Abstract
Gene therapy targeting of kidneys has been largely unsuccessful. Recently, a recombinant adeno-associated virus (rAAV) vector was used to target adult mouse kidneys. Our hypothesis is that a pseudotyped rAAV 2/9 vector can produce fetal kidney-specific expression of the green fluorescent protein (GFP) gene following maternal tail vein injection of pregnant mice. Pregnant mice were treated with rAAV2/9 vectors with either the ubiquitous cytomegalovirus promoter or the minimal NPHS1 promoter to drive kidney-specific expression of GFP. Kidneys from dams and pups were analyzed for vector DNA, gene expression, and protein. Vector DNA was identified in kidney tissue out to 12 weeks at low but stable levels, with levels higher in dams than that in pups. Robust GFP expression was identified in the kidneys of both dams and pups treated with the cytomegalovirus (CMV)-enhanced green fluorescent protein (eGFP) vector. When treated with the NPHS1-eGFP vector, dams and pups showed expression of GFP only in kidneys, localized to the glomeruli. An 80-fold increase in GFP mRNA expression in dams and a nearly 12-fold increase in pups was found out to 12 weeks of life. Selective targeting of the fetal kidney with a gene therapy vector was achieved by utilizing the pseudotyped rAAV 2/9 vector containing the NPHS1 promoter.
Introduction
Congenital anomalies of the kidney and urinary tract constitute ~20 to 30% of all disorders identified in the prenatal period1 and play a causative role in a majority of cases of end-stage renal disease in pediatric patients.2 The morbidity and costs associated with congenital anomalies of the kidney and urinary tract are enormous, with an estimated 1 billion dollars per year burden to the healthcare system. The genetic etiologies of several inherited structural kidney diseases have been elucidated and have their origins in early renal development.3,4 Some of these conditions are pre- or perinatally lethal. Many can also be diagnosed prenatally, and with advancing gestational age, these conditions have significant progression of their phenotype with irreversible damage.5,6
In-utero gene therapy may improve or prevent progression of defects in kidney function that would otherwise not be treated until after birth. Several routes of administration have been utilized for in-utero gene therapy. The majority of studies have utilized maternal laparotomy followed by direct fetal (intravascular,7–9 intraperitoneal,10–15 intramuscular,8,9,11,16 and intrahepatic8,9,17), intraamniotic,9,18 or intraplacental9 injection. A few studies have shown successful gene transfer to fetuses following intravenous administration of cationic liposomes,19 DNA/lipid complexes,20,21 and T7 phage22 to the mothers.
Recently, a recombinant adeno-associated virus (rAAV) serotype 9 vector was shown to have tissue tropism for the adult mouse kidney.23 AAVs are nonpathogenic, nonenveloped, single-stranded, DNA helper–dependent parvoviruses that have been identified as promising vectors for gene delivery because of several important advantages over other vectors including: (i) broad host cell range; (ii) the ability to transduce dividing as well as growth-arrested cells; (iii) low pathogenicity and immunogenicity in humans; (iv) the ability to be produced in high quantities; (v) the ability to alter vector tropism by capsid pseudotyping; (vi) the ability to produce long-term gene expression by integration or remaining as an episome.24 Additionally, AAVs in the maternal circulation cross the placenta and can infect fetal tissues.25
The kidney is a well-differentiated organ with specialized compartments composed of glomeruli, tubules, vasculature, and interstitium. Because of this complex anatomy, it has proven to be a difficult target for gene therapy vectors both in human and animal experiments. For a thorough review of gene transfer strategies focusing on the kidney, see the study by Isaka.26 The following are studies that have successfully targeted the kidney: Schievenbusch et al.24 utilized the rAAV9 vector containing the KSP promoter to drive expression of GFP in 6-week-old COL4A3 null mice, Luo et al.27 utilized several recombinant vectors including rAAV2/7 and rAAV2/9 containing the human G6Pase minimal promoter to drive expression of the G6Pase gene in newborn mice, and Sénac et al.28 utilized the rAAV9 vector containing the β-actin promoter to drive expression of methylmalonyl-CoA mutase in newborn Mut null mice. All three were able to successfully produce transgene expression in the kidneys; however, there was expression in other tissues as well, most often, the liver. To date, there have been no successful studies that have demonstrated kidney-specific transgene expression or in-utero transgene studies to target the fetal kidney. Currently, there are no gene therapy trials targeting the kidneys, although there are several utilizing transduced autologous cells (http://www.clinicaltrials.gov).
We hypothesized that a pseudotyped rAAV 2/9 vector can produce sustained fetal kidney expression of the GFP gene following maternal tail vein injection of pregnant mice. Furthermore, with the addition of a kidney-specific promoter (minimal nephrin promoter), we proposed that this would allow expression to be restricted to the kidneys, specifically the glomerulus.
Results
Targeting of maternal and fetal kidneys with rAAV 2/9 pseudotyped vector with ubiquitous promoter
Immunofluorescence and immunohistochemistry.
Prior to undertaking the in-utero administration of the rAAV 2/9-cytomegalovirus (CMV)-enhanced green fluorescent protein (eGFP) vector, we sought to replicate previous reports of AAV9 affinity for kidney tissue. Following tail vein injection of the rAAV 2/9-CMV-eGFP vector to adult mice, immunofluorescence (IF) and immunohistochemistry (IH) staining of harvested tissues for GFP showed consistent staining of all tissues analyzed (adrenal, bladder, brain, heart, kidney, liver, lung, ovary, pancreas, salivary gland, skeletal muscle, spleen, testis, thymus, thyroid, and uterus). Specifically, the AAV2/9-CMV-eGFP vector showed significant tropism for the kidney, localizing to the glomeruli and tubules (Figure 1a,b).
Figure 1.

Immunofluorescence staining for GFP in adult mouse kidneys. Adult mice (n = 2) were treated with 1 × 1012 viral genomes rAAV 2/9-CMV-eGFP by tail vein injection. Eight weeks postinjection, kidneys were snap frozen and whole mounted for imaging, then cryosectioned (10 μm) and processed for immunofluorescence staining. Bars = 2 mm (a) and 500 μm (b). CMV, cytomegalovirus; eGFP, enhanced green fluorescent protein.
Based on these findings, the rAAV 2/9-CMV-eGFP vector (8.4 × 1012 vg) was administered by tail vein injection to pregnant dams at embryonic day (E) 12.5. Control mice were injected with rAAV2-CMV-eGFP (1.3 × 1012 vg) or saline also at E12.5. The rAAV2-CMV-eGFP vector was chosen because in previous studies, AAV2 has demonstrated limited transfection efficiency in the kidney.29
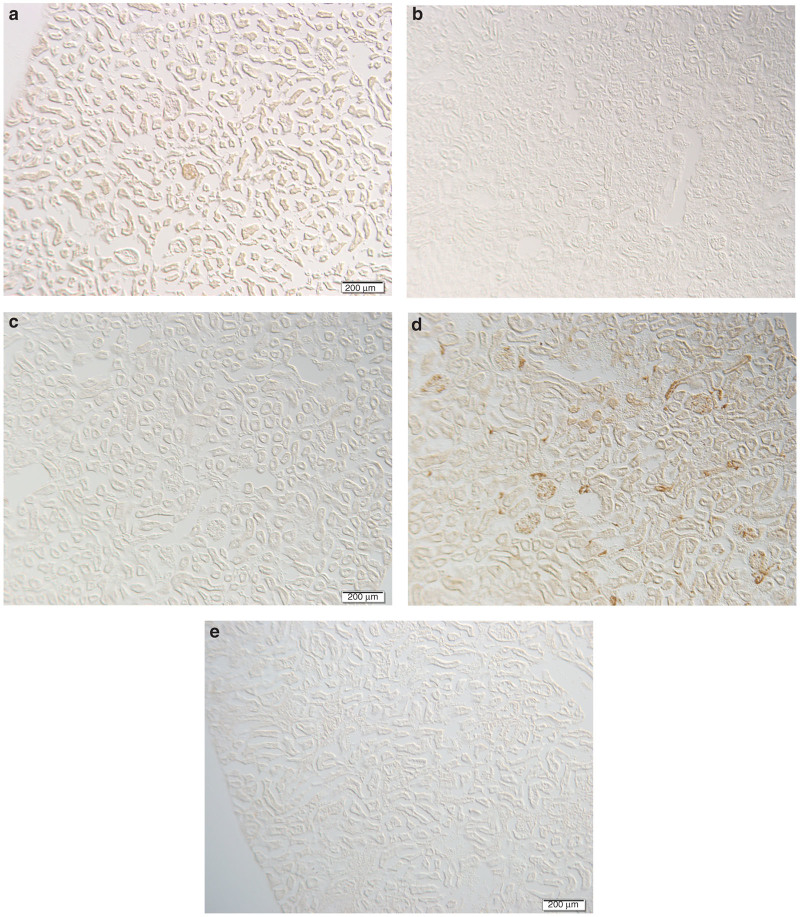
When kidneys from the rAAV 2/9-CMV-eGFP–treated pups were examined, robust staining was identified (Figure 2a, IH and Figure 3a, IF). Specifically, glomeruli and tubules were clearly shown to be expressing GFP. In contrast, the kidneys of pups from rAAV2-CMV-eGFP–treated animals demonstrated no identifiable staining (Figure 2b, IH and Figure 3b, IF). Kidneys taken from the treated dams showed the same patterns of IF and IH staining as the pups, with absence of staining in kidneys of the dams treated with rAAV2-CMV-eGFP (Figure 2c, IH and Figure 3c, IF), similar to saline-injected controls (Figure 2e, IH and Figure 3e, IF). Robust staining was also seen in the kidneys of the dams treated with rAAV 2/9-CMV-eGFP (Figure 2d, IH and Figure 3d, IF).
Figure 2.
Immunohistochemistry staining for GFP in kidneys of treated pups at 12 weeks. Pregnant adult dams at E12.5 were treated with 8.4 × 1012 viral genomes rAAV 2/9-CMV-eGFP (n = 1), saline control (n = 1), or 1.3 × 1012 viral genomes rAAV2-CMV-eGFP (n = 1) by tail vein injection. Pups (a: rAAV2/9; n = 12), (b: rAAV2; n = 9), and saline n = 10 (not shown) were sacrificed at 12 weeks of life. Dams (c: rAAV2; d: rAAV2/9, and e: saline control) were sacrificed at 8 weeks postinjection. Kidneys were snap frozen, whole mounted for imaging, cryosectioned (10 μm), and processed for immunohistochemistry. Bar = 200 μm. CMV, cytomegalovirus; eGFP, enhanced green fluorescent protein.
Figure 3.
Immunofluorescence staining for GFP in kidneys of treated pups at 8 weeks. Pregnant adult dams at E12.5 were treated with 8.4 × 1012 viral genomes rAAV 2/9-CMV-eGFP (n = 1), saline control (n = 1), or 1.3 × 1012 viral genomes rAAV2-CMV-eGFP (n = 1) by tail vein injection. Pups (a: rAAV2/9; n = 12), (b: rAAV2; n = 9), and saline n = 10 (not shown) were sacrificed at 8 weeks of life. Dams (c: rAAV2; d: rAAV2/9, and e: saline control) were sacrificed at 8 weeks postinjection. Kidneys were snap frozen, whole mounted for imaging, cryosectioned (10 μm), and processed for immunofluorescence staining for DAPI (blue), GFP (green), WT-1 (red), and merge slide. Bars = 50 μm. CMV, cytomegalovirus; eGFP, enhanced green fluorescent protein.
Quantitative PCR for viral transfection.
We quantified the number of viral genomes in renal tissues to determine the viral gene transduction efficiency. At the time of sacrifice, DNA was isolated from kidneys of dams and pups treated with the rAAV2-CMV-eGFP or rAAV2/9-CMV-eGFP vectors or saline-treated controls. Viral genomes were measured by quantitative PCR and compared with a standard curve of GFP plasmids (Figure 4). As expected, maternal tissues contained several orders of magnitude higher viral genomes per microgram DNA (rAAV2/9-CMV-eGFP: 7.61 × 105 and rAAV2-CMV-eGFP: 2.61 × 104) than that found in pups (rAAV2/9-CMV-eGFP: 7.95–2,150 and rAAV2-CMV-eGFP: 57.1–988). Saline-treated dams and pups did not demonstrate any eGFP expression as expected. DNA was also isolated from the kidneys of the second litter of pups for each dam to determine if there was germline transmission of viral vector DNA. No viral genomes could be detected by quantitative PCR in the kidneys of any of the pups of either second litter (data not shown).
Figure 4.
Transfection efficiency of rAAV vectors following in-utero maternal systemic delivery. Pregnant adult dams at E12.5 were treated with 1.3 × 1012 viral genomes rAAV2-CMV-eGFP (n = 1) or 8.4 × 1012 viral genomes rAAV 2/9-CMV-eGFP (n = 1) by tail vein injection. Pups were sacrificed at 2, 4, 6, 8, 10, and 12 weeks of life. Dams were sacrificed at 8 weeks postpartum. Quantitative real-time PCR was performed on DNA isolated from kidneys for viral genome copies. Transfection efficiency is expressed in viral genomes per microgram of extracted DNA. White bars represent mice treated with rAAV2-CMV-eGFP (n = 9) and black bars represent mice treated with rAAV2/9-CMV-eGFP (n = 12). Error bars are mean + SD. CMV, cytomegalovirus; eGFP, enhanced green fluorescent protein.
Survival.
We did not directly assess the vector-related toxicity in these mice, but there were not apparent differences in the mice treated with each of the vectors, nor were there apparent differences noticed on the anatomic survey at necropsy on dams or pups. Both treatment dams and saline controls produced appropriately sized litters (mean litter size was 10 pups). All pups grew well and survived to scheduled sacrifice out to 12 weeks of life. Previous studies have shown the safety of recombinant AAVs.
Targeting of maternal and fetal kidneys with rAAV 2/9 vector with kidney-specific promoter
After confirmation that the rAAV 2/9 vector was able to transduce both maternal and fetal kidneys following maternal tail vein injection, the rAAV 2/9-NPHS1-eGFP vector (1.0 × 1012 vg) was administered by tail vein injection to pregnant dams at E12.5. This vector contains the minimal Nephrin promoter, as described by Moeller et al.30, driving expression of the eGFP gene. Control dams were injected with rAAV 2/9-CMV-eGFP (1.0 × 1012 vg) or saline sham also at E12.5.
Immunofluorescence and immunohistochemistry.
When kidneys of pups treated with rAAV 2/9-NPHS1-eGFP were examined by IH, significant staining of renal glomeruli was observed compared with sham-injected pups at 2, 4, 8, and 12 weeks of life (Figure 5a–h). More robust staining was identified in treated dams compared with the pups as shown in Figure 5i–j. When analyzed by IF with costaining with WT-1 and DAPI (Figure 6a–c), similar staining of the glomeruli was identified that was more robust in the dams and absent in saline-injected controls.
Figure 5.
Immunohistochemistry staining for GFP in kidneys of treated pups and dams. Pregnant adult dams at E12.5 were treated with saline sham (a, c, e, g, i) or 1.0 × 1012 viral genomes rAAV 2/9-NPHS1-eGFP (b, d, f, h, j) by tail vein injection. Pups were sacrificed at 2 (a, b), 4 (c, d), 8 (e, f), and 12 (g, h) weeks of life. (i, j) Dams were sacrificed at 8 weeks postpartum. Kidneys were snap frozen, whole mounted for imaging, cryosectioned (10 μm), and processed for immunohistochemistry. Bars = 200 μm. CMV, cytomegalovirus; eGFP, enhanced green fluorescent protein.
Figure 6.
Immunofluorescence staining for GFP in treated pups and dams at 8 weeks. Pregnant adult dams at E12.5 were treated with 1.0 × 1012 viral genomes rAAV 2/9-NPHS1-eGFP or saline sham by tail vein injection. Pups (a: experimental and b: saline control) and (c) experimental dams were sacrificed at 8 weeks postpartum. Kidneys were snap frozen, whole mounted for imaging, cryosectioned (10 μm), and processed for immunofluorescence staining for DAPI (blue), WT-1 (green), GFP (red), and merge slide. Bars = 50 μm. CMV, cytomegalovirus; eGFP, enhanced green fluorescent protein.
Quantitative PCR for viral transduction and expression.
In order to determine the vector transduction efficiency and tissue tropism, DNA and RNA were isolated at the time of sacrifice from liver, kidney, spleen, brain, heart, and lung of dams treated with the rAAV 2/9-NPHS1-eGFP or rAAV 2/9-CMV-eGFP vectors or saline sham at E12.5 by tail vein injection. The dams were chosen for analysis because the previous experiment had shown that levels of transduction were significantly higher in the dams. Viral genomes in extracted DNA were measured by quantitative PCR and compared with a standard curve of GFP plasmids (Figure 7). As expected, the eGFP vector was not identified in any of the saline-injected dams. The rAAV 2/9 vector showed modest transduction efficiency in all tissues analyzed with maximal levels seen in the liver and the least in the brain. Similar levels of vector transduction were seen with both vectors.
Figure 7.
Tissue tropism and transduction efficiency of rAAV 2/9 vectors in dams. Pregnant adult dams at E12.5 were treated with 1.0 × 1012 viral genomes of rAAV 2/9-NPHS1-eGFP (n = 3) or rAAV 2/9-CMV-eGFP (n = 3) vectors by tail vein injection. Dams were sacrificed at 8 weeks postpartum. Quantitative real-time PCR was performed on DNA isolated from liver, kidney, spleen, brain, heart, and lung for viral genome copies. Transduction efficiency is expressed in viral genomes per microgram of extracted DNA. White bars represent mice treated with rAAV 2/9-NPHS1-eGFP and black bars represent mice treated with rAAV 2/9-CMV-eGFP. Error bars are mean + SD. CMV, cytomegalovirus; eGFP, enhanced green fluorescent protein.
We performed quantitative real-time PCR on RNA samples from maternal tissues to determine the tissue specificity of the NPHS1 promoter. As expected, no expression of eGFP was identified in saline-injected dams, and robust expression of eGFP was identified in all tissues treated with the vector containing the CMV promoter (Figure 8). Significant expression of eGFP was identified in the kidneys of the dams treated with vector containing the NPHS1 promoter, whereas minimal expression that was not statistically significant from saline controls was identified in other tissues analyzed.
Figure 8.

Expression of rAAV 2/9 vector in treated dams. Pregnant adult dams at E12.5 were treated with 1.0 × 1012 viral genomes rAAV 2/9-NPHS1-eGFP (n = 3) or rAAV 2/9-CMV-eGFP (n = 3) vectors or saline sham (n = 2) by tail vein injection. Dams were sacrificed at 8 weeks postpartum. Quantitative real-time PCR was performed on cDNA of isolated liver, kidney, spleen, brain, heart, and lung mRNA. White bars represent rAAV 2/9-NPHS1-eGFP and black bars represent rAAV 2/9-CMV-eGFP. Expression of eGFP was normalized to saline controls using 2ΔΔCT (*P < 0.05). Error bars are mean + SD. CMV, cytomegalovirus; eGFP, enhanced green fluorescent protein.
Finally, RNA was extracted from the liver, kidney, spleen, brain, heart, and lung of pups treated with saline sham or the rAAV 2/9-NPHS1-eGFP or rAAV 2/9-CMV-eGFP vectors in-utero at E12.5 by tail vein injection (Figure 9). As with the dams above, no expression of eGFP was identified in saline-injected dams or pups. Treatment with the rAAV 2/9-CMV-eGFP vector showed robust expression in all tissues analyzed, with up to 400-fold increased expression over saline-injected controls. A similar, but significantly less robust, pattern of expression was seen in the kidneys of pups treated with the rAAV 2/9-NPHS1-eGFP vector with a nearly 12-fold increase over saline-injected controls. No significant expression of eGFP was identified in any other tissues.
Figure 9.
Expression of rAAV 2/9 vectors following maternal injection. Pregnant adult dams at E12.5 were treated with 1.0 × 1012 viral genomes rAAV 2/9-NPHS1-eGFP (n = 3) or rAAV 2/9-CMV-eGFP (n = 3) vectors or saline sham (n = 2) by tail vein injection. Pups (NPHS1: n = 31/CMV: n = 29/saline: n = 19) were sacrificed at 2, 4, 6, 8, 10, and 12 weeks of life. Quantitative real-time PCR was performed on cDNA of isolated liver, kidney, spleen, brain, heart, and lung mRNA. White bars represent rAAV 2/9-NPHS1-eGFP and black bars represent rAAV 2/9-CMV-eGFP. Expression of eGFP was normalized to saline controls using 2ΔΔCT (*P < 0.05). Error bars are mean + SD. CMV, cytomegalovirus; eGFP, enhanced green fluorescent protein.
Discussion
The driving hypothesis for this experiment was that the pseudotyped rAAV 2/9 vector can produce fetal kidney expression of the eGFP gene following maternal tail vein injection of pregnant mice. Toward that end, we first administered a rAAV 2/9-CMV-eGFP vector to adult mice to replicate previous experiments showing kidney tissue tropism by the AAV serotype 9 vector. The pseudotyped AAV 2/9 vector showed ubiquitous staining of all tissues examined, including robust staining of the kidneys. We then injected pregnant dams at E12.5 by tail vein injection with the rAAV 2/9-CMV-eGFP or rAAV2-CMV-eGFP (acting as a negative control) vectors to determine if it would cross the placenta in sufficient concentration to target the fetal kidneys and whether we could induce stable expression of eGFP following birth. We were able to demonstrate stable expression of eGFP out to 12 weeks postinjection in the dam and pup kidneys. Finally, we replaced the ubiquitous CMV promoter in our vector with the minimal NPHS1 promoter to produce kidney-specific glomerular expression in both pups and dams that was again stable out to 12 weeks postinjection.
In-utero gene therapy offers the potential to treat or prevent progression of many congenital diseases. Several recent studies have focused on direct fetal administration of viral vectors in a number of animal models including mice,7–13,16,17 rabbits,18 sheep,14 and nonhuman primates.15 Although the administration of vector in large mammals can be performed under ultrasound guidance, small mammals have required maternal laparotomy, which is associated with an increased risk of morbidity and mortality for both dams and pups. In contrast to these reports, we administered vectors by maternal tail vein injection and assessed the transplacental transfection efficiency in the fetuses. We chose to utilize tail vein injection because the vitelline circulation is not accessible at this early gestational age (E11.5–E12.5). This gestational age was chosen because between E11.5 and E12.5, the ureteric bud epithelium and condensations of mesenchymal cells invade the metanephric mesenchyme. By E13.5, the S-shaped bodies derived from the cap mesenchyme have become infiltrated by endothelial precursors to form the glomerular tuft, which consists of the capillary loops, the mesangium, the glomerular basement membrane, and the podocyte cells.31 Using this model, we were able to deliver a gene therapy vector to the fetal kidneys resulting in stable expression.
Although previous studies have successfully utilized systemic maternal administration of gene therapy vectors such as cationic liposomes,19 DNA/lipid complexes,20,21 and T7 phage,22 the transduction/transfection efficiency was variable. In contrast to these previous studies, we utilized a rAAV, which is known to cross the placenta in humans and has been found in as high as 27% of amniotic fluid samples in one study.32 Recombinant AAVs offer a number of advantages including safety, the ability to transduce nondividing cells, and a broad array of tissue tropisms.
A recent study in mice showed that rAAV9 transduced adult kidneys with significant efficiency.23 Historically, renal tissue has been very difficult to target. Here, we replicated the previous study by showing consistent staining in all tissues examined in both male and female adult mice treated with tail vein injection of a pseudotyped rAAV 2/9 vector. Specifically, the vector showed significant tropism for the kidneys and localized to the glomeruli and tubules. In a previous study by Zincarelli et al.29, the AAV2 vector had poor tissue tropism for the kidneys, so we utilized this vector as a negative control. When pregnant dams were treated with either rAAV2 or our pseudotyped rAAV 2/9 vector, transfection of vector DNA for both serotypes was found, but while the kidneys of the rAAV2-treated dam were negative for IF and IH staining, the rAAV 2/9-treated dam demonstrated robust IF and IH staining of the glomeruli and renal tubules. Additionally, we demonstrated low but persistent presence of both rAAV2 and rAAV 2/9 DNA in the kidneys of pups exposed in-utero. Second litters were evaluated for both dams and neither showed evidence of the viral vectors, consistent with absence of germ line cell transmission of integrated vector.
Recent advances in the identification of genes responsible for congenital anomalies of the kidney and urinary tract have yielded several new targets for gene therapy. However, many of these conditions have their onset prior to birth and have a high rate of mortality and morbidity despite optimal treatment. Specifically, nephrotic syndrome, type 1 results from defects in the slit diaphragm structure resulting in massive proteinuria and end-stage renal disease. The gene mutated in this condition, NPHS1, has been well characterized, and recently, a 1.25 kB minimal promoter region was isolated that may limit expression to the glomerular basement membrane and podocytes.30
Using the pseudotyped AAV 2/9 vector, we were able to deliver eGFP expression to all tissues analyzed when its expression is driven by the ubiquitous CMV promoter. When this promoter was replaced by the minimal NPHS1 promoter, we were able to achieve kidney-specific transgene expression stable out to 12 weeks as well as delivering the vector to fetal kidneys by a simple maternal tail vein injection. Such a vector may allow us to guide gene delivery into the glomerular component of the kidney and raises the tantalizing possibility of abrogating or ameliorating the genetic defect.
A limitation of this study is the low level of expression in the pups compared with the maternal kidneys. We believe that this is a direct result of the route of administration. AAVs have a high affinity for the liver, and tail vein administration would ensure that a significant proportion of the vector would pass through the liver prior to the uterus. We are currently pursuing other options of administration in the mouse model to optimize both dosage and route of administration. However, when applied to a larger mammalian model, ultrasound-targeted administration of this vector will likely not be subject to this limitation. Additionally, we did not assess for the development of a maternal immune response to the vector. This will be evaluated in future studies as well as longer time courses to determine if subsequent administration of the vector is possible.
In conclusion, this study demonstrates the first use of a rAAV vector to produce kidney-specific transgene expression. Previous studies have shown transgene delivery to the kidney that was also expressed in the liver. Furthermore, we were able to demonstrate that this vector could be systemically administered to pregnant dams with successful transgene expression in the fetal kidneys that is stably expressed out to 12 weeks of life. This was accomplished by the use of the pseudotyped rAAV 2/9 vector containing the NPHS1 minimal promoter region driving eGFP expression. Although further evaluation of the dosing, vector, and route of administration are needed, maternal tail vein injection of rAAV 2/9 represents a possible avenue for the delivery of gene therapy vectors, as it readily crosses the placental interface and produces stable expression. The pseudotyped rAAV 2/9 vector containing the minimal NPHS1 promoter may be a useful tool for the targeting of genetic fetal therapies for congenital anomalies of the kidney and urinary tract and early-onset renal diseases as well as in further elucidation of the embryonic development of the kidney.
Materials and Methods
rAAV vector construction and production
rAAV 2/9-CMV-eGFP is a single-stranded pseudotyped 2/9 AAV vector containing the CMV promoter driving expression of the eGFP gene. rAAV 2/9-NPHS1-eGFP is a single-stranded pseudotyped 2/9 AAV vector containing the 1.25 kb upstream promoter region of the mouse NPHS1 (Nephrin) gene29 driving expression of the eGFP gene. These viral vectors were prepared at the University of Iowa Gene Transfer Vector Core as described previously.33 Sf9 cells were cultured in suspension using the WAVE bioreactor (GE Life Sciences, Piscataway, NJ) at densities of 1 × 106 cells/ml in 400 ml of media. Sf9 cells were coinfected with BacRep, BacCap, and BacGFP at a multiplicity of infection for 5 for each construct.
rAAV2-CMV-eGFP is a single-stranded serotype 2 AAV vector containing the CMV promoter driving expression of the eGFP gene. HEK293 cells were cultured in Dulbecco’s modified Eagle’s medium-F12 supplemented with 10% fetal calf serum (Life Technologies, Carlsbad, CA) in suspension. HEK293 cells were triple transfected with pHelper, pRep2/Cap9, and pCMVeGFP plasmids at a multiplicity of infection of 5 each.
For both vectors, the cells/media were centrifuged 68 to 72 hours posttransfection, and the media was discarded. The cells were resuspended in lysis buffer (50 mmol/l Tris, 200 mmol/l NaCl, 1.0 mmol/l MgCl and 1.0 mmol/l CaCl at pH 8.5). The cells were nitrogen fractioned, and the virus released using a cell disruption bomb (Parr Instrument Company, Moline, IL). The viral lysate was centrifuged to remove the cell debris, and the vector was purified by iodixanol gradient and ion exchange chromatography using Mustang Q membranes (Pall Life Sciences, Ann Arbor, MI). Virus was eluted with 250 mmol/l NaCl and 20 mmol/l Tris, pH 8.0. Physical titers were determined by Q-PCR. Viral vectors were purified by CsCl purification and dialyzed in phosphate-buffered saline supplemented with 5% sorbitol and F68.
Animal procedures
Tail vein injections were performed by placing the animals in the Broom-style Rodent Restrainer (Plas Labs, Lansing, MI), and their tails were warmed before the injection. The injections were carried out using 28.5 gauge needles. All injection volumes were normalized to 150 µl of normal saline. All the mice recovered from the injections quickly without loss of mobility or interruption of grooming activity. Females were checked daily for the presence of vaginal plugs, that if observed were recorded as E0.5. Sacrifice was by ketamine/xylazine intraperitoneal injection at 100/10 mg/kg followed by intracardiac perfusion with phosphate-buffered saline. All experiments involving animals were conducted in accordance with the Institutional Animal Care and Use Committee of the University of Iowa.
Vector administration
Adult mice with ubiquitous promoter.
One male and one female C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) were injected at 18 weeks of life with 1 × 1012 vg rAAV 2/9-CMV-eGFP. At 8 weeks postinjection, the animals were sacrificed.
Pregnant mice with ubiquitous promoter.
Recombinant AAV vectors were administered to 17-week-old pregnant C57BL/6 dams at E12.5 by tail vein injections. Injections consisted of 8.4 × 1012 vg of rAAV 2/9-CMV-eGFP (n = 1), saline control (n = 1), or 1.3 × 1012 vg of rAAV2-CMV-eGFP (n = 1). Pups were weaned at 21 days. Dams were sacrificed at 8 weeks postinjection. For rAAV 2/9-CMV-eGFP–exposed pups (n = 12), two pups were sacrificed at 2, 4, 6, 8, 10, and 12 weeks of life. For rAAV2-CMV-eGFP–exposed pups (n = 9), two pups were sacrificed at 2, 4, and 6 weeks, and one pup was sacrificed at 8, 10, and 12 weeks. Saline-injected pups (n = 10) were sacrificed at similar time points with one pup at 10 and 12 weeks.
Pregnant mice with kidney-specific promoter.
In order to limit vector expression to fetal kidneys, we injected 17-week-old pregnant C57BL6 dams at E12.5 by tail vein injection. Injections consisted of 1.0 × 1012 vg of rAAV 2/9-NPHS1-eGFP (n = 3) or rAAV 2/9-CMV-eGFP (n = 3) vectors or saline sham (n = 2). Pups were again weaned at 21 days, dams were sacrificed at 8 weeks postinjection, and pups were sacrificed at 2, 4, 6, 8, 10, and 12 weeks of life (NPHS1: n = 31/CMV: n = 29/saline: n = 19).
Assessment of vector integration in germline cells.
In order to verify that the rAAV vector did not alter the maternal germline cells, the dams were allowed to breed again following delivery of the first litter. The second litters (n = 9) for each of the two dams were sacrificed following a similar schedule as the first litters (two pups sacrificed at 4, 6, 8, and 10 weeks and one pup at 12 weeks of life).
Tissue processing and analysis
At the time of sacrifice, kidney, liver, lung, heart, brain, and spleen tissues were harvested and divided for snap freezing for RNA/DNA collection or whole mount. Tissues for IH IF staining were immediately placed in 4% paraformaldehyde for 2 hours followed by transfer to 30% sucrose overnight prior to embedding in Tissue-Tek OCT Compound (Sakura Finetek, Torrance, CA) and frozen.
Immunohistochemistry
Ten micrometer frozen sections of formalin-fixed tissues were treated with 0.1% Triton X-100 followed by 0.3% hydrogen peroxide in methanol to quench the endogenous peroxidase activity. Blocking was performed with 5% normal goat serum plus 5% bovine serum albumin followed by overnight incubation with rabbit anti-GFP antibody (1:400) followed by overnight incubation with secondary goat antirabbit antibody (1:200) (Invitrogen, Carlsbad, CA). Anti-GFP antibodies were detected by avidin–biotin complex formation using the peroxidase-coupled Vectastain Elite ABC reagent (Vector Laboratories, Burlingame, CA) and detection by DAB peroxidase substrate kit (Vector). Sections were cleared in ethanol and permanently mounted.
Immunofluorescence
Ten micrometer frozen sections of formalin-fixed tissues were treated with 0.1% Triton X-100. Blocking was performed with 5% goat serum plus 5% bovine serum albumin followed by overnight incubation with rabbit anti-GFP antibody (1:400; Invitrogen). Anti-GFP antibodies were detected by fluorescence-conjugated goat antirabbit antibody IgG Alexa Fluor 488 (1:500; Invitrogen) and treatment with mounting medium (Vector). Tissues were then examined with a Zeiss 510 confocal microscope using FITC fluorescence filter (Carl Zeiss Microimaging, Thornwood, NY).
To confirm the location of the GFP expression in the kidney, multiple immunofluorescence study was performed using a podocyte marker, rabbit anti-wt1 (1:200; Santa Cruz), and goat anti-GFP (1:400; Invitrogen) antibody overnight at 4 °C, followed by incubation with Alexa Fluor 488-labeled donkey antirabbit IgG (1:200; Invitrogen) and Alexa Fluor 546-labeled donkey antigoat IgG (1:200; Invitrogen) at room temperature for 2 hours. Sections were washed, mounted with VECTASHIELD Mounting Medium with DAPI (4’,6-diamidino-2-phenylindole) (Vector Laboratories), and then examined by laser confocal microscopy (LSM 710; Carl Zeiss AG).
Quantification of transfection efficiency by qPCR
Total genomic DNA was isolated from kidney tissue from dams and pups using the DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA) in accordance with the manufacturer’s protocol. Total DNA concentration was determined by spectrophotometry using NanoDrop (Thermo Scientific, Ashville, NC) and used for real-time quantitative PCR to assess for viral genome copies per sample. Briefly, a 20 μl PCR volume contained 9 μl of DNA, 10 μl TaqMan Fast Universal PCR Master Mix (2×) (Applied Biosystems, Foster City, CA), and 1 μl of primers (200 nmol/l for forward and reverse primers and probe). The primers used for eGFP were: 5′-GAGCGCACCATCTTCTTCAAG-3′ (forward), 5′-FAM-ACGACGGCAACTACA-NFQ-3′ (probe), and 5′-TGTCGCCCTCGAACTTCAC-3′ (reverse). All samples were run in triplicate on the CFX96 Real Time System (Bio-Rad, Taunton, MA) at the following thermocycler conditions: 10 minutes at 95 °C followed by 39 cycles of 95 °C for 15 seconds and 60 °C for 60 seconds. Quantitation of viral eGFP was compared to standard curve of eGFP plasmid 103–1010 copies per microliter, and results were reported as viral GFP copies per microgram of mouse DNA.
Quantification of vector expression
RNA was extracted from dam and pup kidneys using TRIzol reagent (Life Technologies, Grand Island, NY) according to manufacturer’s recommendations. RNA was quantitated using the NanoDrop spectrophotometer. Reverse transcription of one microgram kidney RNA aliquots was performed using Super Script III First Strand Synthesis kit (Life Sciences) in accordance with the manufacturers’ protocols. Duplex real-time quantitative PCR was performed in triplicate on kidney cDNA samples using CFX96 Real-Time PCR Detection System (Bio-Rad) to look at eGFP expression with GAPDH used as an endogenous control (Applied Biosystems). Briefly, cDNA samples were diluted to 10 ng/μl, and 5 μl was used for each reaction. Reaction efficiency for each probe was verified with a standard curve of positive control cDNA samples on each plate. Reactions were run with the following thermocycler conditions: preincubation at 95 °C for 10 minutes followed by 40 cycles of denaturation at 95 °C for 15 seconds and annealing and extension at 60 °C for 1 minute. Each animal was an n of 1 in analysis. The fold difference in eGFP expression was calculated based on the 2ΔΔCT values and analyzed using one-way analysis of variance followed by Bonferroni t-tests with the software package SPSS (IBM, Armonk, NY) to determine statistically significant changes in gene expression.
Acknowledgments
This work was supported by the University of Iowa Foundation Pediatric Nephrology Research Fund, and P.D.B. is supported by grants from the National Institutes of Health (NIH P30DK079328), the Elizabeth Sueppel Fund, and the University of Texas Southwestern O’Brien Kidney Research Core Center. The authors thank Beverly Davidson, Yong Hong Chen, and the University of Iowa Gene Transfer Vector Core Lab for their assistance in the design and production of the vectors used in this publication.
The authors declare no conflict of interest.
References
- Schedl A. Renal abnormalities and their developmental origin. Nat Rev Genet. 2007;8:791–802. doi: 10.1038/nrg2205. [DOI] [PubMed] [Google Scholar]
- Song R, Yosypiv IV. Genetics of congenital anomalies of the kidney and urinary tract. Pediatr Nephrol. 2011;26:353–364. doi: 10.1007/s00467-010-1629-4. [DOI] [PubMed] [Google Scholar]
- Ahrabi AK, Jouret F, Marbaix E, Delporte C, Horie S, Mulroy S. Glomerular and proximal tubule cysts as early manifestations of Pkd1 deletion. Nephrol Dial Transplant. 2010;25:1067–1078. doi: 10.1093/ndt/gfp611. [DOI] [PubMed] [Google Scholar]
- Martinovic-Bouriel J, Benachi A, Bonnière M, Brahimi N, Esculpavit C, Morichon N. PAX2 mutations in fetal renal hypodysplasia. Am J Med Genet A. 2010;152A:830–835. doi: 10.1002/ajmg.a.33133. [DOI] [PubMed] [Google Scholar]
- Nakai H, Asanuma H, Shishido S, Kitahara S, Yasuda K. Changing concepts in urological management of the congenital anomalies of kidney and urinary tract, CAKUT. Pediatr Int. 2003;45:634–641. doi: 10.1046/j.1442-200x.2003.01782.x. [DOI] [PubMed] [Google Scholar]
- Kuwayama F, Miyazaki Y, Ichikawa I. Embryogenesis of the congenital anomalies of the kidney and the urinary tract. Nephrol Dial Transplant. 2002;17 suppl. 9:45–47. doi: 10.1093/ndt/17.suppl_9.45. [DOI] [PubMed] [Google Scholar]
- Schachtner S, Buck C, Bergelson J, Baldwin H. Temporally regulated expression patterns following in utero adenovirus-mediated gene transfer. Gene Ther. 1999;6:1249–1257. doi: 10.1038/sj.gt.3300939. [DOI] [PubMed] [Google Scholar]
- Bilbao R, Reay DP, Li J, Xiao X, Clemens PR. Patterns of gene expression from in utero delivery of adenoviral-associated vector serotype 1. Hum Gene Ther. 2005;16:678–684. doi: 10.1089/hum.2005.16.678. [DOI] [PubMed] [Google Scholar]
- Mitchell M, Jerebtsova M, Batshaw ML, Newman K, Ye X. Long-term gene transfer to mouse fetuses with recombinant adenovirus and adeno-associated virus (AAV) vectors. Gene Ther. 2000;7:1986–1992. doi: 10.1038/sj.gt.3301332. [DOI] [PubMed] [Google Scholar]
- Koppanati BM, Li J, Xiao X, Clemens PR. Systemic delivery of AAV8 in utero results in gene expression in diaphragm and limb muscle: treatment implications for muscle disorders. Gene Ther. 2009;16:1130–1137. doi: 10.1038/gt.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard S, MacKenzie TC, Radu AP, Hayashi S, Peranteau WH, Chirmule N. Long-term transgene expression in cardiac and skeletal muscle following fetal administration of adenoviral or adeno-associated viral vectors in mice. J Gene Med. 2003;5:941–950. doi: 10.1002/jgm.421. [DOI] [PubMed] [Google Scholar]
- Lipshutz GS, Titre D, Brindle M, Bisconte AR, Contag CH, Gaensler KM. Comparison of gene expression after intraperitoneal delivery of AAV2 or AAV5 in utero. Mol Ther. 2003;8:90–98. doi: 10.1016/s1525-0016(03)00132-1. [DOI] [PubMed] [Google Scholar]
- Lipshutz GS, Gruber CA, Cao Y, Hardy J, Contag CH, Gaensler KM. In utero delivery of adeno-associated viral vectors: intraperitoneal gene transfer produces long-term expression. Mol Ther. 2001;3:284–292. doi: 10.1006/mthe.2001.0267. [DOI] [PubMed] [Google Scholar]
- David AL, McIntosh J, Peebles DM, Cook T, Waddington S, Weisz B. Recombinant adeno-associated virus-mediated in utero gene transfer gives therapeutic transgene expression in the sheep. Hum Gene Ther. 2011;22:419–426. doi: 10.1089/hum.2010.007. [DOI] [PubMed] [Google Scholar]
- Binny C, McIntosh J, Della Peruta M, Kymalainen H, Tuddenham EG, Buckley SM. AAV-mediated gene transfer in the perinatal period results in expression of FVII at levels that protect against fatal spontaneous hemorrhage. Blood. 2012;119:957–966. doi: 10.1182/blood-2011-09-377630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatino DE, Mackenzie TC, Peranteau W, Edmonson S, Campagnoli C, Liu YL. Persistent expression of hF.IX After tolerance induction by in utero or neonatal administration of AAV-1-F.IX in hemophilia B mice. Mol Ther. 2007;15:1677–1685. doi: 10.1038/sj.mt.6300219. [DOI] [PubMed] [Google Scholar]
- Lipshutz GS, Flebbe-Rehwaldt L, Gaensler KM. Adenovirus-mediated gene transfer in the midgestation fetal mouse. J Surg Res. 1999;84:150–156. doi: 10.1006/jsre.1999.5588. [DOI] [PubMed] [Google Scholar]
- Boyle MP, Enke RA, Adams RJ, Guggino WB, Zeitlin PL. In utero AAV-mediated gene transfer to rabbit pulmonary epithelium. Mol Ther. 2001;4:115–121. doi: 10.1006/mthe.2001.0428. [DOI] [PubMed] [Google Scholar]
- Ochiya T, Takahama Y, Baba-Toriyama H, Tsukamoto M, Yasuda Y, Kikuchi H. Evaluation of cationic liposome suitable for gene transfer into pregnant animals. Biochem Biophys Res Commun. 1999;258:358–365. doi: 10.1006/bbrc.1999.0590. [DOI] [PubMed] [Google Scholar]
- Tsukamoto M, Ochiya T, Yoshida S, Sugimura T, Terada M. Gene transfer and expression in progeny after intravenous DNA injection into pregnant mice. Nat Genet. 1995;9:243–248. doi: 10.1038/ng0395-243. [DOI] [PubMed] [Google Scholar]
- Kikuchi N, Nakamura S, Ohtsuka M, Kimura M, Sato M. Possible mechanism of gene transfer into early to mid-gestational mouse fetuses by tail vein injection. Gene Ther. 2002;9:1529–1541. doi: 10.1038/sj.gt.3301818. [DOI] [PubMed] [Google Scholar]
- Srivastava AS, Chauhan DP, Carrier E. In utero detection of T7 phage after systemic administration to pregnant mice. Biotechniques. 2004;37:81–83. doi: 10.2144/04371ST04. [DOI] [PubMed] [Google Scholar]
- Schievenbusch S, Strack I, Scheffler M, Nischt R, Coutelle O, Hösel M. Combined paracrine and endocrine AAV9 mediated expression of hepatocyte growth factor for the treatment of renal fibrosis. Mol Ther. 2010;18:1302–1309. doi: 10.1038/mt.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Asokan A, Samulski RJ. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol Ther. 2006;14:316–327. doi: 10.1016/j.ymthe.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Lipps BV, Mayor HD. Transplacental infection with adeno-associated virus type 1 in mice. Intervirology. 1980;14:118–123. doi: 10.1159/000149172. [DOI] [PubMed] [Google Scholar]
- Isaka Y. Gene therapy targeting kidney diseases: routes and vehicles. Clin Exp Nephrol. 2006;10:229–235. doi: 10.1007/s10157-006-0442-7. [DOI] [PubMed] [Google Scholar]
- Luo X, Hall G, Li S, Bird A, Lavin PJ, Winn MP. Hepatorenal correction in murine glycogen storage disease type I with a double-stranded adeno-associated virus vector. Mol Ther. 2011;19:1961–1970. doi: 10.1038/mt.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sénac JS, Chandler RJ, Sysol JR, Li L, Venditti CP. Gene therapy in a murine model of methylmalonic acidemia using rAAV9-mediated gene delivery. Gene Ther. 2012;19:385–391. doi: 10.1038/gt.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16:1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB. Two gene fragments that direct podocyte-specific expression in transgenic mice. J Am Soc Nephrol. 2002;13:1561–1567. doi: 10.1097/01.asn.0000015614.68893.0b. [DOI] [PubMed] [Google Scholar]
- Dressler GR. Advances in early kidney specification, development and patterning. Development. 2009;136:3863–3874. doi: 10.1242/dev.034876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burguete T, Rabreau M, Fontanges-Darriet M, Roset E, Hager HD, Köppel A. Evidence for infection of the human embryo with adeno-associated virus in pregnancy. Hum Reprod. 1999;14:2396–2401. doi: 10.1093/humrep/14.9.2396. [DOI] [PubMed] [Google Scholar]
- Urabe M, Ding C, Kotin RM. Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum Gene Ther. 2002;13:1935–1943. doi: 10.1089/10430340260355347. [DOI] [PubMed] [Google Scholar]