Abstract
Pulmonary arterial hypertension (PAH) is commonly associated with chronic hypoxemia in disorders such as chronic obstructive pulmonary disease (COPD). Prostacyclin analogs are widely used in the management of PAH patients; however, clinical efficacy and long-term tolerability of some prostacyclin analogs may be compromised by concomitant activation of the E-prostanoid 3 (EP3) receptor. Here, we found that EP3 expression is upregulated in pulmonary arterial smooth muscle cells (PASMCs) and human distal pulmonary arteries (PAs) in response to hypoxia. Either pharmacological inhibition of EP3 or Ep3 deletion attenuated both hypoxia and monocrotaline-induced pulmonary hypertension and restrained extracellular matrix accumulation in PAs in rodent models. In a murine PAH model, Ep3 deletion in SMCs, but not endothelial cells, retarded PA medial thickness. Knockdown of EP3α and EP3β, but not EP3γ, isoforms diminished hypoxia-induced TGF-β1 activation. Expression of either EP3α or EP3β in EP3-deficient PASMCs restored TGF-β1 activation in response to hypoxia. EP3α/β activation in PASMCs increased RhoA-dependent membrane type 1 extracellular matrix metalloproteinase (MMP) translocation to the cell surface, subsequently activating pro–MMP-2 and promoting TGF-β1 signaling. Activation or disruption of EP3 did not influence PASMC proliferation. Together, our results indicate that EP3 activation facilitates hypoxia-induced vascular remodeling and pulmonary hypertension in mice and suggest EP3 inhibition as a potential therapeutic strategy for pulmonary hypertension.
Introduction
Pulmonary arterial hypertension (PAH), a rare but often fatal disease characterized by an average pulmonary arterial (PA) pressure of greater than 25 mmHg, contributes to unacceptably high morbidity and mortality of adult and pediatric patients with lung diseases (1). PA remodeling is the pathogenic hallmark of all forms of pulmonary hypertension. Deposition of extracellular matrix (ECM), such as fibronectin and collagen, and proliferation, migration, and hypertrophy of vascular smooth muscle cells (VSMCs) result in PA hypertrophy and muscularization, leading to increased pulmonary vascular resistance in PAH (2). Current therapies such as vasodilators, endothelin receptor antagonists, and phosphodiesterase inhibitors mainly aim to relieve symptoms without significant improvements in overall prognosis; therefore, these therapies do little to ameliorate the underlying vascular remodeling in PAH.
COX-derived prostaglandins (PGs) play an essential role in the maintenance of pulmonary vascular tone and modulation of pulmonary vascular remodeling in response to inflammatory stimulations through activation of their specific receptors (3). COX-1 is ubiquitously expressed in lung tissue, while COX-2 can be induced in the smooth muscle layer of pulmonary blood vessels by chronic hypoxia (4, 5). Disruption or knockdown of COX-2 exacerbates hypoxia- and monocrotaline-induced (MCT-induced) pulmonary hypertension and enhances the contractility of VSMCs (6, 7). In vitro, both thromboxane A2 (TxA2) and low concentrations of PGE2 evoke contraction of PAs through the thromboxane (TP) and E prostanoid 3 (EP3) receptors, respectively (8–10), and the nitric oxide synthase inhibitor NG-nitro-L-arginine methyl ester (L-NAME) could augment the contractile function of PGE2 (11). In contrast, prostacyclin analogs (prostaglandin I2, PGI2) induce PA relaxation, inhibit platelet aggregation, and ameliorate PA remodeling through suppression of pulmonary arterial smooth muscle cell (PASMC) proliferation (12, 13). Additionally, enhanced secretion of TxA2 and reduced secretion of PGI2 were observed in patients with both primary and secondary pulmonary hypertension (14, 15). Blockade of TP-mediated signaling significantly suppresses the hypoxia-induced hyperreactivity of the PA response to phenylephrine (16). Disruption of the I prostanoid (IP) receptor in mice results in more severe pulmonary hypertension and vascular remodeling after chronic exposure to hypoxia (17). In contrast, activation of the IP receptor by overexpression of PGI2 synthase protects against the development of hypoxia-induced PAH in rodents (18, 19). Therefore, restoration and activation of IP signaling using analogs of PGI2, such as iloprost, treprostinil, and beraprost, represent effective strategies in the treatment of PAH (20). However, the clinical efficacy and safety of these analogs may be altered because of their heterogeneous affinities for the various PG receptors. For example, in addition to the IP receptor, iloprost, treprostinil, and beraprost differentially bind to the EP receptors, including EP3 (21–26). Likewise, diminished relaxation of PAs in response to iloprost, treprostinil, and beraprost was reported in MCT-induced PAH in rats via a mechanism that involves stimulation of the contractile EP3 receptor (27, 28). Furthermore, the EP3 receptor appears to be functionally upregulated in the PAs of MCT-treated rats (28), indicating that EP3 receptor may be involved in the pathogenesis of PAH.
In this report, we demonstrate that PGE2 generation and EP3 expression increased in both rodent and human PASMCs, in human PAs in response to hypoxia, and in PAs from mouse models of hypoxia-induced PAH. Disruption of Ep3 attenuated both hypoxia-induced and hypoxia plus SU5416–induced (HySu-induced) PAH and pulmonary vascular remodeling in mice through suppression of Rho-dependent extracellular MMP-2/TGF-β1 signaling. More important, EP3 antagonist treatment attenuated the development of pulmonary hypertension in the MCT rat model. These observations indicate that EP3 is a potential therapeutic target for the management of PAH.
Results
EP3 is upregulated in PAs in multiple experimental PAH animal models.
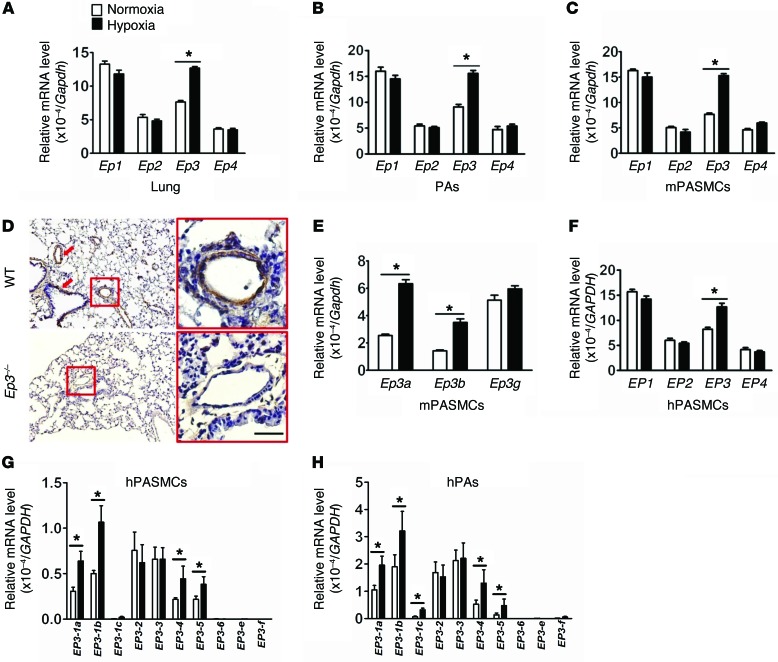
COX-2 expression is increased in lung tissues from PAH patients and in PAs from hypoxia-induced PAH mouse models (4, 5). As such, we also observed the substantial induction of all PG products, including PGE2 (Supplemental Figure 1; supplemental material available online with this article; doi:10.1172/JCI77656DS1) in lungs in response to chronic hypoxia. All PGE2 receptors (EP1–4) were abundantly expressed in the lung (Figure 1A), PAs (Figure 1B), and cultured murine PASMCs (mPASMCs) (Figure 1C). Interestingly, Ep3 expression was strikingly elevated in lungs, in PAs from chronic hypoxia–treated mice (Figure 1, A and B), and in cultured mPASMCs exposed to hypoxia (Figure 1C). In situ hybridization showed strong Ep3 expression in vessel wall and tunica muscularis bronchiorum in the lungs (Figure 1D). All 3 mouse Ep3 splice variants (a, b, and g) were coexpressed in mPASMCs, and only Ep3a and Ep3b levels significantly increased in PASMCs under hypoxic conditions (Figure 1E). Similarly, we observed EP3 upregulation induced by hypoxia in human PASMCs (hPASMCs) and human PA (hPA) tissue (Figure 1, F–H). Ten splice mRNAs and 8 isoforms of human EP3 have been identified (29, 30). Only EP3-1a, EP3-1b, EP3-1c, EP3-4, and EP3-5 were significantly upregulated in hPAs in response to hypoxia (Figure 1, G and H).
Figure 1. EP3 expression is upregulated in PASMCs in response to hypoxia.
(A) Relative mRNA levels of Ep1–4 in lung tissues from mice exposed to chronic hypoxia (n = 6). (B) Relative mRNA levels of Ep1–4 in PAs of hypoxia-treated mice (n = 6). (C) Relative mRNA levels of Ep1–4 in primary mPASMCs exposed to 1% O2 (n = 6). (D) Ep3 in situ hybridization in lung tissues from WT and Ep3–/– mice. Red arrows indicate strong staining. Scale bar: 20 μm (E) Relative mRNA levels of Ep3 isoforms in mPASMCs treated with normoxia or hypoxia (n = 6). (F) Relative mRNA levels of EP1–4 in primary hPASMCs exposed to 1% O2 (n = 6). *P < 0.05 versus normoxia. (G) Relative mRNA levels of EP3 isoforms in hPASMCs exposed to hypoxia. (H) Relative mRNA levels of EP3 isoforms in human PAs exposed to hypoxia. hPASMCs and human PAs in culture were exposed to 1% O2 for 24 hours before subjection to real-time quantitative RT-PCR analysis (n = 6 per group). *P < 0.05 versus normoxia by 2-tailed Student’s t test.
In addition, we also detected significant induction of EP3 expression in PDGF-BB–treated hPASMCs (Supplemental Figure 2). Taken together, EP3 expression in PAs is upregulated in response to hypoxia, indicating that the PGE2/EP3 axis might be involved in the progression of PAH.
Deletion of EP3 attenuates the development of both hypoxia- and HySu-induced PAH in mice.
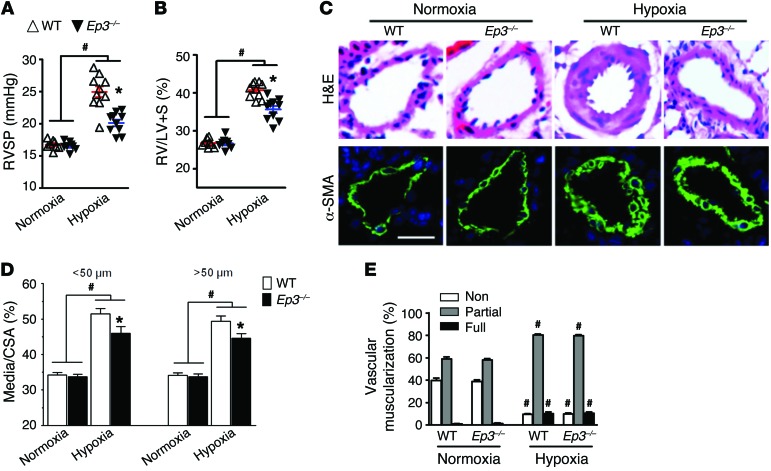
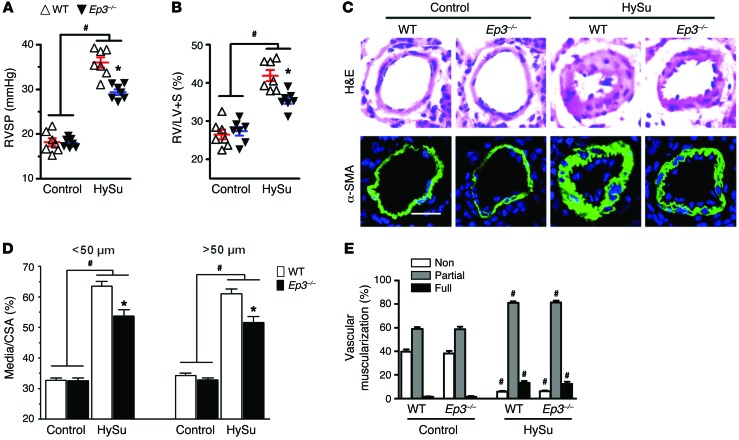
Next, we tested whether disruption of Ep3 would modulate the progression of PAH in different murine models. After a 3-week exposure to chronic hypoxia (10% O2), mice developed significant elevation in right ventricular systolic pressure (RVSP) (Figure 2A) and in the ratio of the weight of the free right ventricular wall to the weight of the left ventricular wall plus the septum (RV/LV+S) (Figure 2B) compared with what was observed in normoxic controls. Intriguingly, Ep3-deficient (Ep3–/–) mice displayed a significant reduction in RVSP (20.14 ± 0.50 mmHg vs. 24.98 ± 0.84 mmHg, P < 0.05) (Figure 2A) and in RV/LV+S (35.60 ± 0.92% vs. 40.67 ± 0.64%, P < 0.05) (Figure 2B) compared with that in WT controls. Moreover, Ep3 deletion attenuated pulmonary vascular remodeling induced by hypoxia through a reduction of pulmonary vascular wall thickness (Figure 2, C and D) and muscularization (Figure 2E). We then tested another classical experimental PAH model induced by HySu with more intense pulmonary vascular remodeling in Ep3–/– mice. Consistent with the hypoxia model, Ep3-deficient mice displayed similar protection against the development of PAH and PA remodeling in the HySu-induced PAH model (Figure 3, A–E). However, we did not detect any significant difference in hypoxia-induced inflammatory cell infiltration around the PAs between Ep3–/– and WT mice (Supplemental Figure 3).
Figure 2. Absence of Ep3 attenuates pulmonary hypertension and pulmonary vascular remodeling in chronic hypoxia–induced PAH in mice.
(A) RVSP in Ep3–/– and WT controls after exposure to hypoxia. (B) Ratio of RV/LV+S in Ep3–/– and WT control mice after exposure to hypoxia. (C) Representative images of H&E staining and α-SMA (green) immunostaining of lung tissues from hypoxia-treated Ep3–/– and WT mice. Scale bar: 20 μm. (D) Quantification of the ratio of vascular medial thickness to total vessel size (Media/cross-sectional area [CSA]) for the hypoxia model. (E) Proportion of non-, partially, or fully muscularized pulmonary arterioles (20–50 μm in diameter) from hypoxia-treated mice. (A–E) n = 8–10 mice. *P < 0.05 versus WT and #P < 0.05 versus normoxic controls by 2-way ANOVA with Bonferroni’s post-hoc analysis.
Figure 3. Disruption of EP3 ameliorates PAH and pulmonary vascular remodeling in HySu-induced PAH in mice.
(A) RVSP in Ep3–/– and WT mice after HySu treatment. (B) RV/LV+S in Ep3–/– and WT mice after HySu treatment. (C) Representative images of H&E staining and α-SMA (green) immunostaining of lung sections from HySu-treated Ep3–/– and WT mice. Scale bar: 20 μm. (D) Quantification of the ratio of vascular medial thickness to total vessel size for the HySu treatment model. (E) Proportion of non-, partially, or full muscularized pulmonary arterioles (20–50 μm in diameter) from HySu-treated mice. (A–E) n = 7 mice. *P < 0.05 versus WT and #P < 0.05 versus control by 2-way ANOVA with Bonferroni’s post-hoc analysis.
To further dissect the role of the EP3 receptor in endothelial cells (ECs) or VSMCs in the pathogenesis of PAH, EC Ep3–/– (Ep3fl/fl Tie2-Cre, Supplemental Figure 4A) and VSMC Ep3–/– (EP3fl/fl Sm22-Cre Supplemental Figure 4A) mice were subjected to HySu treatment. Again, Ep3 mRNA was expressed in both ECs and VSMCs of the flox control mice, but was diminished in ECs from Ep3fl/fl Tie2-Cre mice and in VSMCs from Ep3fl/fl Sm22-Cre mice (Supplemental Figure 4B). We observed that Ep3 ablation in VSMCs, but not in ECs, ameliorated PAH development in mice by reducing RVSP, RV/LV+S, and PA remodeling (Supplemental Figure 4, C–F).
Pharmacological blockade of EP3 suppresses progression of MCT-induced pulmonary hypertension and pulmonary vascular remodeling in rats.
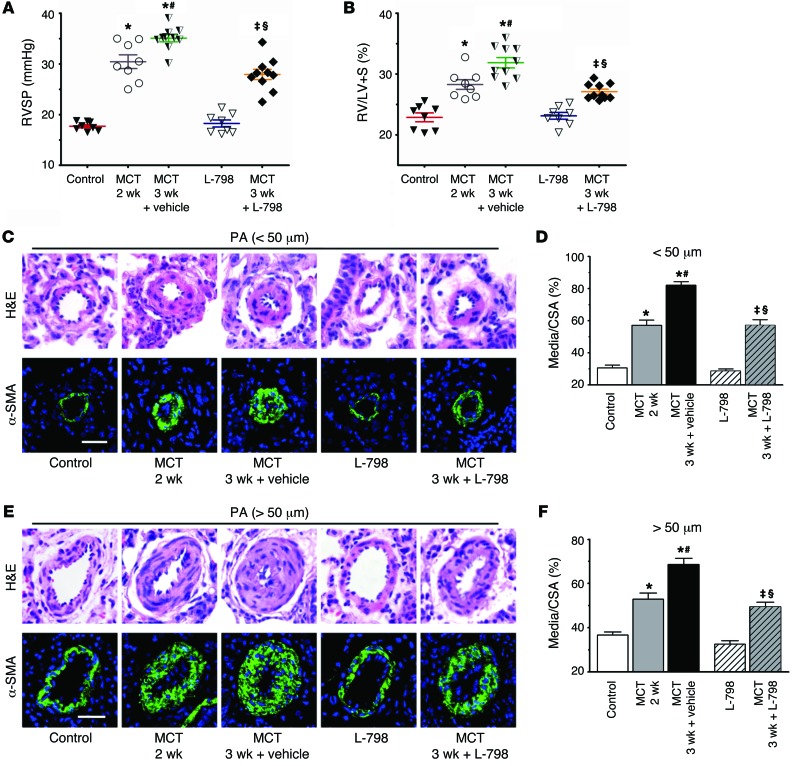
We then explored the therapeutic effect of the selective EP3 inhibitor L-798106 on pulmonary vascular remodeling in a model of established pulmonary hypertension induced by MCT in rats. As shown in Supplemental Figure 5A, L-798106 reaches peak plasma concentration after 1 hour when administered as a single peritoneal injection. It exhibits linear proportional pharmacokinetics over the effective and safe dose range (30–1,000 nM) (31, 32), and the elimination half-life (t1/2) is approximately 8 hours (Supplemental Figure 5A). A dose of 200 mg/kg L-798106 was administered to the MCT-treated rats twice a day at the beginning of the third week after MCT treatment (Supplemental Figure 5B). As anticipated, MCT-treated rats displayed much higher RVSP, RV/LV+S ratios, and thickness of pulmonary vascular walls 3 weeks after injection, indicating that MCT-induced PAH models were established (Figure 4, A–F). Analogous to the genetic deficiency of Ep3 (Figures 2 and 3), L-798106 administration significantly reduced the augmented RVSP, RV/LV+S ratio, and pulmonary vascular wall thickness in MCT-treated rats (Figure 4, A–F). These results indicate that a selective EP3 inhibitor can alleviate the progression of pulmonary vascular remodeling of established pulmonary hypertension.
Figure 4. Inhibition of EP3 alleviates progression of MCT-induced PAH in rats.
(A and B) Effect of L-798106 administration on RVSP and RV/LV+S of MCT-induced PAH rats. (C) Representative images of H&E staining and α-SMA (green) immunostaining of PAs (20–50 μm in diameter). (D) Quantification of vascular medial thickness for images in C. (E) Representative images of H&E staining and α-SMA immunostaining of PAs (51–100 μm in diameter). (F) Quantification of vascular medial thickness for images in E. Scale bars: 20 μm. n = 8–10/group. *P < 0.05 versus control; #P < 0.05 versus MCT treatment after 2 weeks; ‡P < 0.05 versus L-798106; §P < 0.05 versus MCT treatment for 3 weeks plus vehicle. Data were calculated by 2-tailed Student’s t test or 1-way ANOVA with Bonferroni’s post-hoc analysis as appropriate. L-798, L-798106.
EP3 mediates accumulation of ECM proteins in PAs through activation of TGF-β1 signaling in response to hypoxia.
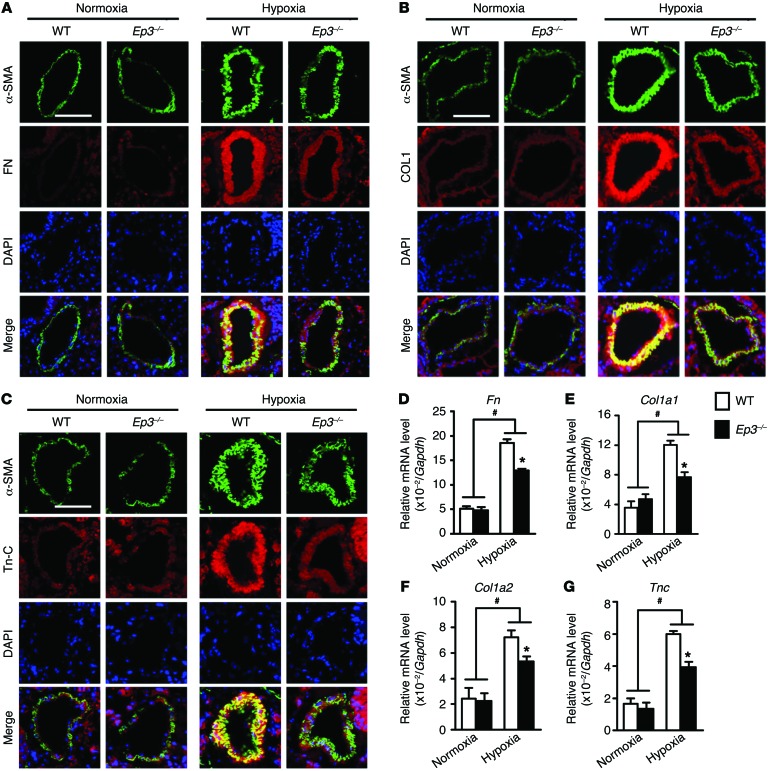
Pathological lesions in PAH patients, including neomuscularization and fibrotic changes (ECM deposition), predominantly affect the distal pulmonary arterioles (33). Immunostaining for α smooth muscle actin (α-SMA) demonstrated that there was no significant difference in neomuscularization of distal pulmonary arterioles, as calculated by α-SMA+ cells, between Ep3–/– mice and their littermate controls after exposure to hypoxia (Figure 2 and Supplemental Figure 6A). We then explored whether the EP3 receptor mediates PASMC proliferation in response to hypoxia. As shown in Supplemental Figure 6, B–D, neither genetic deletion nor knockdown nor pharmacological activation of the EP3 receptor significantly influenced the proliferation of mPASMCs or hPASMCs. Consistently, in hypoxia- and MCT-induced PAH models, PCNA+ PASMCs were not altered by Ep3 deletion or inhibition (Supplemental Figure 6, E–H). However, the accumulation of ECM proteins (collagen I, fibronectin, and tenascin C) induced by chronic hypoxic stress was significantly reduced in PAs of Ep3-deficient mice (Figure 5, A–C), and this reduction was reflected at the mRNA transcriptional level in lung tissues (Figure 5, D–G) and by Masson’s trichrome staining in hypoxia- and MCT-treated animals (Supplemental Figure 7). Likewise, we observed a significant reduction of both mRNA and protein expression levels of fibronectin, collagen I, and tenascin C in Ep3–/– mPASMCs under hypoxic conditions compared with levels in WT mPASMCs (Supplemental Figure 8).
Figure 5. EP3 mediates accumulation of ECM proteins in PAs in response to hypoxia.
(A–C) Immunofluorescence staining (red) of fibronectin (FN), collagen I (COL1), and tenascin C (Tn-C). Slides were double stained with α-SMA (green) and counterstained with DAPI (blue) for nuclei. Scale bars: 20 μm. (D–G) Relative mRNA levels of Fn, collagen 1α1 (Col1a1), collagen 1α2 (Col1a2), and Tn-C (Tnc) in lungs from normoxia- or hypoxia-treated Ep3–/– and WT mice (n = 4). *P < 0.05 versus WT and #P < 0.05 versus normoxia by 2-way ANOVA with Bonferroni’s post-hoc analysis.
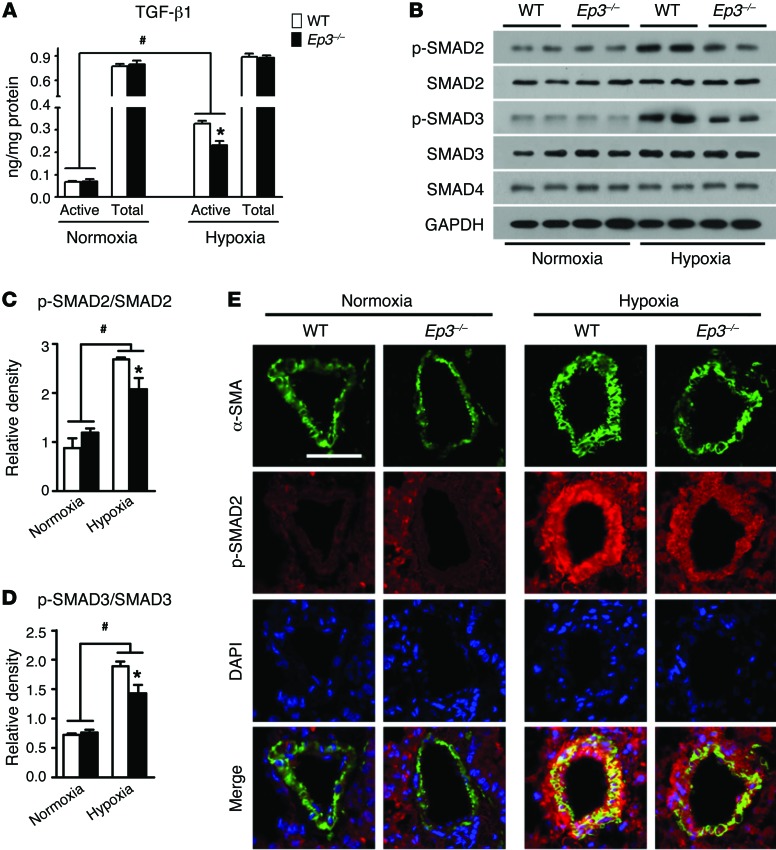
Since a notable reduction of ECM deposition was observed in PAs from Ep3–/– PAH mice, we next determined whether TGF-β1 signaling is altered in Ep3–/– PASMCs in response to hypoxia. Both bone morphogenetic proteins (BMPs) and TGF-β isoforms that mediate signaling are believed to be involved in PA remodeling in PAH (34). Consistent with previous reports (35, 36), we observed that hypoxia suppressed the expression of the inhibitor of differentiation 1 (ID1) (Supplemental Figure 9A), a downstream target of the BMP receptor 2 pathway, and hypoxia induced the expression of PAI-1, a target of TGF-β1 signaling (Supplemental Figure 9B), in mPASMCs. Meanwhile, Ep3 deletion attenuated hypoxia-stimulated PAI-1 expression without significant influence on ID1 expression, indicating that the EP3 receptor may modulate TGF-β1 signaling in mPASMCs. ELISA and Western blot analyses showed that the levels of culture–active TGF-β1 and phosphorylation of SMAD2/3 were depressed in hypoxia-stimulated Ep3–/– PASMCs (Figure 6, A–D). PDGF-BB can activate TGF-β1 signaling and drive collagen synthesis in PASMCs (36). We then examined the effect of Ep3 deletion on PDGF-BB–induced TGF-β1 signaling in mPASMCs. Again, Ep3 disruption suppressed the induction of active TGF-β1 in culture medium, suppressed cellular phosphorylation of SMAD2 stimulated by PDGF-BB in mPASMCs (Supplemental Figure 10, A and B), and restrained both protein and mRNA expression of the TGF-β1 downstream targets fibronectin, collagen I, and tenascin C (Supplemental Figure 10, C–G). Accordingly, in PAs from chronic hypoxia–treated Ep3–/– mice, SMAD2 phosphorylation levels also decreased (Figure 6E), further supporting the conclusion that EP3-mediated TGF-β1 activation contributes to PA remodeling in PAH.
Figure 6. Ep3 deficiency suppresses TGF-β1 signaling pathway in hypoxia-treated PASMCs and PAs.
(A) Content of active TGF-β1 and total TGF-β1 in Ep3–/– and WT PASMC medium after normoxia or hypoxia treatment. (B) Western blot analysis of phosphorylated (p-) SMAD2/3 in Ep3–/– and WT PASMCs after normoxia or hypoxia treatment. (C and D) Quantification of SMAD2 and SMAD3 phosphorylation (n = 6). *P < 0.05 versus WT and #P < 0.05 versus normoxia by 2-way ANOVA with Bonferroni’s post-hoc analysis. (E) Immunofluorescence staining of p-SMAD2 (red) and α-SMA (green) of lung sections from normoxia- or hypoxia-treated Ep3–/– and WT mice. Scale bar: 20 μm.
Both Ep3a and Ep3b, but not Ep3g, mediate activation of TGF-β1 signaling in the PASMC response to hypoxia.
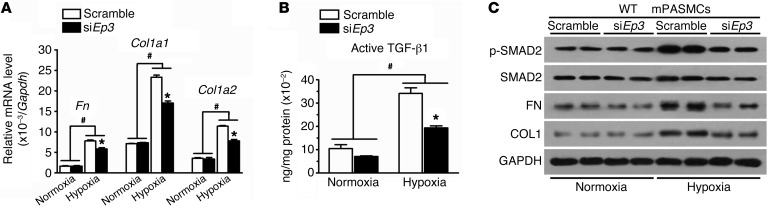
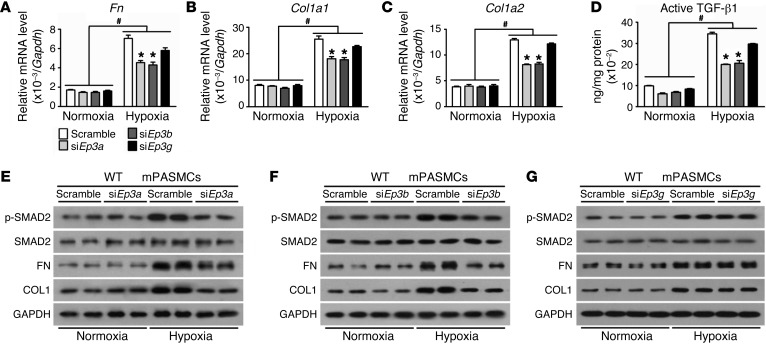
By using common Ep3 siRNA (siEp3) (31), Ep3 expression was reduced by 75% in primary mPASMCs, resulting in an approximately 40% to 50% reduction in transcription of fibronectin and collagen 1, suppression of latent TGF-β1 activation, and suppression of SMAD2 phosphorylation in the PASMC response to hypoxia (Figure 7, A–C). Since all 3 Ep3 splice variants are expressed in PAs and primary mPASMCs (Figure 1E), we next evaluated the EP3 variant that mediates TGF-β1 signaling activation triggered by hypoxia. PASMCs from WT mice were subjected to transfection with siRNA specifically designed for each variant. We observed that knockdown of Ep3a and Ep3b reduced active TGF-β1 content in the culture medium and intracellular phosphorylation of SMAD2 and suppressed hypoxia-induced expression of fibronectin and collagen I at both mRNA and protein levels in PASMCs (Figure 8, A–F). However, silencing of Ep3g did not significantly influence TGF-β1 signaling in PASMCs (Figure 8, A–D, and G).
Figure 7. Knockdown of Ep3 attenuates hypoxia-induced TGF-β1 activation in PASMCs.
(A) Effect of Ep3 knockdown on mRNA levels of Fn, Col1a1, and Col1a2 in normoxia- or hypoxia-treated PASMCs. (B) Effect of Ep3 knockdown on active TGF-β1 content in culture medium of normoxia- or hypoxia-treated PASMCs (n = 4). *P < 0.05 versus scramble and #P < 0.05 versus normoxia by 2-way ANOVA with Bonferroni’s post-hoc analysis. (C) Effect of Ep3 knockdown on TGF-β1 signaling in normoxia- or hypoxia-treated PASMCs.
Figure 8. Knockdown of Ep3a/b variants attenuates hypoxia-induced TGF-β1 activation in PASMCs.
(A–C) Effect of single Ep3a/b/g variant knockdown on mRNA expression of Fn, Col1a1, and Col1a2 in normoxia- or hypoxia-treated PASMCs. (D) Effect of single Ep3a/b/g variant knockdown on active TGF-β1 content in culture medium of normoxia- or hypoxia-treated PASMCs (n = 4). *P < 0.05 versus scramble and #P < 0.05 versus normoxia by 2-way ANOVA with Bonferroni’s post-hoc analysis. (E–G) Western blot analysis of p-SMAD2, FN, and collagen I protein in PASMCs transfected with siRNA specifically designed for Ep3a (E), Ep3b (F), and Ep3g (G), respectively.
Next, we sought to determine whether reexpression or overexpression of Ep3 variants could restore the activation of TGF-β1 signaling in PASMCs. Primary PASMCs isolated from Ep3–/– mice were transfected with murine Ep3a, Ep3b, and Ep3g cDNAs that were HA tagged at the extracellular N terminus. Overexpression efficiency was determined by real-time PCR 24 hours after transfection (Supplemental Figure 11A). Reexpression of Ep3a and Ep3b in PASMCs rescued hypoxia-induced activation of TGF-β1 signaling, as evidenced by increased phosphorylation of SMAD2 and upregulation of fibronectin and collagen I (Supplemental Figure 11, B–G), whereas Ep3g overexpression showed very little influence on TGF-β1 signaling in PASMCs (Supplemental Figure 11, B–E, and H). Taken together, these results indicate that both Ep3a and Ep3b, but not Ep3g, mediate activation of TGF-β1 signaling in response to hypoxia.
Ep3a/b mediates RhoA/Rho-associated kinase–dependent activation of MMP-2.
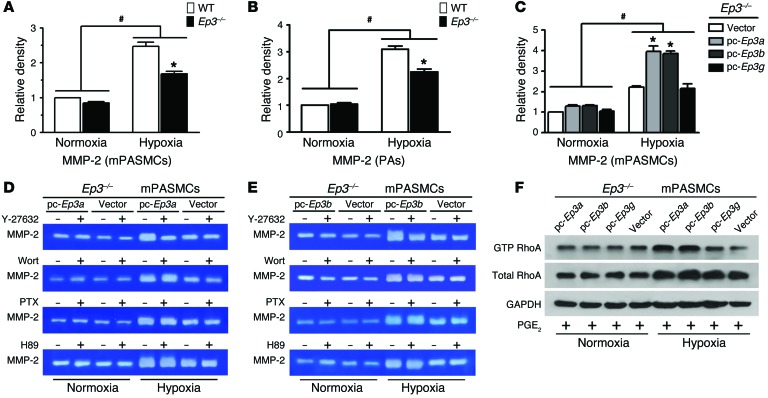
TGF-β1 is synthesized and secreted into the ECM as an inactive latent precursor that is composed of an N-terminal latency–associated peptide (LAP) and a C-terminal mature TGF-β1. Proteolytic cleavage of LAP is required for activation of TGF-β1. Many factors such as an acidic microenvironment, thrombospondin 1 (Tsp-1), integrin, and MMP-2/9 can activate TGF-β1 (37–39). Interestingly, MMP-2 expression in cultured mPASMCs and PAs was depressed in Ep3–/– mice under hypoxic stress (Supplemental Figure 12, A and B). Furthermore, we were unable to detect significant alternations of expression of other TGF-β1 activators (Tsp-1, αV integrin, and MMP-9) in Ep3–/– cells or controls (Supplemental Figure 12). A gelatin zymographic assay also showed reduced MMP-2 activity in the culture of hypoxia-treated Ep3–/– PASMCs (1.68 ± 0.07 AU versus WT, 2.47 ± 0.12 AU; P <0.05) (Supplemental Figure 13A and Figure 9A) and in the PAs of Ep3–/– mice subjected to chronic hypoxia (2.24 ± 0.10 AU versus WT, 3.09 ± 0.12 AU; P < 0.05) (Supplemental Figure 13B and Figure 9B). Accordingly, overexpression of Ep3a and Ep3b significantly elevated hypoxia-induced MMP-2 activation in the culture of mPASMCs (Ep3a, 3.970.26 AU; Ep3b, 3.87 ± 0.12 AU; vs. vector, 2.21 ± 0.06 AU; P < 0.05) (Supplemental Figure 13C and Figure 9C), whereas overexpression of Ep3g in PASMCs failed to significantly alter MMP-2 activity. Thus, activation of both Ep3a and Ep3b variants promotes gene transcription and secretion of MMP-2 in PAs in response to a chronically hypoxic environment.
Figure 9. Ep3a/b variants mediate RhoA/ROCK-dependent activation of extracellular MMP-2 in cultured mPASMCs.
(A) Culture MMP-2 activity of PASMCs from Ep3–/– and WT mice. (B) MMP-2 activity in PAs from Ep3–/– and WT mice after normoxia or hypoxia treatment (n = 6). *P < 0.05 versus WT and #P < 0.05 versus normoxia by 2-way ANOVA with Bonferroni’s post-hoc analysis. (C) Culture MMP-2 activity in EP3-deficient PASMCs reexpressing Ep3a, Ep3b, or Ep3g (n = 3). *P < 0.05 versus vector and #P < 0.05 versus normoxia by 2-way ANOVA with Bonferroni’s post-hoc analysis. Effect of Y-27632, wortmannin (Wort), PTX, and H89 on culture MMP-2 activity in EP3-deficient PASMCs with forced expression of Ep3a (D) or Ep3b (E). (F) Western blot analysis of active RhoA in Ep3-deficient PASMCs with forced expression of Ep3a, Ep3b, or Ep3g in the presence of PGE2. pc, pcDNA3.1 vector.
The EP3 receptor modulates multiple intracellular signaling pathways by coupling different types of heterotrimeric G proteins, including Gαs, Gαi, and Gα12/13 (29). To further examine the underlying mechanisms involved in the regulation of EP3-mediated TGF-β1 signaling in PASMCs, the following treatments were applied to PASMCs overexpressing EP3α/β: wortmannin, a phosphatidylinositol 3-kinase inhibitor; pertussis toxin (PTX), a Gαi protein blocker; H-89, an inhibitor of Gαs protein downstream cAMP-dependent protein kinase (PKA); and Y-27632, a Rho-associated kinase (ROCK) inhibitor. The elevated activity of MMP-2, induced by overexpression of both Ep3a and Ep3b in PASMCs under hypoxic conditions, was greatly decreased by pretreatment with the ROCK inhibitor Y-27632 (Figure 9, D and E), while no significant effects were detected using wortmannin, PTX, or H-89 (Figure 9, D and E). Meanwhile, active RhoA levels were notably increased in Ep3a/b-overexpressing PASMCs compared with those in controls (Figure 9F), which further supports the notion that Ep3a/b-mediated activation of MMP-2 is RhoA/ROCK dependent.
Ep3a/b modulates hypoxia-induced MMP-2/TGF-β1 activation by facilitating membrane translocation of MT1-MMP via Rho/ROCK-dependent actin remodeling.
Activation of RhoA/ROCK signaling is known to be involved in the regulation of actin cytoskeletal remodeling (40), which has recently been recognized to mediate the trafficking of membrane type 1–MMP (MT1-MMP) to the cell surface (41–43). MT1-MMP is a transmembrane protease that cleaves extracellular pro–MMP-2. On the basis of the reduced extracellular MMP-2 activity and reduced Rho/ROCK signaling observed in Ep3–/– PASMCs under hypoxic conditions, we hypothesized that EP3 mediates intracellular MT1-MMP movement toward the membrane and subsequently activates the MMP-2/TGF-β1 signaling pathway by modulating Rho/ROCK activity in PASMCs. Upon hypoxic stimulation, MT1-MMP protein moved to the cell membrane (Supplemental Figure 14A). Ep3–/– PASMCs displayed much less membrane MT1-MMP but more MT1-MMP in the cytoplasm in response to hypoxia compared with that in WT controls (Supplemental Figure 14, A and B). In contrast, complementation of Ep3a and Ep3b, but not Ep3g, rescued the levels of membrane-localized MT1-MMP in mPASMCs in response to hypoxia (Supplemental Figure 14, C and D). Thus, these results suggest that PGE2-EP3α/β invokes MMP-2 activation through the regulation of MT1-MMP membrane translocation.
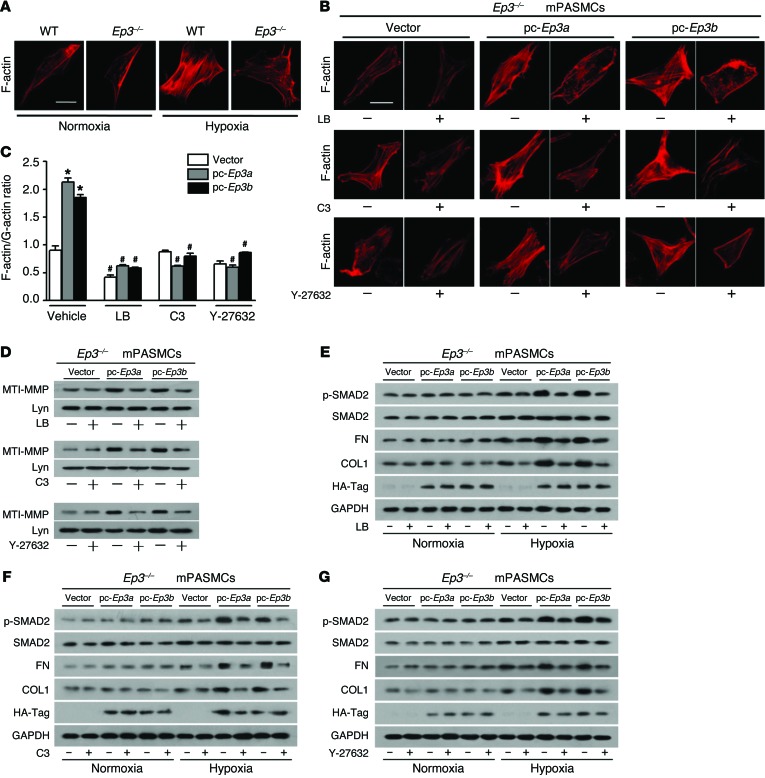
Next, we sought to determine whether movement of MT1-MMP regulated by the EP3 receptor is dependent on RhoA/ROCK-mediated actin polymerization. Consistent with reduced RhoA activity (Figure 9F), polymerization of F-actin was also decreased in Ep3–/– PASMCs exposed to hypoxia, as evidenced by rhodamine phalloidin staining (Figure 10A). Reexpression of either Ep3a or Ep3b in Ep3–/– PASMCs restored polymerization of F-actin (Figure 10B), which could be attenuated by the specific Rho inhibitor C3 transferase (C3), the ROCK inhibitor Y-27632, and the actin polymerization inhibitor latrunculin B (LB) (Figure 10B). We further measured and quantified the relative levels of monomeric (globular) versus polymeric (filamentous) forms of actin in PASMCs under hypoxic conditions. In agreement with immunofluorescence observations, reexpression of Ep3a/b in PASMCs led to an augmented ratio of F-actin to G-actin, which, likewise, was also suppressed by pretreatment with C3, Y-27632, or LB (Supplemental Figure 15 and Figure 10C). These results indicate that Ep3a/b mediates a significant shift toward filamentous stress fiber formation (F-actin polymerization). Similarly, induction of MT1-MMP protein, phosphorylation of SMAD2, and expression of fibronectin and collagen I in PASMCs, all of which were provoked by Ep3a/b overexpression, were completely blunted by pretreatment with C3, Y-27632, or LB (Figure 10, D–G). Thus, these data indicate that Ep3a/b variants regulate MT1-MMP movement toward the membrane to activate MMP-2/TGF-β1 signaling in PASMCs through Rho/ROCK-dependent cytoskeletal remodeling.
Figure 10. Ep3a/b variants mediate membrane localization of MT1-MMP through RhoA/ROCK-dependent cytoskeletal remodeling.
(A) Rhodamine phalloidin staining of F-actin in Ep3–/– and WT PASMCs after normoxia or hypoxia exposure. (B) Effect of LB, C3, and Y-27632 on F-actin polymerization in Ep3-deficient PASMCs with forced expression of Ep3a or Ep3b. (C) Quantification of F-actin/G-actin ratio in hypoxia-treated, Ep3a/b-complemented PASMCs in the presence or absence of LB, C3, or Y-27632 (n = 3). *P < 0.05 versus vector and #P < 0.05 versus vehicle by 2-way ANOVA with Bonferroni’s post-hoc analysis. (D) Immunoblot analysis of MT1-MMP membrane expression in Ep3a/b-overexpressing PASMCs with or without pretreatment with LB, C3, or Y-27632. (E–G) Immunoblot analyses of p-SMAD2, fibronectin, and collagen I in Ep3a/b-complemented PASMCs with or without pretreatment with LB, C3, or Y-27632.
Ep3a/b variants are coupled via Gα12 to activate Rho/ROCK/MMP-2/TGF-β1 signaling in PASMCs in response to hypoxia.
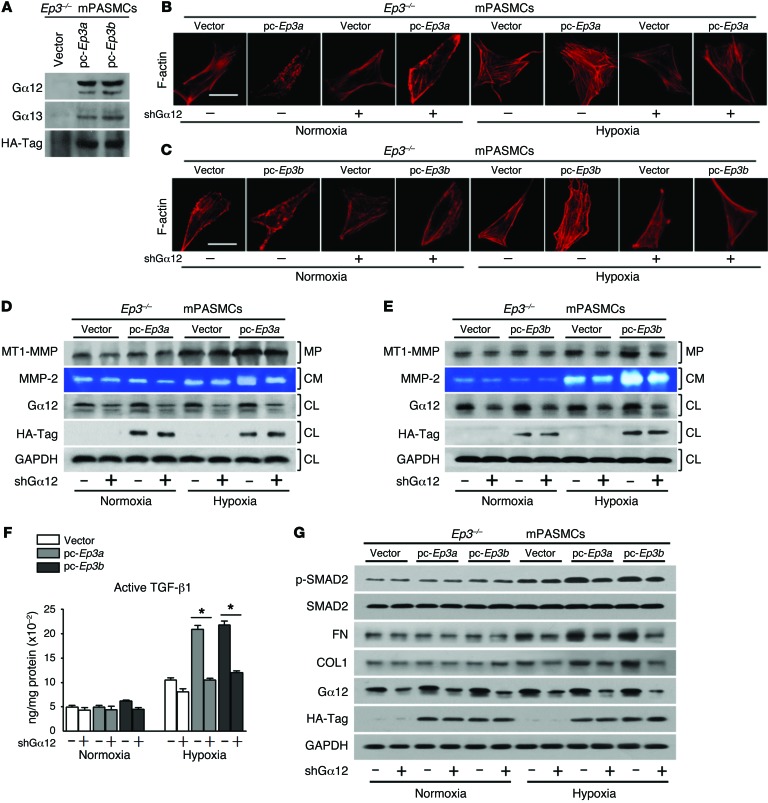
Evidence indicates that EP3-mediated Rho/ROCK activation occurs through coupling to Gα12/13 (44, 45). To determine whether the G protein interaction occurs in PASMCs, HA-tagged Ep3a or Ep3b was introduced into cultured PASMCs. Coimmunoprecipitation using an HA-Tag antibody displayed both Gα12 and Gα13 in the HA-EP3 immunocomplex, with a greater abundance of Gα12 than of Gα13 (Figure 11A). Then, shRNAs, designed to specifically knock down the expression of Gα12 or Gα13, were transfected into Ep3a- and Ep3b-overexpressing mPASMCs to determine which of the 2 G proteins transduces EP3-mediated Rho/MMP-2/TGF-β1 signaling. As shown in Figure 11, silencing Gα12 significantly suppressed the following processes: induction of F-actin polymerization (Figure 11, B and C); augmentation of MT1-MMP membrane levels (Figure 11, D and E); upregulation of extracellular MMP-2 activity (Figure 11, D and E); enhanced cleavage of latent TGF-β1 in culture (Figure 11F); and boosted expression of fibronectin and collagen I (Figure 11G) in mPASMCs caused by Ep3a/b overexpression under hypoxic stimulatory conditions. However, we did not observe a significant effect of Gα13 silencing on EP3-mediated TGF-β1 signaling (Supplemental Figure 16). Therefore, these results suggest that Ep3a/b variants mediate Rho-dependent TGF-β1 signaling in PASMCs in response to hypoxia via coupling to Gα12 (Figure 12). In primary cultured hPASMCs, Rho-dependent TGF-β1 signaling was also suppressed by inhibition of the EP3 receptor (Supplemental Figure 17).
Figure 11. Ep3a/b mediates ROCK/MT1-MMP/MMP-2/TGF-β1 signaling in PASMCs via Gα12 upon hypoxic stress.
(A) Western blot analysis of Ep3a/b binding to Gα12 or Gα13. (B and C) Effect of Gα12 knockdown on F-actin polymerization in Ep3-deficient PASMCs with reexpression of Ep3a or Ep3b. (D and E) Effect of Gα12 knockdown on MT1-MMP membrane localization and MMP-2 activity in the culture medium (CM) of Ep3a- or Ep3b-reexpressing PASMCs. MP, membrane protein; CL, cell lysate. (F) Effect of Gα12 knockdown on active TGF-β1 content in culture medium of Ep3a/b-reexpressing PASMCs (n = 4). *P < 0.05 by 2-tailed Student’s t test. (G) Effect of Gα12 knockdown on Ep3a/b-mediated TGF-β1 signaling in PASMCs.
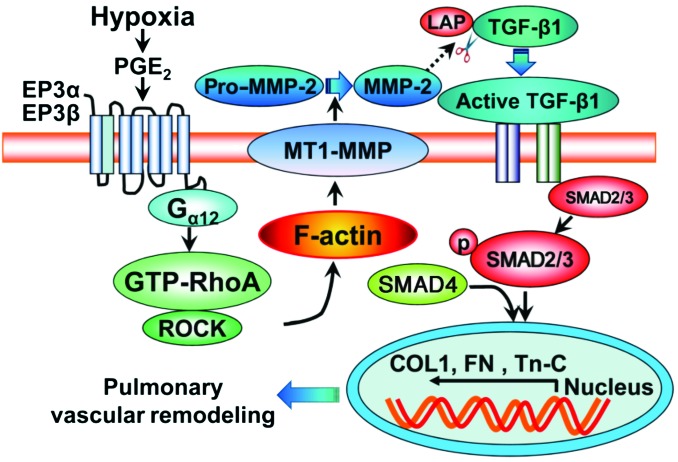
Figure 12. Schematic diagram of Ep3a/b-mediated PA remodeling through Rho-dependent MT1-MMP/MMP-2/TGF-β1 signaling.
Discussion
The EP3 receptor can mediate vasoconstriction of human arteries including PAs (46). In this study, we found that EP3 expression was upregulated in human and mouse PASMCs in response to hypoxia and was significantly elevated in the PAs of hypoxia-induced PAH mouse models. Furthermore, we demonstrated that the EP3 receptor was involved in the development of PAH in 3 rodent models through both pharmacological inhibition and genetic deletion approaches. In addition, activation of the EP3 receptor, including both Ep3a and Ep3b splice variants, boosted hypoxia-stimulated TGF-β1 signaling and fibrotic protein expression in PASMCs and PAs by increasing extracellular MMP-2 activity. More interestingly, EP3α/β-mediated MMP-2/TGF-β1 activation was attributed to localization of MT1-MMP toward the cell membrane through Rho/ROCK-dependent actin cytoskeletal remodeling. Therefore, inhibition of the EP3 receptor confers protection against PAH through suppression of Rho/ROCK-dependent TGF-β1 signaling.
PGs, the metabolic products of arachidonic acid via phospholipase A2 (PLA2) and COX catalysis, are implicated in the regulation of vascular homeostasis, including pulmonary vascular tone. Pharmacological inhibition and genetic deletion of cytosolic PLA2 attenuate hypoxia-induced pulmonary vasoconstriction and PA resistance in mice (47, 48). Moreover, COX-2 can be induced greatly by hypoxia in PAs and PASMCs (7). Both nonselective and COX-2–selective NSAIDs suppress hypoxic vasoconstriction in PAs (47); however, disruption of the Cox2 gene exaggerates hypoxia-induced pulmonary hypertension and vascular remodeling in rodents (7), indicating that COX-2–derived PGs may be involved in the development of PAH. Patients with PAH produce fewer vasodilators but excessive vasoconstrictors (33). For instance, reduced circulating levels of the vasodilator PGI2 and elevated levels of vasoconstrictive thromboxane were observed in PAH patients (14). We found that the vasoconstrictive EP3 receptor, which also mediates the contraction of PAs (9, 10), was strikingly upregulated in human and murine PASMCs in response to hypoxia in vitro and in PAs in rodent PAH models. Interestingly, we found that 5 of 10 different human EP3 splice variants (EP3-1a, EP3-1b, EP3-1c, EP3-4, and EP3-5) in PAs were markedly elevated in response to hypoxia. Others failed to detect the EP3 upregulation in cultured PASMCs from MCT-PAH rats, probably due to the different experimental approaches adopted (49). Moreover, the EP3 receptor is also involved in the mediation of pulmonary vascular contraction induced by isoprostanes, which are oxidative metabolites of the polyunsaturated fatty acids that are found to be markedly elevated in PAH patients (50, 51). We were unable to detect any significant influences of EP3 receptor activation, inhibition, or genetic deletion on PASMC proliferation in vitro or in vivo and observed no notable impact of EP3 ablation on PASMC hypertrophy in PAH animals. Indeed, disruption or inhibition of the EP3 receptor attenuates overdeposition of collagen and thickening of the smooth muscle layer in pulmonary arterioles and attenuates the increase in RVSP and the thickness of the right ventricular wall in both hypoxic and MCT-induced PAH models. These observations suggest that elevated expression of the EP3 receptor in PAs contributes to pulmonary vascular remodeling in PAH.
The increased activation of TGF-β1 signaling in PAs was reported in idiopathic PAH patients (52) and in different PAH animal models (53, 54), and inhibition of the TGF-β1 receptor activin receptor–like kinase 1 prevents the development of PAH (55). Similarly, we observed elevated TGF-β1 signaling in the PAs of hypoxia-induced PAH models. Interestingly, inhibition or loss of the EP3 receptor suppressed hypoxia-induced activation of TGF-β1 signaling in PASMCs and PAs, implying that targeting the EP3 receptor confers protection against PAH by suppressing TGF-β1 signaling. Although 3 EP3 splice isoforms are coexpressed in vasculature, only Ep3a and Ep3b, not Ep3g, seem to be involved in the mediation of hypoxia-induced TGF-β1 activation in PASMCs and maintenance of cell polarity, as previously described (31). These phenomena are perhaps due to the unique C terminus of EP3γ, which is crucial for its activation of the GTP-binding protein and cellular distribution (56, 57).
The dissociation or activation of TGF-β from LAP is a critical regulatory event (58). We found that extracellular MMP-2 activity was notably reduced in PAs from hypoxia-stressed Ep3–/– mice and in the culture of Ep3–/– PASMCs under hypoxic conditions and that reexpression of Ep3a/b boosted MMP-2 activity in PASMC culture, which is in agreement with TGF-β1–signaling alterations. These observations indicate that Ep3a/b-mediated extracellular MMP-2 activation cleaves LAP and promotes TGF-β1 signaling upon hypoxic stress. Moreover, TGF-β1 signaling may also regulate MMP-2 transcription (59), as reduced transcription of MMP-2 was detected in Ep3–/– PASMCs. Interestingly, blockade of Gα12 downstream ROCK (60) or knockdown of Gα12 reduced the exaggerated MMP-2 activation caused by overexpression of Ep3a/b in PASMCs, further supporting the idea that the Ep3a/b-triggered MMP-2/TGF-β1 cascade in response to hypoxic stress is dependent on activation of the Gα12/RhoA pathway. Similarly, MMP-2–mediated TGF-β1 activation and subsequent matrix-associated protein deposition are also implicated in arterial stiffness in aged arteries (61). Moreover, PGE2/EP3-mediated arterial contraction seems to require ROCK activation (62), which may also contribute to the development of PAH.
Rho GTPase is a master regulator controlling cytoskeletal-dependent functions such as cytokinesis, actin cytoskeletal organization, cell adhesion, and migration (63). Ep3 deficiency suppressed hypoxia-induced GTP-RhoA levels in PASMCs, while overexpression of Ep3a/b by transfection augmented RhoA activity upon hypoxia exposure. Accordingly, overexpression of Ep3a/b increased F-actin polymerization and subsequent MT1-MMP movement toward the cell membrane, which was inhibited by the actin polymerization inhibitor LB. These observations indicate that EP3α/β-mediated actin cytoskeletal remodeling regulates intracellular MT1-MMP transportation through activation of Rho GTPase. CD44, a hyaluronic acid receptor, is believed to interact with the hemopexin-like domain of MT1-MMP (41) and to link MT1-MMP with actin filaments through direct CD44 binding to F-actin (41, 43). As such, leptin mediates myocardial matrix remodeling by regulating the cell-surface location of MT1-MMP in cardiac fibroblasts via Rho GTPase–mediated actin polymerization, which facilitates the development of heart failure in obesity (43, 64). Thus, EP3α/β activation, which stimulates the MMP-2/TGF-β1–signaling cascade in the PASMC response to hypoxic stress, induces Rho/ROCK-dependent actin cytoskeletal reorganization, an event that may be implicated in PAH pathogenesis. Consequently, long-term treatment with ROCK inhibitors markedly reduces PA pressure and ameliorates PA remodeling in MCT- and hypoxia-induced PAH in rodents (65–67). In humans with PAH, intake of ROCK inhibitors also leads to significant improvement of pulmonary hemodynamics (68).
In summary, we showed that the vasoconstrictive receptor EP3 is upregulated in PAs and PASMCs in response to hypoxia. Additionally, we demonstrated that pharmacological inhibition or genetic deletion of Ep3 retards the progress of both hypoxia- and MCT-induced PAH in rodents through suppression of the Rho/ROCK-dependent MMP-2/TGF-β1 signaling pathway. Thus, blockade of the EP3 receptor may be a promising strategy for the management of PAH.
Methods
Further information can be found in the Supplemental Methods.
Animals.
All the mice were maintained on a C57BL6 genetic background. WT littermates were generated as experimental controls from EP3 receptor heterozygous matings. VSMC- and EC-specific Ep3-KO mice were generated by crossing Ep3fl/fl mice (69) with Sm22-Cre and Tie2-Cre transgenic mice, respectively.
Chronic hypoxia–induced pulmonary hypertension model in mice.
Eight- to 10-week-old male Ep3-KO (Ep3–/–) mice and their age- and weight-matched WT littermates were exposed to room air (normoxia) or 10% oxygen (hypoxia) in a ventilated chamber, as described previously (36). All animals had access to standard mouse chow and water, and they were subjected to an alternating 12-hour light/12-hour dark cycle under controlled temperature conditions. The chamber was opened twice a week for 10 to 15 minutes for cleaning and supplementing food and water. After 3 weeks, pulmonary hypertension and pulmonary vascular remodeling were assessed.
SU5416 combined with chronic hypoxia–induced pulmonary hypertension model in mice.
Eight- to 10-week-old Ep3–/–, VSMC-Ep3–/–, and EC-Ep3–/– male mice and age-matched controls received a single weekly s.c. injection of the VEGFR inhibitor SU5416 (20 mg/kg), which was suspended in carboxymethylcellulose (CMC) solution (0.5% [w/v] carboxymethylcellulose sodium, 0.9% [w/v] sodium chloride, 0.4% [v/v] polysorbate 80, and 0.9% [v/v] benzyl alcohol in deionized water). Control mice received vehicle instead. The animals were exposed to chronic hypoxia (10% O2) in a ventilated chamber for 3 weeks (70). At the end of the treatment period, mice were anesthetized, and hemodynamic changes were assessed.
MCT-induced pulmonary hypertension model in rats.
Eight-week-old male Sprague-Dawley rats were injected once s.c. with or without MCT (60 mg/kg). At the beginning of the third week, MCT-injected rats received L-798106 (200 mg/kg) or vehicle twice a day.
Statistics.
All data are expressed as the mean ± SEM. Data were analyzed using GraphPad Prism software, version 5.0 (GraphPad Software). The 2-tailed unpaired Student’s t test was performed to compare 2 data sets. Multiple comparisons were tested with ANOVA followed by Bonferroni’s post test. A P value of less than 0.05 was considered statistically significant.
Study approval.
All animals were maintained and handled in accordance with the guidelines approved by the IACUC of the Institute for Nutritional Sciences, Chinese Academy of Sciences. Human lung sections (~1 × 1 × 1 cm) were obtained from patients undergoing lobectomy or pneumonectomy for bronchial carcinoma. Lung tissue distant from the tumor was selected to isolate distal PAs. All the lungs were judged by the pathologist to have no vascular pathological changes under microscopy. PA sections were cut and incubated in DMEM under normoxic or hypoxic conditions for 24 hours, then subjected to RNA extraction. Written informed consent was obtained from all subjects prior to their participation in this study. Approval for these studies was obtained from the ethics committee of Ruijin Hospital, which is affiliated with Shanghai Jiaotong University.
Supplementary Material
Acknowledgments
This work was supported by the Ministry of Science and Technology of China (2012CB945100 and 2011CB503906); the National Natural Science Foundation of China (91439204 and 31371154); a Natural Science Foundation of China (NSFC)/Canadian Institutes of Health Research (CIHR) joint grant (NSFC81161120538 and CIHR-CCI117951); the Science and Technology Commission of Shanghai Municipality (14JC1407400 and 13ZR1425700); and by the One Hundred Talents Program of the Chinese Academy of Sciences (2010OHTP10). Y. Yu is a Fellow at the Jiangsu Collaborative Innovation Center for Cardiovascular Disease Translational Medicine. R.M. Breyer is a recipient of a Merit Award (1BX000616) from the Department of Veterans Affairs. C.D. Funk holds a Canada Research Chair and is a recipient of an Ontario Heart and Stroke Foundation Career Investigator award.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information:J Clin Invest. 2015;125(3):1228–1242. doi:10.1172/JCI77656.
References
- 1.Benza RL, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation. 2010;122(2):164–172. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 2.Shimoda LA, Laurie SS. Vascular remodeling in pulmonary hypertension. J Mol Med (Berl). 2013;91(3):297–309. doi: 10.1007/s00109-013-0998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lourenco AP, Fontoura D, Henriques-Coelho T, Leite-Moreira AF. Current pathophysiological concepts and management of pulmonary hypertension. Int J Cardiol. 2012;155(3):350–361. doi: 10.1016/j.ijcard.2011.05.066. [DOI] [PubMed] [Google Scholar]
- 4.Cathcart MC, et al. Cyclooxygenase-2-linked attenuation of hypoxia-induced pulmonary hypertension and intravascular thrombosis. J Pharmacol Exp Ther. 2008;326(1):51–58. doi: 10.1124/jpet.107.134221. [DOI] [PubMed] [Google Scholar]
- 5.Loukanov T, Jaschinski C, Kirilov M, Klimpel H, Karck M, Gorenflo M. Cyclooxygenase-2 expression in lung in patients with congenital heart malformations and pulmonary arterial hypertension. Thorac Cardiovasc Surg. 2013;61(4):307–311. doi: 10.1055/s-0033-1337446. [DOI] [PubMed] [Google Scholar]
- 6.Seta F, Rahmani M, Turner PV, Funk CD. Pulmonary oxidative stress is increased in cyclooxygenase-2 knockdown mice with mild pulmonary hypertension induced by monocrotaline. PLoS One. 2011;6(8):e23439. doi: 10.1371/journal.pone.0023439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fredenburgh LE, et al. Absence of cyclooxygenase-2 exacerbates hypoxia-induced pulmonary hypertension and enhances contractility of vascular smooth muscle cells. Circulation. 2008;117(16):2114–2122. doi: 10.1161/CIRCULATIONAHA.107.716241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKenzie C, MacDonald A, Shaw AM. Mechanisms of U46619-induced contraction of rat pulmonary arteries in the presence and absence of the endothelium. Br J Pharmacol. 2009;157(4):581–596. doi: 10.1111/j.1476-5381.2008.00084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kozlowska H, et al. EP(3) receptor-mediated contraction of human pulmonary arteries and inhibition of neurogenic tachycardia in pithed rats. Pharmacol Rep. 2012;64(6):1526–1536. doi: 10.1016/S1734-1140(12)70950-7. [DOI] [PubMed] [Google Scholar]
- 10.Qian YM, Jones RL, Chan KM, Stock AI, Ho JK. Potent contractile actions of prostanoid EP3-receptor agonists on human isolated pulmonary artery. Br J Pharmacol. 1994;113(2):369–374. doi: 10.1111/j.1476-5381.1994.tb16997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jourdan KB, Evans TW, Curzen NP, Mitchell JA. Evidence for a dilator function of 8-iso prostaglandin F2 α in rat pulmonary artery. Br J Pharmacol. 1997;120(7):1280–1285. doi: 10.1038/sj.bjp.0701052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olschewski H, et al. Prostacyclin and its analogues in the treatment of pulmonary hypertension. Pharmacol Ther. 2004;102(2):139–153. doi: 10.1016/j.pharmthera.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Yang J, et al. Smad-dependent and smad-independent induction of id1 by prostacyclin analogues inhibits proliferation of pulmonary artery smooth muscle cells in vitro and in vivo. Circ Res. 2010;107(2):252–262. doi: 10.1161/CIRCRESAHA.109.209940. [DOI] [PubMed] [Google Scholar]
- 14.Christman BW, et al. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N Engl J Med. 1992;327(2):70–75. doi: 10.1056/NEJM199207093270202. [DOI] [PubMed] [Google Scholar]
- 15.Tuder RM, et al. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med. 1999;159(6):1925–1932. doi: 10.1164/ajrccm.159.6.9804054. [DOI] [PubMed] [Google Scholar]
- 16.Delannoy E, Courtois A, Freund-Michel V, Leblais V, Marthan R, Muller B. Hypoxia-induced hyperreactivity of pulmonary arteries: role of cyclooxygenase-2, isoprostanes, and thromboxane receptors. Cardiovasc Res. 2010;85(3):582–592. doi: 10.1093/cvr/cvp292. [DOI] [PubMed] [Google Scholar]
- 17.Hoshikawa Y, et al. Prostacyclin receptor-dependent modulation of pulmonary vascular remodeling. Am J Respir Crit Care Med. 2001;164(2):314–318. doi: 10.1164/ajrccm.164.2.2010150. [DOI] [PubMed] [Google Scholar]
- 18.Geraci M, et al. Pulmonary prostacyclin synthase overexpression by adenovirus transfection and in transgenic mice. Chest. 1998;114(1):99S. doi: 10.1378/chest.114.1_supplement.99s. [DOI] [PubMed] [Google Scholar]
- 19.Geraci MW, et al. Pulmonary prostacyclin synthase overexpression in transgenic mice protects against development of hypoxic pulmonary hypertension. J Clin Invest. 1999;103(11):1509–1515. doi: 10.1172/JCI5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mubarak KK. A review of prostaglandin analogs in the management of patients with pulmonary arterial hypertension. Respir Med. 2010;104(1):9–21. doi: 10.1016/j.rmed.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Tam FS, Chan K, Borreau JP, Jones RL. The mechanisms of enhancement and inhibition of field stimulation responses of guinea-pig vas deferens by prostacyclin analogues. Br J Pharmacol. 1997;121(7):1413–1421. doi: 10.1038/sj.bjp.0701275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiriyama M, Ushikubi F, Kobayashi T, Hirata M, Sugimoto Y, Narumiya S. Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Br J Pharmacol. 1997;122(2):217–224. doi: 10.1038/sj.bjp.0701367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79(4):1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 24.Orie NN, Clapp LH. Role of prostanoid IP and EP receptors in mediating vasorelaxant responses to PGI2 analogues in rat tail artery: Evidence for Gi/o modulation via EP3 receptors. Eur J Pharmacol. 2011;654(3):258–265. doi: 10.1016/j.ejphar.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Abramovitz M, et al. The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim Biophys Acta. 2000;1483(2):285–293. doi: 10.1016/S1388-1981(99)00164-X. [DOI] [PubMed] [Google Scholar]
- 26.Whittle BJ, Silverstein AM, Mottola DM, Clapp LH. Binding and activity of the prostacyclin receptor (IP) agonists, treprostinil and iloprost, at human prostanoid receptors: treprostinil is a potent DP1 and EP2 agonist. Biochem Pharmacol. 2012;84(1):68–75. doi: 10.1016/j.bcp.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 27.Kuwano K, Hashino A, Noda K, Kosugi K, Kuwabara K. A long-acting and highly selective prostacyclin receptor agonist prodrug, 2-{4-[(5,6-diphenylpyrazin-2-yl)(isopropyl)amino]butoxy}-N-(methylsulfonyl)acetam ide (NS-304), ameliorates rat pulmonary hypertension with unique relaxant responses of its active form, {4-[(5,6-diphenylpyrazin-2-yl)(isopropyl)amino]butoxy}acetic acid (MRE-269), on rat pulmonary artery. J Pharmacol Exp Ther. 2008;326(3):691–699. doi: 10.1124/jpet.108.138305. [DOI] [PubMed] [Google Scholar]
- 28.Morrison K, Studer R, Ernst R, Haag F, Kauser K, Clozel M. Differential effects of Selexipag [corrected] and prostacyclin analogs in rat pulmonary artery. J Pharmacol Exp Ther. 2012;343(3):547–555. doi: 10.1124/jpet.112.197152. [DOI] [PubMed] [Google Scholar]
- 29.Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol. 2001;41:661–690. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- 30.Kotelevets L, Foudi N, Louedec L, Couvelard A, Chastre E, Norel X. A new mRNA splice variant coding for the human EP3-I receptor isoform. Prostaglandins Leukot Essent Fatty Acids. 2007;77(3):195–201. doi: 10.1016/j.plefa.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, et al. Cyclooxygenase-2-derived prostaglandin E2 promotes injury-induced vascular neointimal hyperplasia through the E-prostanoid 3 receptor. Circ Res. 2013;113(2):104–114. doi: 10.1161/CIRCRESAHA.113.301033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones RL, Woodward DF, Wang JW, Clark RL. Roles of affinity and lipophilicity in the slow kinetics of prostanoid receptor antagonists on isolated smooth muscle preparations. Br J Pharmacol. 2011;162(4):863–879. doi: 10.1111/j.1476-5381.2010.01087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest. 2012;122(12):4306–4313. doi: 10.1172/JCI60658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Humbert M, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43(12):13S–24S. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 35.Wu X, Chang MS, Mitsialis SA, Kourembanas S. Hypoxia regulates bone morphogenetic protein signaling through C-terminal-binding protein 1. Circ Res. 2006;99(3):240–247. doi: 10.1161/01.RES.0000237021.65103.24. [DOI] [PubMed] [Google Scholar]
- 36.Ma W, et al. Calpain mediates pulmonary vascular remodeling in rodent models of pulmonary hypertension, and its inhibition attenuates pathologic features of disease. J Clin Invest. 2011;121(11):4548–4566. doi: 10.1172/JCI57734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stetler-Stevenson WG, Aznavoorian S, Liotta LA. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993;9:541–573. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- 38.Wipff PJ, Hinz B. Integrins and the activation of latent transforming growth factor beta1 — an intimate relationship. Eur J Cell Biol. 2008;87(8):601–615. doi: 10.1016/j.ejcb.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 39.Leask A, Abraham DJ. TGF-β signaling and the fibrotic response. FASEB J. 2004;18(7):816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 40.Lee SH, Dominguez R. Regulation of actin cytoskeleton dynamics in cells. Mol Cells. 2010;29(4):311–325. doi: 10.1007/s10059-010-0053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mori H, et al. CD44 directs membrane-type 1 matrix metalloproteinase to lamellipodia by associating with its hemopexin-like domain. EMBO J. 2002;21(15):3949–3959. doi: 10.1093/emboj/cdf411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clark ES, Whigham AS, Yarbrough WG, Weaver AM. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res. 2007;67(9):4227–4235. doi: 10.1158/0008-5472.CAN-06-3928. [DOI] [PubMed] [Google Scholar]
- 43.Schram K, Ganguly R, No EK, Fang X, Thong FS, Sweeney G. Regulation of MT1-MMP and MMP-2 by leptin in cardiac fibroblasts involves Rho/ROCK-dependent actin cytoskeletal reorganization and leads to enhanced cell migration. Endocrinology. 2011;152(5):2037–2047. doi: 10.1210/en.2010-1166. [DOI] [PubMed] [Google Scholar]
- 44.Hatae N, Sugimoto Y, Ichikawa A. Prostaglandin receptors: advances in the study of EP3 receptor signaling. J Biochem. 2002;131(6):781–784. doi: 10.1093/oxfordjournals.jbchem.a003165. [DOI] [PubMed] [Google Scholar]
- 45.Macias-Perez IM, Zent R, Carmosino M, Breyer MD, Breyer RM, Pozzi A. Mouse EP3 α, β, and γ receptor variants reduce tumor cell proliferation and tumorigenesis in vivo. J Biol Chem. 2008;283(18):12538–12545. doi: 10.1074/jbc.M800105200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31(5):986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan G, et al. Regulation of rat intrapulmonary arterial tone by arachidonic acid and prostaglandin E2 during hypoxia. PLoS One. 2013;8(8):e73839. doi: 10.1371/journal.pone.0073839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ichinose F, et al. Cytosolic phospholipase A(2) in hypoxic pulmonary vasoconstriction. J Clin Invest. 2002;109(11):1493–1500. doi: 10.1172/JCI14294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lai YJ, et al. Role of the prostanoid EP4 receptor in iloprost-mediated vasodilatation in pulmonary hypertension. Am J Respir Crit Care Med. 2008;178(2):188–196. doi: 10.1164/rccm.200710-1519OC. [DOI] [PubMed] [Google Scholar]
- 50.Cracowski JL, et al. Increased lipid peroxidation in patients with pulmonary hypertension. Am J Respir Crit Care Med. 2001;164(6):1038–1042. doi: 10.1164/ajrccm.164.6.2104033. [DOI] [PubMed] [Google Scholar]
- 51.Cracowski JL, et al. Independent association of urinary F2-isoprostanes with survival in pulmonary arterial hypertension. Chest. 2012;142(4):869–876. doi: 10.1378/chest.11-1267. [DOI] [PubMed] [Google Scholar]
- 52.Botney MD, Bahadori L, Gold LI. Vascular remodeling in primary pulmonary hypertension. Potential role for transforming growth factor-β. Am J Pathol. 1994;144(2):286–295. [PMC free article] [PubMed] [Google Scholar]
- 53.Arcot SS, Lipke DW, Gillespie MN, Olson JW. Alterations of growth factor transcripts in rat lungs during development of monocrotaline-induced pulmonary hypertension. Biochem Pharmacol. 1993;46(6):1086–1091. doi: 10.1016/0006-2952(93)90675-M. [DOI] [PubMed] [Google Scholar]
- 54.Perkett EA, Lyons RM, Moses HL, Brigham KL, Meyrick B. Transforming growth factor-β activity in sheep lung lymph during the development of pulmonary hypertension. J Clin Invest. 1990;86(5):1459–1464. doi: 10.1172/JCI114862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Long L, et al. Altered bone morphogenetic protein and transforming growth factor-β signaling in rat models of pulmonary hypertension: potential for activin receptor-like kinase-5 inhibition in prevention and progression of disease. Circulation. 2009;119(4):566–576. doi: 10.1161/CIRCULATIONAHA.108.821504. [DOI] [PubMed] [Google Scholar]
- 56.Irie A, Sugimoto Y, Namba T, Asano T, Ichikawa A, Negishi M. The C-terminus of the prostaglandin-E-receptor EP3 subtype is essential for activation of GTP-binding protein. Eur J Biochem. 1994;224(1):161–166. doi: 10.1111/j.1432-1033.1994.tb20007.x. [DOI] [PubMed] [Google Scholar]
- 57.Hasegawa H, Katoh H, Yamaguchi Y, Nakamura K, Futakawa S, Negishi M. Different membrane targeting of prostaglandin EP3 receptor isoforms dependent on their carboxy-terminal tail structures. FEBS Lett. 2000;473(1):76–80. doi: 10.1016/S0014-5793(00)01508-8. [DOI] [PubMed] [Google Scholar]
- 58.Rifkin DB. Latent transforming growth factor-β (TGF-β) binding proteins: orchestrators of TGF-β availability. J Biol Chem. 2005;280(9):7409–7412. doi: 10.1074/jbc.R400029200. [DOI] [PubMed] [Google Scholar]
- 59.Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1(3):169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 60.Kozasa T, Hajicek N, Chow CR, Suzuki N. Signalling mechanisms of RhoGTPase regulation by the heterotrimeric G proteins G12 and G13. J Biochem. 2011;150(4):357–369. doi: 10.1093/jb/mvr105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang M, et al. Matrix metalloproteinase 2 activation of transforming growth factor-β1 (TGF-β1) and TGF-β1-type II receptor signaling within the aged arterial wall. Arterioscler Thromb Vasc Biol. 2006;26(7):1503–1509. doi: 10.1161/01.ATV.0000225777.58488.f2. [DOI] [PubMed] [Google Scholar]
- 62.Kobayashi K, Murata T, Hori M, Ozaki H. Prostaglandin E2-prostanoid EP3 signal induces vascular contraction via nPKC and ROCK activation in rat mesenteric artery. Eur J Pharmacol. 2011;660(2):375–380. doi: 10.1016/j.ejphar.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 63.Thumkeo D, Watanabe S, Narumiya S. Physiological roles of Rho and Rho effectors in mammals. Eur J Cell Biol. 2013;92(10):303–315. doi: 10.1016/j.ejcb.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 64.Schram K, Wong MM, Palanivel R, No EK, Dixon IM, Sweeney G. Increased expression and cell surface localization of MT1-MMP plays a role in stimulation of MMP-2 activity by leptin in neonatal rat cardiac myofibroblasts. J Mol Cell Cardiol. 2008;44(5):874–881. doi: 10.1016/j.yjmcc.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 65.Abe K, et al. Long-term treatment with a Rho-kinase inhibitor improves monocrotaline-induced fatal pulmonary hypertension in rats. Circ Res. 2004;94(3):385–393. doi: 10.1161/01.RES.0000111804.34509.94. [DOI] [PubMed] [Google Scholar]
- 66.Oka M, et al. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res. 2007;100(6):923–929. doi: 10.1161/01.RES.0000261658.12024.18. [DOI] [PubMed] [Google Scholar]
- 67.Abe K, et al. Long-term inhibition of Rho-kinase ameliorates hypoxia-induced pulmonary hypertension in mice. J Cardiovasc Pharmacol. 2006;48(6):280–285. doi: 10.1097/01.fjc.0000248244.64430.4a. [DOI] [PubMed] [Google Scholar]
- 68.Fukumoto Y, et al. Double-blind, placebo-controlled clinical trial with a rho-kinase inhibitor in pulmonary arterial hypertension. Circ J. 2013;77(10):2619–2625. doi: 10.1253/circj.CJ-13-0443. [DOI] [PubMed] [Google Scholar]
- 69.Lazarus M, et al. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat Neurosci. 2007;10(9):1131–1133. doi: 10.1038/nn1949. [DOI] [PubMed] [Google Scholar]
- 70.Wang Z, Lakes RS, Golob M, Eickhoff JC, Chesler NC. Changes in large pulmonary arterial viscoelasticity in chronic pulmonary hypertension. PLoS One. 2013;8(11):e78569. doi: 10.1371/journal.pone.0078569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.