287:2511–2518. doi: 10.1001/jama.287.19.2511. [DOI] [PubMed] [Google Scholar]
- 5.Wolfsdorf JI, Glaser N, Sperling MA American Diabetes Association. Diabetic ketoacidosis in infants, children, and adolescents: A consensus statement from the American Diabetes Association. Diabetes Care. 2006;29:1150–1159. doi: 10.2337/diacare.2951150. [DOI] [PubMed] [Google Scholar]
- 6.Wolfsdorf JI, Allgrove J, Craig ME, Edge J, Glaser N, Jain V, et al. Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr Diabetes. 2014;15(Suppl 20):154–179. doi: 10.1111/pedi.12165. [DOI] [PubMed] [Google Scholar]
- 7.Cengiz E, Connor CG, Ruedy KJ, Beck RW, Kollman C, Klingensmith GJ, et al. Pediatric diabetes consortium T1D New Onset (NeOn) study: clinical outcomes during the first year following diagnosis. Pediatr Diabetes. 2014;15:287–293. doi: 10.1111/pedi.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunger DB, Sperling MA, Acerini CL, Bohn DJ, Daneman D, Danne TP, et al. European Society for Paediatric Endocrinology/Lawson Wilkins Pediatric Endocrine Society consensus statement on diabetic ketoacidosis in children and adolescents. Pediatrics. 2004;113:133–140. doi: 10.1542/peds.113.2.e133. [DOI] [PubMed] [Google Scholar]
- 9.Bowden SA, Duck MM, Hoffman RP. Young children (<5 yr) and adolescents (>12 yr) with type 1 diabetes mellitus have low rate of partial remission: diabetic ketoacidosis is an important risk factor. Pediatr Diabetes. 2008;9:197–201. doi: 10.1111/j.1399-5448.2008.00376.x. [DOI] [PubMed] [Google Scholar]
- 10.Klingensmith GJ, Tamborlane WV, Wood J, Haller MJ, Silverstein J, Cengiz E, et al. Diabetic ketoacidosis at diabetes onset: still an all too common threat in youth. J Pediatr. 2013;162:330–334. doi: 10.1016/j.jpeds.2012.06.058. [DOI] [PubMed] [Google Scholar]
- 11.Rewers A, Klingensmith G, Davis C, Petitti DB, Pihoker C, Rodriguez B, et al. Presence of diabetic ketoacidosis at diagnosis of diabetes mellitus in youth: the Search for Diabetes in Youth Study. Pediatrics. 2008;121:1258–1266. doi: 10.1542/peds.2007-1105. [DOI] [PubMed] [Google Scholar]
- 12.Randall L, Begovic J, Hudson M, Smiley D, Peng L, Pitre N, et al. Recurrent diabetic ketoacidosis in inner-city minority patients: behavioral, socioeconomic, and psychosocial factors. Diabetes Care. 2011;34:1891–1896. doi: 10.2337/dc11-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Umpierrez G, Freire AX. Abdominal pain in patients with hyperglycemic crises. J Crit Care. 2002;17:63–67. doi: 10.1053/jcrc.2002.33030. [DOI] [PubMed] [Google Scholar]
- 14.Jayashree M, Singhi S. Diabetic ketoacidosis: predictors of outcome in a pediatric intensive care unit of a developing country. Pediatr Crit Care Med. 2004;5:427–433. doi: 10.1097/01.pcc.0000137987.74235.5e. [DOI] [PubMed] [Google Scholar]
- 15.Lawrence SE, Cummings EA, Gaboury I, Daneman D. Population-based study of incidence and risk factors for cerebral edema in pediatric diabetic ketoacidosis. J Pediatr. 2005;146:688–692. doi: 10.1016/j.jpeds.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 16.Glaser N, Barnett P, McCaslin I, Nelson D, Trainor J, Louie J, et al. Risk factors for cerebral edema in children with diabetic ketoacidosis. The Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. N Engl J Med. 2001;344:264–269. doi: 10.1056/NEJM200101253440404. [DOI] [PubMed] [Google Scholar]
- 17.Graham J, Hagopian WA, Kockum I, Li LS, Sanjeevi CB, Lowe RM, et al. Genetic effects on age-dependent onset and islet cell autoantibody markers in type 1 diabetes. Diabetes. 2002;51:1346–1355. doi: 10.2337/diabetes.51.5.1346. [DOI] [PubMed] [Google Scholar]
- 18.Pihoker C, Gilliam LK, Hampe CS, Lernmark A. Autoantibodies in diabetes. Diabetes. 2005;54(Suppl 2):S52–S61. doi: 10.2337/diabetes.54.suppl_2.s52. [DOI] [PubMed] [Google Scholar]
- 19.Isermann B, Ritzel R, Zorn M, Schilling T, Nawroth PP. Autoantibodies in diabetes mellitus: current utility and perspectives. Exp Clin Endocrinol Diabetes. 2007;115:483–490. doi: 10.1055/s-2007-981452. [DOI] [PubMed] [Google Scholar]
- 20.Savino A, Pelliccia P, Schiavone C, Primavera A, Tumini S, Mohn A, et al. Serum and urinary nitrites and nitrates and Doppler sonography in children with diabetes. Diabetes Care. 2006;29:2676–2681. doi: 10.2337/dc06-0346. [DOI] [PubMed] [Google Scholar]
- 21.Flood RG, Chiang VW. Rate and prediction of infection in children with diabetic ketoacidosis. Am J Emerg Med. 2001;19:270–273. doi: 10.1053/ajem.2001.24473. [DOI] [PubMed] [Google Scholar]


