Abstract
Background:
Irrational antibiotic prescribing as a global health problem has a major influence on medical care quality and healthcare expenditure. This study was aimed to determine the pattern of antibiotic use and to assess the seasonality and physician-related factors associated with variability in antibiotic prescribing in Isfahan province of Iran.
Methods:
This cross-sectional survey was conducted on all prescriptions issued by general physicians from rural and urban areas in 2011. Associations between season of prescribing and physician-related variables including gender, practice location and time since graduation with antibiotic prescriptions and also the pattern of antibiotic prescribing were assessed using Chi-square tests and multiple logistic regression models.
Results:
Of the 7439709 prescriptions issued by 3772 general practitioners, 51% contained at least one antibiotic. Penicillins were the most frequently prescribed antibiotics, followed by cephalosporins and macrolides. Over-prescription of penicillins was associated with female gender (odds ratio [OR], 2.61; 95% confidence interval [CI] 2.13–3.19) and with moderate duration of time in practice (10–20 years) (OR, 1.42; 95% CI 1.14–1.76). Higher rates of cephalosporins prescription were observed in urban areas than rural areas and by male physicians. Seasonal peak was detected for penicillins and cephalosporins prescriptions in autumn.
Conclusions:
These findings showed the widespread use of antibiotics by general practitioners that was associated with the physicians’ gender, time since graduation and practice location and also season of prescribing. More researches are needed on other factors related to the overprescribing of antibiotics and they could be used to project educational programs for improvement of antibiotic prescribing quality in our country.
Keywords: Antibiotics, general practice, prescriptions patter, rational drug use
INTRODUCTION
Irrational antibiotic prescribing is an important health problem which affects control of infectious diseases worldwide.[1,2] This problem increases anti-microbial resistance, toxicity risks, and therapeutic costs.[3,4] There are reported concerns about the inappropriate and over-prescribing of antibiotics especially broad-spectrum anti-microbial agents by physicians in primary care as the main driver of resistance.[5] Large volume of antibiotic self-medication by patients particularly taking in under-dose and for treatment of viral respiratory infections also promotes antibiotic resistance and threatens the public health.[6] The emergence of resistant microbial pathogens and associated infections is a major complication, which has troubled the management of infectious diseases worldwide.[7] According to the World Health Organization statement, anti-microbial resistance results in prolonged illness and greater risk of death, compromises the success of some treatments like organ transplantation, cancer chemotherapy and major surgery, increases health-care costs and threatens health security.[8]
In Iran, there are some evidence of overuse and inappropriately prescribing of antibiotics.[9,10] Antibiotics are the most used drugs in Iran, and about half of patients received an antibiotic during doctor-patients encounters in general practice.[11,12] However, little is known about the factors associated with antibiotic prescribing. Various socio-cultural and demographic factors including patient-and physician-related factors can influence antibiotic prescribing.[13,14] The aim of the present study was to assess the physician-related factors associated with variability in antibiotic prescription in Isfahan province of Iran. Since most antibiotic prescribing occurs among outpatients, antibiotic prescribing was evaluated in outpatient prescriptions in this study.[15] About the physician-related factors, various hypotheses have been developed to explain the differences in antibiotic prescribing among physicians.[16] In this investigation, the role of gender, practice location and practice experience (time in practice) were evaluated on the pattern of antibiotic prescribing. Although some evidence have presented that younger and female physicians are more likely to prescribe antibiotics appropriately,[16,17] there are also some reports for nonsignificant difference in antibiotic prescribing pattern regarding the physician's gender or time in practice.[18]
Wide geographic variability has been reported in the prescribing of various antibiotic classes in the researches.[17] There are also diverse reports for antibiotic over-prescription by urban or rural practicing physicians.[19,20] In this study, the seasonality of antibiotic prescription that may be associated with seasonal changes in drug resistance levels was also assessed.[21]
METHODS
Study design and participants
In this cross-sectional study, all prescriptions data from general physicians were collected by Rational Use of Drugs Committee in Food and Drug Deputy of Isfahan University of Medical Sciences from rural and urban locations of Isfahan province in center of Iran in 2011. In this retrospective survey, the prescriptions data including 7439709 prescriptions issued by 3772 general physicians was obtained electronically using (Mana Faravaran Software Group) Rx Analyzer or Noskhehpardaz software from the Social Security Insurance Organization and Iran Health Insurance Organization (two Iranian public insurance organizations). This software has installed in all pharmacies in the country and has the ability to record unlimited number of prescriptions regarding various medicine information and also prescriber information. It has capability of analyzing of the prescriptions according to the specific prescribing indicators such as average number of drugs per encounter, percentage of patients receiving antibiotics, percentage of patients receiving injectable drugs, and percentage of the most frequently prescribed drugs.
Variables assessment
All prescriptions containing antibiotics were included in this study. Percentage of patients receiving antibiotics and pattern of anti-bacterial prescribing including the most frequently prescribed antibiotic classes were determined in the prescriptions of general practitioners. The antibiotics were classified according to the American Hospital Formulary Service pharmacologic-therapeutic classification system into penicillins, cephalosporins (first, second and third generations), macrolides, aminoglycosides, quinolones, sulfonamides, tetracyclines and others (chloramphenicols, metronidazole, anti-tuberculars and urinary anti-infectives). The less frequently prescribing antibiotics including chloramphenicols, anti-tuberculars, and urinary anti-infectives were not regarded in statistical evaluation.
Seasonality of antibiotic prescription and physician-related variables including gender, practice location and rurality, and time since graduation were assessed. The physicians’ demographic information was obtained from the Isfahan Medical Council. The association between the selected factors with antibiotic prescribing was first evaluated and then we investigated whether these physicians’ characteristics and also season of prescription are predictors of inappropriate prescribing.
Statistical analysis
The prescriptions data were evaluated using professional computer software for gathering and analyzing prescription data (Rx Analyzer or Noskhehpardaz, Mana Faravaran Software Group, Tehran, Iran) and also SPSS version 16, SPSS Inc, Chicago, USA
Data were reported as frequency (percentage). The Chi-square test was used for examining the associations of physician-related factors and also seasonality with antibiotic prescribing pattern. The effects of related factors on the prescribing pattern of different classes of antibiotics were also evaluated by multiple logistic regressions and the odds ratio (OR) with 95% confidence interval (CI) were determined for the assessment of over-prescription on different level of covariates. P <0.05 was considered as statistically significant.
RESULTS
The total population of Isfahan were 4186678 persons (the percentage of urban was 74.3%) in 2011. The prevalence rate of prescriptions was 3750/1000 inhabitants/year.
Of the 7439709 prescriptions issued by 3772 general practitioners, 51% contained at least one antibiotic. It is noteworthy that there is also over the counter purchase of antibiotics in Iran. Demographic characteristics are presented in Table 1. Male physicians outnumbered females (74.5%). Most physicians were working on urban places (67.4%) and have 10–20 years experiences. The pattern of antibiotic prescribing showed that penicillins were the most frequently prescribed antibiotics and 23.55% of patients received penicillins, followed by cephalosporins (13.4%), macrolides (5.26%) and quinolones (3.19%) [Figure 1]. Amoxicillin (7.5%) happened to be the most prescribed penicillins, followed by co-amoxiclav (5.3%), penicillin 6.3.3 (5.23%), benzathine penicillin G (2.7%), procaine penicillin G (2.47%) and ampicillin (0.35%). The frequency of prescribed cephalosporins was as cephalexin (2%) and cefazolin (0.5) in the first generation, and cefixime (8.2%) and ceftriaxone (2.7%) in the third generation. The mainly prescribed macrolides were azithromycin (4.16%) and erythromycin (1.1%). Ciprofloxacin (3.14%) and ofloxacin (0.05) were the mainly prescribed quinolones.
Table 1.
Demographic information of general physicians in Isfahan province of Iran, 2011 (n =3772)
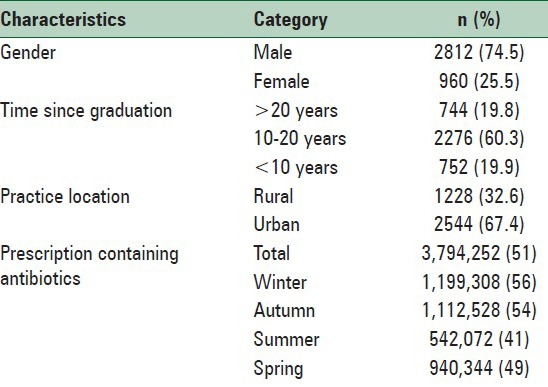
Figure 1.
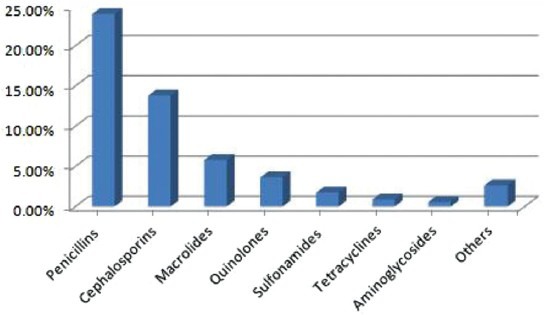
Pattern of antibiotic prescribing by general physicians in Isfahan province of Iran, 2011
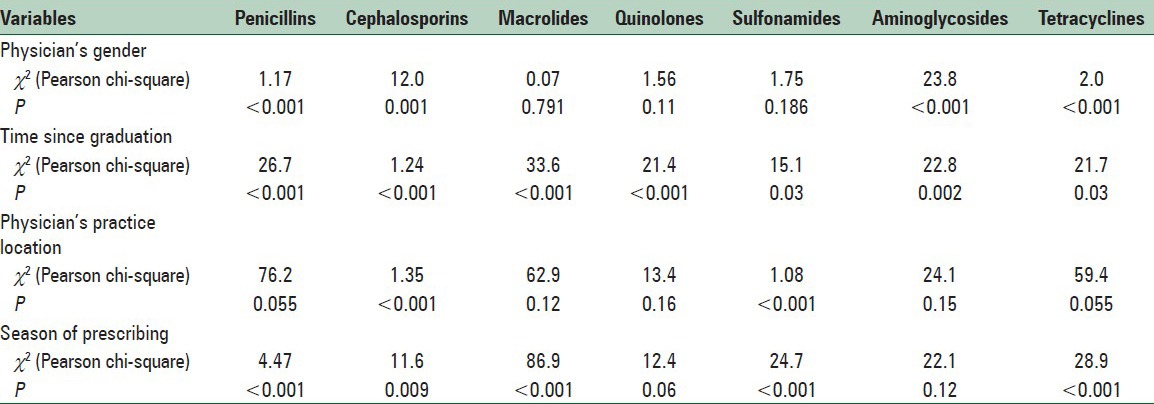
In the relationship analysis [Table 2], antibiotic prescribing was significantly related to the demographic characteristics such as gender, time since graduation, physicians’ practice location factors and season of prescribing.
Table 2.
Association of season of prescribing and physician-related factors with antibiotic prescription in Isfahan, Iran, 2011
The results of multivariate logistic regression and their OR according to the season of prescribing and physician-related factors with a different pattern of antibiotic prescription are shown in Table 3. There were significant differences between higher-prescribing and not higher-prescribing according to the gender of physicians, time since graduation and physicians’ practice location factors.
Table 3.
Multivariable logistic regression (OR, 95% CI) of the season of prescribing and physician-related factors associated with variability in antibiotic prescription in Isfahan, Iran, 2011
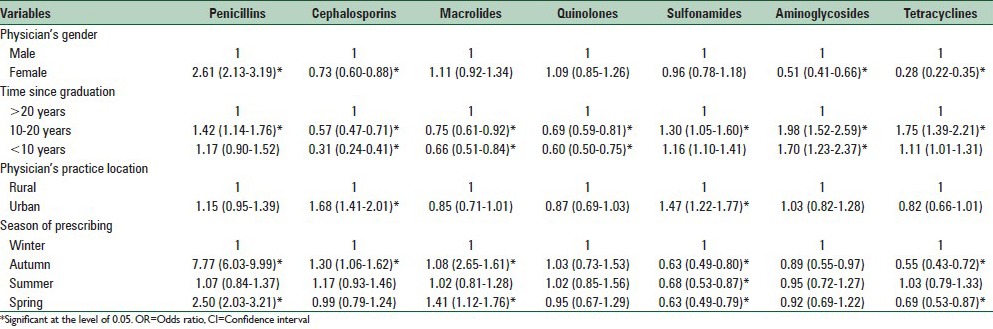
Female physicians significantly more often prescribed penicillins (OR, 2.61; 95% CI, 2.13–3.19) but less prescribed cephalosporins (OR.,73; CI, 0.63–0.88) especially third generation cephalosporins (data were not shown), aminoglycosides and tetracyclines.
There was higher-prescribing of most classes of antibiotics except cephalosporins and macrolides by physicians with moderate duration of time in practice (10–20 years) while younger physicians significantly less prescribed most classes of antibiotics. There were higher rates of prescribing of cephalosporins in urban areas than rural areas. Seasonal peak was observed for penicillins and cephalosporins prescriptions in autumn. Macrolides were significantly more prescribed during autumn and spring while sulfonamides and tetracyclines were less prescribed during all seasons other than winter [Table 3].
DISCUSSION
The results of this assessment showed a wide range of antibiotic prescribing by general physicians in Isfahan province of Iran. Prescriptions with antibiotics were about one-half of all prescriptions (51%). Our previous study also revealed that general practitioners prescribed more antibiotics than medical specialists.[10] Different rates of antibiotic prescription from 17.5% to 67% have been reported in developing countries that are much higher than developed countries.[22,23,24] The inappropriate antibiotic prescription has significantly declined over the last decade in developed countries such as United States, and persuasive interventional approaches are needed to improve antibiotic use in other countries.[25]
The most common antibiotic categories prescribed by general physicians were penicillins, followed by cephalosporins and macrolides. Other researches also reported similar results for the main choices of antibiotic classes in developing countries.[26,27] Excessive use and irrational prescribing of broad-spectrum antibiotics result in antibiotic resistance. Unfortunately, large volumes of broad-spectrum antibiotics are incorrectly prescribed for mild and viral infections of upper respiratory tract in our country. Besides adverse effects and dangerous allergic reactions, emergence of extended-spectrum beta-lactamase-producing infections might threaten the public health.[28] Recently some national policies have been used for limitation of the ceftriaxone use in Iran but other third-generation cephalosporins should be also used cautiously for outpatients.
In this study, some physician-related variables including gender, practice location and rurality, and time since graduation were evaluated and other physicians’ characteristics, patients’ characteristics, socioeconomic, educational and facility factors were not recorded because of limitation of our electronic data. Our finding showed gender variability in antibiotic prescribing by general practitioners. Moghadamnia et al., also reported significant difference in antibiotic prescribing patterns between female and male physicians in Iran.[29] Female gender has been shown to positively affect physician adherence to guidelines, for example, in antibiotic prophylaxis.[30] There are some reports about possible differences in the treatment approaches of the male and female physicians however the reasons for these differences is not clear.[31,32]
Regarding the time in practice, most antibiotic categories were significantly less prescribed by younger physicians especially cephalosporins. Other studies also suggested increasing in inappropriate antibiotic prescribing with lengthening time in practice.[33] More recent medical graduates have been found to follow standard protocols in their practices. Excessive antibiotic prescribing may be due to acceptance of patients demand for antibiotics by older physicians.[16] Other data in our research were similar rate of antibiotic prescribing in rural and urban areas except for cephalosporins and sulfonamide prescribing, which were higher in urban areas. The results of investigations have suggested the geographic region of physicians’ practice as a predictor of broad-spectrum antibiotic prescribing.[34] Although higher rates of antibiotic prescription have been found in rural setting in some studies due to the disease pattern or patients’ demand for quick relief,[19,20] there are also several reports for antibiotic over-prescription by urban practicing physicians.[35,36] In our findings, similarity between rural and urban areas in prescribing of most antibiotic classes may be attributed to the resemble pattern of infectious diseases in our province and cultural growth in rural regions. However, larger cephalosporins prescription in urban areas may be due to the patients’ knowledge and more pressure for taking broad-spectrum antibiotics.
Our results also revealed seasonal variability in antibiotic prescribing. Although higher rates of penicillins and cephalosporins prescriptions were observed during autumn, more antibiotics were prescribed totally in the winter. Our previous study also showed higher uses of antibiotics, corticosteroids, and injectable drugs in the winter and lower use in the summer.[9] Sun et al., also reported increased antibiotic use in the winter and its strong correlation with resistance.[21] However, in the report of Pathak et al., the peak antibiotic prescribing rates have been observed during summer and rainy season in India.[37]
CONCLUSIONS
The findings of this study confirmed antibiotic over-prescription and provided some implications for understanding the variability in antibiotic prescribing in the central province of Iran. Widespread use and inappropriate antibiotic prescriptions promote antibiotic resistance and increase morbidity and mortality. Hence, there is an urgent need to various educational, managerial and regulatory strategies to promote rational antibiotic use. More efforts are required to identify the role of other physician's factor and also patients’ characteristics, socioeconomic, educational and facility factors on antibiotic prescription in our country. Since various factors can influence antibiotic prescribing, wide variability is observed in antibiotic use worldwide. Therefore, interventional strategies should be performed focusing on specific variables that predict inappropriate antibiotic prescribing in each country to reduce prescription rates and improve the quality of prescribing effectively.
ACKNOWLEDGMENT
This research project numbered 288224 was financially supported by Research Council of Isfahan University of Medical Sciences, Isfahan, Iran.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Laing R, Hogerzeil H, Ross-Degnan D. Ten recommendations to improve use of medicines in developing countries. Health Policy Plan. 2001;16:13–20. doi: 10.1093/heapol/16.1.13. [DOI] [PubMed] [Google Scholar]
- 2.Coenen S, Ferech M, Haaijer-Ruskamp FM, Butler CC, Vander Stichele RH, Verheij TJ, et al. European Surveillance of Antimicrobial Consumption (ESAC): Quality indicators for outpatient antibiotic use in Europe. Qual Saf Health Care. 2007;16:440–5. doi: 10.1136/qshc.2006.021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Mar C. Prescribing antibiotics in primary care. BMJ. 2007;335:407–8. doi: 10.1136/bmj.39307.642963.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goossens H, Ferech M, Vander Stichele R, Elseviers M ESAC Project Group. Outpatient antibiotic use in Europe and association with resistance: A cross-national database study. Lancet. 2005;365:579–87. doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- 5.Tan T, Little P, Stokes T Guideline Development Group. Antibiotic prescribing for self limiting respiratory tract infections in primary care: Summary of NICE guidance. BMJ. 2008;337:a437. doi: 10.1136/bmj.a437. [DOI] [PubMed] [Google Scholar]
- 6.Sarahroodi S, Mikaili P. Self-medication with antibiotics: A global challenge of our generation. Pak J Biol Sci. 2012;15:707–8. doi: 10.3923/pjbs.2012.707.708. [DOI] [PubMed] [Google Scholar]
- 7.Sarahroodi S, Arzi A, Sawalha AF, Ashtarinezhad A. Antibiotics self-medication among southern Iranian university students. Int J Pharmacol. 2010;6:48–52. [Google Scholar]
- 8.Antimicrobial resistance. WHO Web Site. 2013. [Last updated on 2014 April]. Available from: http://www.who.int/mediacentre/factsheets/fs194/en/
- 9.Safaeian L, Mahdanian AR, Hashemi-Fesharaki M, Salami S, Kebriaee-Zadeh J, Sadeghian GH. General physicians and prescribing pattern in isfahan, iran. Oman Med J. 2011;26:205–6. doi: 10.5001/omj.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadeghian GH, Safaeian L, Mahdanian AR, Salami S, Kebriaee-Zadeh J. Prescribing quality in medical specialists in isfahan, iran. Iran J Pharm Res. 2013;12:235–41. [PMC free article] [PubMed] [Google Scholar]
- 11.Vessal G, Khabiri A, Mirkhani H, Cookson BD, Askarian M. Study of antibiotic prescribing among dental practitioners in Shiraz, Islamic Republic of Iran. East Mediterr Health J. 2011;17:763–9. [PubMed] [Google Scholar]
- 12.Ataei M, Rahimi W, Rezaei M, Koohboomi J, Zobeiri M. The effect of antibiotics rational use workshop on prescription pattern of general physicians in Kermanshah. Behbood. 2010;14:1–9. [Google Scholar]
- 13.Harbarth S, Albrich W, Brun-Buisson C. Outpatient antibiotic use and prevalence of antibiotic-resistant pneumococci in France and Germany: A sociocultural perspective. Emerg Infect Dis. 2002;8:1460–7. doi: 10.3201/eid0812.010533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butler CC, Hood K, Verheij T, Little P, Melbye H, Nuttall J, et al. Variation in antibiotic prescribing and its impact on recovery in patients with acute cough in primary care: Prospective study in 13 countries. BMJ. 2009;338:b2242. doi: 10.1136/bmj.b2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerber JS, Prasad PA, Fiks AG, Localio AR, Grundmeier RW, Bell LM, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: A randomized trial. JAMA. 2013;309:2345–52. doi: 10.1001/jama.2013.6287. [DOI] [PubMed] [Google Scholar]
- 16.Cadieux G, Tamblyn R, Dauphinee D, Libman M. Predictors of inappropriate antibiotic prescribing among primary care physicians. CMAJ. 2007;177:877–83. doi: 10.1503/cmaj.070151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazzaglia G, Caputi AP, Rossi A, Bettoncelli G, Stefanini G, Ventriglia G, et al. Exploring patient- and doctor-related variables associated with antibiotic prescribing for respiratory infections in primary care. Eur J Clin Pharmacol. 2003;59:651–7. doi: 10.1007/s00228-003-0669-0. [DOI] [PubMed] [Google Scholar]
- 18.Arnold SR, Allen UD, Al-Zahrani M, Tan DH, Wang EE. Antibiotic prescribing by pediatricians for respiratory tract infection in children. Clin Infect Dis. 1999;29:312–7. doi: 10.1086/520207. [DOI] [PubMed] [Google Scholar]
- 19.Bhatnagar T, Mishra CP, Mishra RN. Drug prescription practice: A household study in rural Varanasi. Indian J Prev Soc. 2003;34:33–9. [Google Scholar]
- 20.Huang N, Chou YJ, Chang HJ, Ho M, Morlock L. Antibiotic prescribing by ambulatory care physicians for adults with nasopharyngitis, URIs, and acute bronchitis in Taiwan: A multi-level modeling approach. Fam Pract. 2005;22:160–7. doi: 10.1093/fampra/cmh734. [DOI] [PubMed] [Google Scholar]
- 21.Sun L, Klein EY, Laxminarayan R. Seasonality and temporal correlation between community antibiotic use and resistance in the United States. Clin Infect Dis. 2012;55:687–94. doi: 10.1093/cid/cis509. [DOI] [PubMed] [Google Scholar]
- 22.Hamadeh GN, Dickerson LM, Saab BR, Major SC. Common prescriptions in ambulatory care in Lebanon. Ann Pharmacother. 2001;35:636–40. doi: 10.1345/aph.10175. [DOI] [PubMed] [Google Scholar]
- 23.Chalker J. Improving antibiotic prescribing in Hai Phong Province, Viet Nam: The “antibiotic-dose” indicator. Bull World Health Organ. 2001;79:313–20. [PMC free article] [PubMed] [Google Scholar]
- 24.Roumie CL, Halasa NB, Grijalva CG, Edwards KM, Zhu Y, Dittus RS, et al. Trends in antibiotic prescribing for adults in the United States-1995 to 2002. J Gen Intern Med. 2005;20:697–702. doi: 10.1111/j.1525-1497.2005.0148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnold SR, Bush AJ. Decline in inappropriate antibiotic use over a decade by pediatricians in a Tennessee community. Ambul Pediatr. 2006;6:225–9. doi: 10.1016/j.ambp.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Hashemi SH, Nasrollah A, Rajabi M. Irrational antibiotic prescribing: A local issue or global concern? EXCLI J. 2013;12:384–95. [PMC free article] [PubMed] [Google Scholar]
- 27.Dong L, Yan H, Wang D. Antibiotic prescribing patterns in village health clinics across 10 provinces of Western China. J Antimicrob Chemother. 2008;62:410–5. doi: 10.1093/jac/dkn153. [DOI] [PubMed] [Google Scholar]
- 28.Lan CK, Hsueh PR, Wong WW, Fung CP, Lau YT, Yeung JY, et al. Association of antibiotic utilization measures and reduced incidence of infections with extended-spectrum beta-lactamase-producing organisms. J Microbiol Immunol Infect. 2003;36:182–6. [PubMed] [Google Scholar]
- 29.Moghadamnia AA, Mirbolooki MR, Aghili MB. General practitioner prescribing patterns in Babol city, Islamic Republic of Iran. East Mediterr Health J. 2002;8:550–5. [PubMed] [Google Scholar]
- 30.Wurst KE, Sleath BL. Physician knowledge and adherence to prescribing antibiotic prophylaxis for sickle cell disease. Int J Qual Health Care. 2004;16:245–51. doi: 10.1093/intqhc/mzh033. [DOI] [PubMed] [Google Scholar]
- 31.Keane D, Woodward CA, Ferrier BM, Cohen M, Goldsmith CH. Female and male physicians: Different practice profiles: Will increasing numbers of female GPs affect practice patterns of the future? Can Fam Physician. 1991;37:72–81. [PMC free article] [PubMed] [Google Scholar]
- 32.Straand J, Rokstad KS, Sandvik H. Prescribing systemic antibiotics in general practice. A report from the Møre and Romsdal Prescription study. Scand J Prim Health Care. 1998;16:121–7. doi: 10.1080/028134398750003296. [DOI] [PubMed] [Google Scholar]
- 33.Arnold SR. Revenge of the killer microbe. CMAJ. 2007;177:895–6. doi: 10.1503/cmaj.071270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinman MA, Landefeld CS, Gonzales R. Predictors of broad-spectrum antibiotic prescribing for acute respiratory tract infections in adult primary care. JAMA. 2003;289:719–25. doi: 10.1001/jama.289.6.719. [DOI] [PubMed] [Google Scholar]
- 35.Kumar R, Indira K, Rizvi A, Rizvi T, Jeyaseelan L. Antibiotic prescribing practices in primary and secondary health care facilities in Uttar Pradesh, India. J Clin Pharm Ther. 2008;33:625–34. doi: 10.1111/j.1365-2710.2008.00960.x. [DOI] [PubMed] [Google Scholar]
- 36.Kuyvenhoven M, de Melker R, van der Velden K. Prescription of antibiotics and prescribers’ characteristics. A study into prescription of antibiotics in upper respiratory tract infections in general practice. Fam Pract. 1993;10:366–70. doi: 10.1093/fampra/10.4.366. [DOI] [PubMed] [Google Scholar]
- 37.Pathak A, Mahadik K, Dhaneria SP, Sharma A, Eriksson B, Lundborg CS. Antibiotic prescribing in outpatients: Hospital and seasonal variations in Ujjain, India. Scand J Infect Dis. 2011;43:479–88. doi: 10.3109/00365548.2011.554854. [DOI] [PubMed] [Google Scholar]


