Abstract
Background:
In most of the studies, the association of vascular events is limited to migraine with aura or it is stronger in this group, whereas the link between migraine without aura (MO) and vascular events remained uncertain. Therefore, we decided to evaluate endothelial function by chemical and functional markers of endothelium in MO and compare with normal population.
Methods:
In this study, 39 patients and 25 healthy subjects were enrolled and flow-mediated dilatation (FMD), C-reactive protein (CRP), nitrite and nitrate were measured in these two groups.
Results:
The mean of FMD in healthy people was higher than the migraine patients (mean difference − 7.67%; 95% confidence interval [CI] −9.90-−5.44). The means of nitrite concentration in migraineurs was significantly lower than healthy subjects (mean difference − 2.0 μmol/L; 95% CI − 3.45-−0.54). But the CRP concentrations in both groups were not significantly different (mean difference 0.42 pmol/L; 95% CI − 0.13-0.98).
Conclusions:
This study can show the endothelial dysfunction in migraineurs without aura and suggest that MO could also be a risk for cardiovascular disease.
Keywords: C-reactive protein, endothelial dysfunction, flow-mediated dilatation, migraine without aura, nitric oxide
INTRODUCTION
Migraine especially migraine with aura (MA), is associated with an increased risk for several vascular disorders, such as ischemic stroke, myocardial infarction, and death due to ischemia, as well as with coronary revascularization and angina.[1,2] This can clear the importance of vascular mechanism of migraine. In this theory, the endothelium has some kinds of injury that is called as endothelial dysfunction (ED).[3] ED is a clinical syndrome that is described by impaired reactivity of the vasculature.[4] Evidences suggest that the migraine attack is associated with ED, and attacks with and without aura may have a different effect on the endothelium.[5]
In most studies, the association of vascular events is limited to MA or it is stronger in this group while the link between migraine without aura (MO) and vascular events remained uncertain and so the possible role of ED in patients affected by MO was not explored clearly so far.
Several functional and chemical markers such as flow-mediated dilatation (FMD), nitrite and nitrate (NOx) and C-reactive protein (CRP) are used to evaluate endothelial function.
Flow-mediated dilation is a physiological phenomenon characterized by vessel dilatation in reaction to an increase in the shear stress. A key mediator of FMD is endothelium-derived nitric oxide.[6,7,8,9]
The purpose of this study was to compare the endothelial function by means of FMD, as a functional marker, and also NOx and CRP, as chemical markers of endothelium, in migraineurs without aura and healthy people.
METHODS
The Ethics Committee of Isfahan University of Medical Sciences’ approved the study protocol, and all subjects gave informed approval before study inclusion.
Thirty-nine patients who fulfilled the diagnostic criteria of MO according to International Headache Society (2nd edition)[10] were enrolled in this study between July 2008 and October 2010 mainly by office referral. Twenty-five age-and gender-matched healthy subjects according to medical history, physical examination, and routine laboratory tests without history of migraine were also enrolled as a control group.
Subjects who had hypertension, diabetes mellitus, coronary artery disease, infectious disease, known liver or kidney disorders, hypercholesterolemia, hypertriglyceridemia, gynecologic disorders (such as polymenorrhea, cystic ovary disease), morbid obesity (body mass index >35), current cigarette smoking, abuse of alcohol or other substances, anemia, sinusitis, tension type headache <5 days/month were not included in this study and this rule was performed for all migraineurs and control subjects. Furthermore, all subjects had not used hormonal contraceptives or vasoactive drugs since 3 months before beginning of this study.
At beginning, subjects underwent a complete examination that included: Physical examination, blood sampling for routine biochemistry measurements, as well as NOx, CRP and forearm FMD after at least 8 h fasting.
A volume of 5 ml blood samples were taken from the antecubital vein in the supine position. Sera were separated by centrifugation at 4000 rpm for 10 min and then were aliquoted and stored at –70°C until analysis.
Flow-mediated dilation
A high-resolution B-mode ultrasonographic system (ATL Ultrasound, HDI 5000, Bothell, Washington, USA) with a linear transducer mid-frequency of 7.5 MHz was used to determine FMD of the brachial artery. An experienced ultrasonographer blinded to case and control groups performed all FMDs. At first; subjects lied at rest for 10 min. Then baseline brachial artery diameter was determined by locating probe on 4-5 cm above the antecubital fossa of the nondominant arm. After that, a pneumatic tourniquet of a sphygmomanometer was inflated on the most proximal portion of the forearm to a pressure of 300 mmHg for 5 min. The cuff was then released, and second scan was taken 30 s before and 90 s after cuff deflation and average of them was measured. Artery diameters were determined with ultrasonic calipers from the leading edge of the anterior wall to the leading edge of posterior wall of the brachial artery at the end of diastolic period. Three other observers supervised the procedures. Changes in diameter were computed as a percentage relative to the baseline diameter. None of the subjects was experienced headache during measurements. Also, FMD was not measured during the menstrual phase in female patients. All the FMDs were performed between 10 a.m. and 12 a.m. The interobserver correlation coefficient of the FMD was 87% while the intraobserver correlation coefficient was 89%.
Nitric oxide assay
The serum level of stable nitric oxide (NO) metabolite NOx were measured using a colorimetric assay kit (R and D Systems, Minneapolis, USA) based on Griess reaction. For nitrite measurement, briefly, after serum addition into wells, sulfanilamide solution was added to all experimental samples, and after incubation, the N-1-naphtylethylenediamine dihydrochloride solution was added. Then, absorbance was measured by a μreader in 540 nm wavelength. For nitrate measurement, the enzymatic conversion of nitrate to nitrite by nitrate reductase was done. The reaction was followed by colorimetric detection of nitrite as an azo dye product of the Griess reaction. This assay measures total nitrite by converting nitrate to nitrite. To determine the nitrate concentration, the endogenous nitrite concentration measured from the nitrite assay was subtracted from the nitrite concentration measured in this procedure. The sensitivity of the assay was 0.25 μmol/L.
C-reactive protein assay
The plasma levels CRP were determined by high sensitive ELISA kit according to manufactures’ instruction (IBL, Hamburg, Germany). The sensitivity of the assay was <1 μg/ml. All investigators were blind to all clinical data.
Statistical analysis
The results are expressed as mean ± standard deviation. A normal distribution was proven with the test of Kolmogorov–Smirnov. Differences in FMD, serum NOx and CRP concentrations between case and control groups were analyzed using the independent-sample t-test. P =0.05 was considered as significant. Statistical analyses were performed with SPSS 16 software by a blind analyzer about details of research.
RESULTS
The demographic information
Thirty-nine migraineurs without aura were enrolled in this study as the case group, between July 2008 and October 2010. These people have suffered from migraine headaches for about 68.25 ± 8.89 months. The mean of their headache severity (scale 1-10) was 8.62 ± 0.31. They experienced 10.10 ± 1.41 times migraine attacks/months in average. The mean of their headache duration was 13 ± 2.03 h and also the family history of migraine in first degree in 41.7% of them was positive.
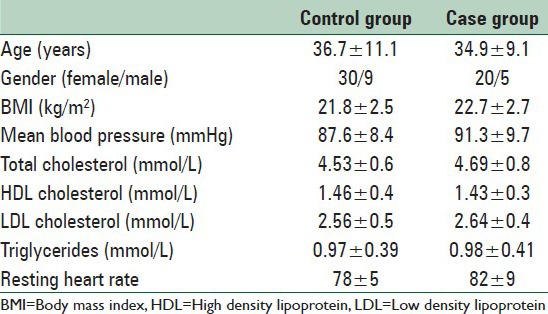
Furthermore, 25 age and sex matched healthy subjects were recruited as the control group. The demographic information of these two groups is summarized in Table 1.
Table 1.
The general patients characteristics
Endothelial function markers
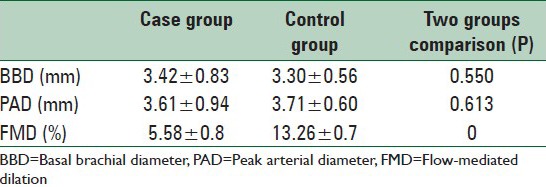
Flow-mediated dilation parameters of the case and control groups have been demonstrated in Table 2. As illustrated in this table, the mean of FMD in control group (healthy people) was dramatically higher than the migraine patients (P = 0.00).
Table 2.
Comparison of the FMD parameters between case and control groups through the study
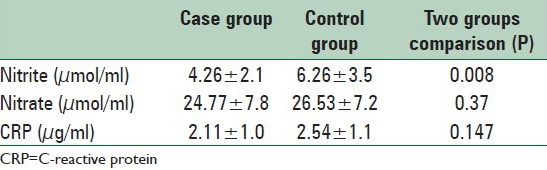
As summarized in Table 3, the nitrite concentrations (the main end metabolite of NO) in the control group was significantly higher than the migraineurs (P = 0.004), while the CRP concentrations in both case and control groups were not significantly different (P = 0.146).
Table 3.
Comparison of the nitrite and CRP concentrations between case and control groups through the study
DISCUSSION
The aim of this study was to consider the ED in MO because this association is not clearly defined in previous studies.
The main finding of the present study is that patients affected by MO show a defective FMD compared to controls as well as decreased nitrite concentration.
The ability of self-regulation of the tone and adjusting the blood flow in reaction to changes in the local environment, illustrates the capacity of blood vessels to respond to physical and chemical stimuli in the lumen. Dilation is the main response of many blood vessels to increasing of flow, or more accurately shear stress. FMD is designated according to this phenomenon. A principal mediator of FMD is endothelium-derived NO.[6,7,8,9]
Previous studies have already investigated the endothelial function and NO vascular response describing either a normal[11,12,13] or a reduced FMD[2,14] in migraine patients respect to controls. However, two studies evaluated only patients affected by MO[2,13] and two studies did not distinguish between MA and MO patients in their analytic approach.[11,14] In one study Vernieri et al. demonstrated that patients affected by MA showed a higher FMD than controls and MO patients reflecting a possible arterial hyper-reactivity to chemical stimuli in MA patients.[15]
Silva et al.[12] measured also blood levels of fasting nitrates and nitrites in controls and patients affected by MA or MO during the asymptomatic period, finding no differences in the three groups.
Evidence of ED in migraine is increasing. It has been shown that level of von Willebrand factor (VWF), a reliable ED biomarker, was significantly higher in migraineurs than in nonheadache controls during the interictal phase.[16,17]
The association of migraine attacks with changes in endothelial biomarkers suggests that migraine attacks may be the cause of the ED. It has been suggested that recurrent migraine attacks lead to inflammation, and hypoxia, and ED. In clinical studies, markers of oxidative stress were higher during migraine attacks.[18] Other studies in patient with MO have been demonstrated that VWF[19] soluble intercellular adhesion molecule[20] levels were also significantly higher than healthy control subjects.
In addition, migraine is associated with reductions in the number and function of endothelial progenitor cells, serving as a marker for dysfunctional endothelium. ED may explain the increased risk of cardiovascular disease and stroke in this patient group.[21]
In a young, relatively healthy cohort of women, a strong relation between biomarkers of endothelial activation and migraine, including VWF activity, high density-CRP, t-PA antigen, and total nitrite/nitrate concentration has been reported.[22] Identification of these alterations in young migraine subjects with a short history of migraine suggests that these alterations are not the result of repeated migraine attacks and may be related to the pathophysiology of migraine.
There is a paucity of studies demonstrating the association of migraine with inflammatory markers. A recently large case–control study has revealed no relationship between migraine and CRP. Indeed, in this study, CRP levels were not increased among migraine sufferers compared with nonmigraineurs;[23] however in a big cross-sectional study of middle-aged women with and without aura, Kurth et al. reported very modest associations between migraine and CRP.[24]
In addition, in our previous study[25] we showed that enalapril improves endothelial function in patients with migraine and therefor it can support the theory of ED in migraineurs without aura.
Another noteworthy point is the relationship between MO and vascular disorders. According to some previous studies there is a relationship between migraine (with or without aura) and vascular events. For example it has been shown that male migraineurs have a significantly increased risk of MI, with a hazard ratio of 1.42 (95% confidence interval, 1.15-1.77; P < 0.001).[26] Also, many studies especially a meta-analysis of data pooled from 11 case–control studies and three cohort studies have shown a relationship between migraine and an increased risk of stroke.
In a meta-analysis Etminan et al. mentioned a twofold risk in both subtypes of migraine.[27] Identifying the populations of migraineurs at a higher risk for vascular events may provide preventive strategies to reduce the risk of ischemic stroke and other vascular events in these individuals.
The limitations of the present study are the small sample size. Furthermore, the endothelium-independent vasodilation was not evaluated in this study.
CONCLUSIONS
This study can show the endothelial dysfunction in migraineurs without aura and suggest that MO could also be a risk for cardiovascular disease.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Yilmaz G, Sürer H, Inan LE, Coskun O, Yücel D. Increased nitrosative and oxidative stress in platelets of migraine patients. Tohoku J Exp Med. 2007;211:23–30. doi: 10.1620/tjem.211.23. [DOI] [PubMed] [Google Scholar]
- 2.Yetkin E, Ozisik H, Ozcan C, Aksoy Y, Turhan H. Decreased endothelium-dependent vasodilatation in patients with migraine: A new aspect to vascular pathophysiology of migraine. Coron Artery Dis. 2006;17:29–33. doi: 10.1097/00019501-200602000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Appenzeller O. Pathogenesis of migraine. Med Clin North Am. 1991;75:763–89. doi: 10.1016/s0025-7125(16)30448-5. [DOI] [PubMed] [Google Scholar]
- 4.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: A marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–75. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 5.Tietjen GE. Migraine as a systemic vasculopathy. Cephalalgia. 2009;29:987–96. doi: 10.1111/j.1468-2982.2009.01937.x. [DOI] [PubMed] [Google Scholar]
- 6.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: Implications for the assessment of endothelial function. J Physiol. 2005;568:357–69. doi: 10.1113/jphysiol.2005.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson EA, Mark AL. Flow-mediated and reflex changes in large peripheral artery tone in humans. Circulation. 1989;79:93–100. doi: 10.1161/01.cir.79.1.93. [DOI] [PubMed] [Google Scholar]
- 8.Laurent S, Lacolley P, Brunel P, Laloux B, Pannier B, Safar M. Flow-dependent vasodilation of brachial artery in essential hypertension. Am J Physiol. 1990;258:H1004–11. doi: 10.1152/ajpheart.1990.258.4.H1004. [DOI] [PubMed] [Google Scholar]
- 9.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–5. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 10.Headache Classification Subcommittee of the International Headache Society. The international classification of headache disorders. Cephalalgia. (2nd edition) 2004;24(Suppl 1):8–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 11.de Hoon JN, Smits P, Troost J, Struijker-Boudier HA, Van Bortel LM. Forearm vascular response to nitric oxide and calcitonin gene-related peptide: Comparison between migraine patients and control subjects. Cephalalgia. 2006;26:56–63. doi: 10.1111/j.1468-2982.2005.00993.x. [DOI] [PubMed] [Google Scholar]
- 12.Silva FA, Rueda-Clausen CF, Silva SY, Zarruk JG, Guzmán JC, Morillo CA, et al. Endothelial function in patients with migraine during the interictal period. Headache. 2007;47:45–51. doi: 10.1111/j.1526-4610.2006.00532.x. [DOI] [PubMed] [Google Scholar]
- 13.Thomsen LL, Daugaard D, Iversen H, Olesen J. Normal radial artery dilatation during reactive hyperaemia in migraine without aura. Endothelium. 1996;4:199–206. [Google Scholar]
- 14.Vanmolkot FH, Van Bortel LM, de Hoon JN. Altered arterial function in migraine of recent onset. Neurology. 2007;68:1563–70. doi: 10.1212/01.wnl.0000260964.28393.ed. [DOI] [PubMed] [Google Scholar]
- 15.Vernieri F, Moro L, Altamura C, Palazzo P, Antonelli Incalzi R, Rossini PM, et al. Patients with migraine with aura have increased flow mediated dilation. BMC Neurol. 2010;10:18. doi: 10.1186/1471-2377-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tietjen GE, Al-Qasmi MM, Athanas K, Dafer RM, Khuder SA. Increased von Willebrand factor in migraine. Neurology. 2001;57:334–6. doi: 10.1212/wnl.57.2.334. [DOI] [PubMed] [Google Scholar]
- 17.Tietjen GE, Al-Qasmi MM, Athanas K, Utley C, Herial NA. Altered hemostasis in migraineurs studied with a dynamic flow system. Thromb Res. 2007;119:217–22. doi: 10.1016/j.thromres.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 18.Tuncel D, Tolun FI, Gokce M, Imrek S, Ekerbiçer H. Oxidative stress in migraine with and without aura. Biol Trace Elem Res. 2008;126:92–7. doi: 10.1007/s12011-008-8193-9. [DOI] [PubMed] [Google Scholar]
- 19.Cesar JM, García-Avello A, Vecino AM, Sastre JL, Alvarez-Cermeño JC. Increased levels of plasma von Willebrand factor in migraine crisis. Acta Neurol Scand. 1995;91:412–3. doi: 10.1111/j.1600-0404.1995.tb07030.x. [DOI] [PubMed] [Google Scholar]
- 20.Sarchielli P, Alberti A, Baldi A, Coppola F, Rossi C, Pierguidi L, et al. Proinflammatory cytokines, adhesion molecules, and lymphocyte integrin expression in the internal jugular blood of migraine patients without aura assessed ictally. Headache. 2006;46:200–7. doi: 10.1111/j.1526-4610.2006.00337.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee ST, Chu K, Jung KH, Kim DH, Kim EH, Choe VN, et al. Decreased number and function of endothelial progenitor cells in patients with migraine. Neurology. 2008;70:1510–7. doi: 10.1212/01.wnl.0000294329.93565.94. [DOI] [PubMed] [Google Scholar]
- 22.Tietjen GE, Herial NA, White L, Utley C, Kosmyna JM, Khuder SA. Migraine and biomarkers of endothelial activation in young women. Stroke. 2009;40:2977–82. doi: 10.1161/STROKEAHA.109.547901. [DOI] [PubMed] [Google Scholar]
- 23.Gudmundsson LS, Aspelund T, Scher AI, Thorgeirsson G, Johannsson M, Launer LJ, et al. C-reactive protein in migraine sufferers similar to that of non-migraineurs: The Reykjavik Study. Cephalalgia. 2009;29:1301–10. doi: 10.1111/j.1468-2982.2009.01865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurth T, Ridker PM, Buring JE. Migraine and biomarkers of cardiovascular disease in women. Cephalalgia. 2008;28:49–56. doi: 10.1111/j.1468-2982.2007.01467.x. [DOI] [PubMed] [Google Scholar]
- 25.Javanmard SH, Sonbolestan SA, Heshmat-Ghahdarijani K, Saadatnia M, Sonbolestan SA. Enalapril improves endothelial function in patients with migraine: A randomized, double-blind, placebo-controlled trial. J Res Med Sci. 2011;16:26–32. [PMC free article] [PubMed] [Google Scholar]
- 26.Kurth T, Gaziano JM, Cook NR, Bubes V, Logroscino G, Diener HC, et al. Migraine and risk of cardiovascular disease in men. Arch Intern Med. 2007;167:795–801. doi: 10.1001/archinte.167.8.795. [DOI] [PubMed] [Google Scholar]
- 27.Etminan M, Takkouche B, Isorna FC, Samii A. Risk of ischaemic stroke in people with migraine: Systematic review and meta-analysis of observational studies. BMJ. 2005;330:63. doi: 10.1136/bmj.38302.504063.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]


