Abstract
Efficient gene transfer to muscle stem cells (satellite cells) has not been achieved despite broad transduction of skeletal muscle by systemically administered adeno-associated virus serotype 2/9 (AAV-9) in mice. We hypothesized that cellular migration during fetal development would make satellite cells accessible for gene transfer following in utero intravascular injection. We injected AAV-9 encoding green fluorescent protein (GFP) marker gene into the vascular space of mice ranging in ages from post-coital day 12 (E12) to postnatal day 1 (P1). Satellite cell transduction was examined using: immunohistochemistry and confocal microscopy, satellite cell migration assay, myofiber isolation and FACS analysis. GFP positive myofibers were detected in all mature skeletal muscle groups and up to 100% of the myofibers were transduced. We saw gestational variation in cardiac and skeletal muscle expression. E16 injection resulted in 27.7 ± 10.0% expression in satellite cells, which coincides with the timing of satellite cell migration, and poor satellite cell expression before and after satellite cell migration (E12 and P1). Our results demonstrate that efficient gene expression is achieved in differentiated myofibers and satellite cells after injection of AAV-9 in utero. These findings support the potential of prenatal gene transfer for muscle based treatment strategies.
Introduction
Potential therapeutic applications of muscle directed gene transfer include transfer of structural protein genes such as dystrophin to treat muscular dystrophy (MD) and the use of muscle as a cellular factory to deliver secreted deficient proteins into the circulation. However, successful gene transfer, at levels required for therapeutic efficiency to muscle stem cells, remains elusive. Challenges to postnatal gene transfer to muscle relate primarily to (i) inaccessibility of the large muscle mass, and satellite cells specifically, to systemically delivered vector; (ii) the low frequency of muscle stem cells (satellite cells); and (iii) when transduction is successful, the immune response to novel transgene or vector related proteins. One strategy that may address these limitations is prenatal gene transfer. In contrast to the adult, the early gestational fetus offers the advantages of (i) small size allowing delivery of relatively large doses of vector on a weight corrected basis; (ii) greater accessibility to satellite cells during migration to form the muscle compartment and a muscle compartment which contains a relatively high frequency of satellite cells; and (iii) the potential for fetal tolerance or a greatly reduced immune response to transferred novel antigens. In previous studies, we have validated these advantages by demonstrating efficient targeting of various stem cell compartments in the developing mouse by altering the timing and mode of delivery of relatively large doses of vector, as well as reduced immune response to vector and transgene associated antigens.1–6
Satellite cells are the main progenitor cells of adult muscle.7 They sit adjacent to the myofiber under the basal lamina and are known to express markers such as Pax 7, α 7 integirin, CD34, and Myf 5. These cells have the ability to self-renew, repair damaged muscles and create new myofibers.8,9 To achieve durable gene expression in the muscle compartment for treatment of the muscular dystrophies, one must presumably achieve gene transfer to a high percentage of satellite cells.10 In skeletal muscle, gene transfer to both developed myofibers and satellite cells has proven an elusive goal. The muscular development of the limbs in mice occurs in three waves starting on post-coital day 11 (E11). However, the migration of adult muscle stem cells, or satellite cells, occurs only in the third wave from E14 until the late prenatal period.11–15 Postnatal administration of AAV-9 results in broad expression in cardiac and striated muscles16–18 but very minimal expression in muscle satellite cells suggesting that the satellite cells in their established position within muscle are relatively inaccessible to systemically administered vector. We hypothesized that in utero administration of AAV9 by the intravascular route during the period of satellite cell migration would result in more effective targeting of satellite cells than can be achieved at later developmental time points.
In this study, we report efficient transgene expression in skeletal muscle satellite cells in mice by the in utero, intravascular injection of AAV-9 at E14 and E16, with reduced ability to transduce that population of cells before their migration at E12 and after their migration at P1. Our data support the concept of developmental targeting of satellite cells in addition to the well-known proclivity of AAV-9 for mature skeletal muscle.
Results
All of our experiments were performed using aliquots of the same lot of vector; therefore the concentration of vector was uniform throughout the experiments. The volumes injected, the approximate weight of the pre and post-natal pups and the dose per weight are listed in Table 1. In summary E12, E14, E16, and P1 injection resulted in a dose of 2.46 × 1011, 1.72 × 1012, 1.56 × 1012, and 3.59 × 1011 viral particles per gram of recipient (vp/g) respectively.
Table 1. Viral dose delivered at each gestational age.
| Gestational age of injection | Volume | Dose of AAV 9 (4.67 × 1010 vp/microliter) | Weight for gestational age | Dose per weight (vp/g) |
|---|---|---|---|---|
| E12 | 1 microliter | 4.67 × 1010 vp | 190 mg | 2.46 × 1011 |
| E14 | 10 microliter | 4.67 × 1011 vp | 270 mg | 1.72 × 1012 |
| E16 | 15 microliter | 7.01 × 1011 vp | 450 mg | 1.56 × 1012 |
| P1 | 10 microliter | 4.67 × 1011 vp | 1,300 mg | 3.59 × 1011 |
Patterns of expression in muscle following early (E12 and E14) injection compared to late (E16 and P1) injection
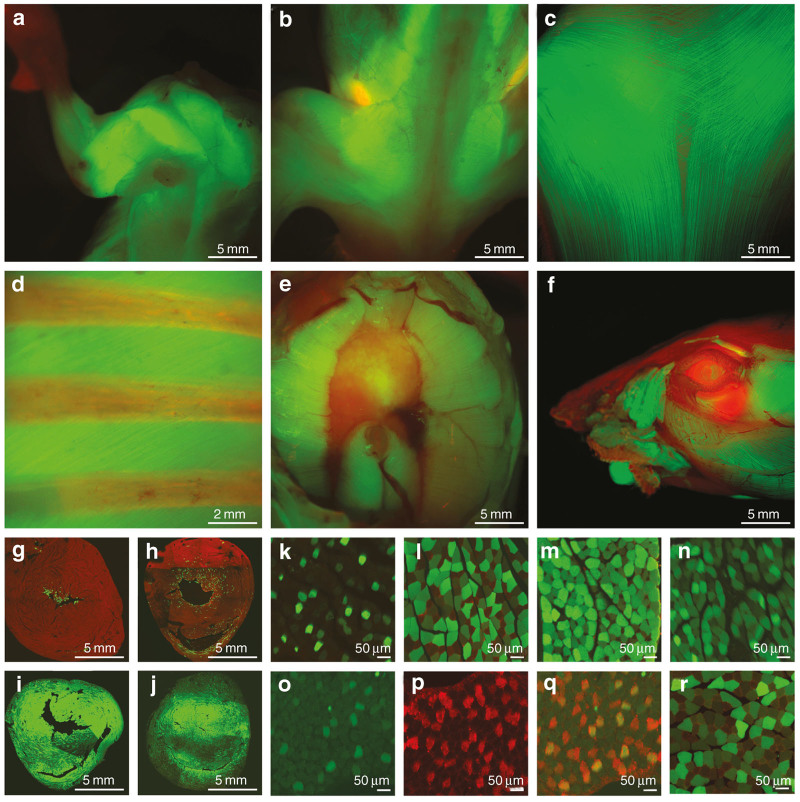
Injections at all gestations tested resulted in broad expression in total body muscle by fluorescent stereomicroscopy. Injections at all time-points resulted in GFP expression in the upper body (the triceps, biceps, deltoids, and latissiumus dorsi muscles) (Figure 1a), lower body muscles (the lower back muscles, flexor and extensor muscles of the lower limb) (Figure 1b), the panniculus carnosus (PC) muscle underneath the skin (Figure 1c), intercostal muscles (with the ribs in red) (Figure 1d), diaphragm from above (Figure 1e) and muscles of the face (Figure 1f).
Figure 1.
Following intravascular injection of AAV9 at E14 with a GFP reporter gene, a mouse examined at P30 under a fluorescent stereomicroscope reveals GFP positive muscles of the (a) upper body, (b) lower body, Panniculus carnosus (PC) muscle underneath the (c) skin, (d) intercostal muscles, (e) diaphragm (Image taken from above the diaphragm) and (f) muscles of the face. Injection at (g) E12 and (h) E14 result in expression of the endocardium of the heart. Injection at (i) E16 and (j) P1 result in expression throughout the entire myocardium. Tibialis anterior muscle, following intravascular injection at (k) E12, (l) E14, (m) E16, and (n) P1. (o–q) E12 injection results in GFP expression in slow twitch fibers (Mab 484 shown in red). (r) E14 injection results in GFP positive myofibers up to 1 year of age.
The gestational timing of in utero injection resulted in a different pattern of expression within muscles. In the heart, earlier injection at E12 and E14 resulted in expression in the endocardial myofibers of the right and left ventricle (Figure 1g,h), where injections at E16 or P1 resulted in expression in nearly 100% of cardiac myofibers (Figure 1i,j). Skeletal muscle following early injection at E12 and E14 resulted in a checkerboard pattern of expression (Figure 1k,l). Injection at or after E16 resulted in expression in greater than 90% of fibers of both types by counting fibers on histologic images (Figure 1m,n). Staining for slow twitch fibers in the E12 injected mouse showed a predominance of expression in slow twitch fibers stained in red for Mab 484 (Figure 1o–q). Injection at E14 resulted in a checkerboard pattern but both fast and slow twitch fibers were transduced. Following E14 injection, transgene expression persisted for up to 12 months (the duration of the study) (Figure 1r).
Evidence of satellite cell transduction
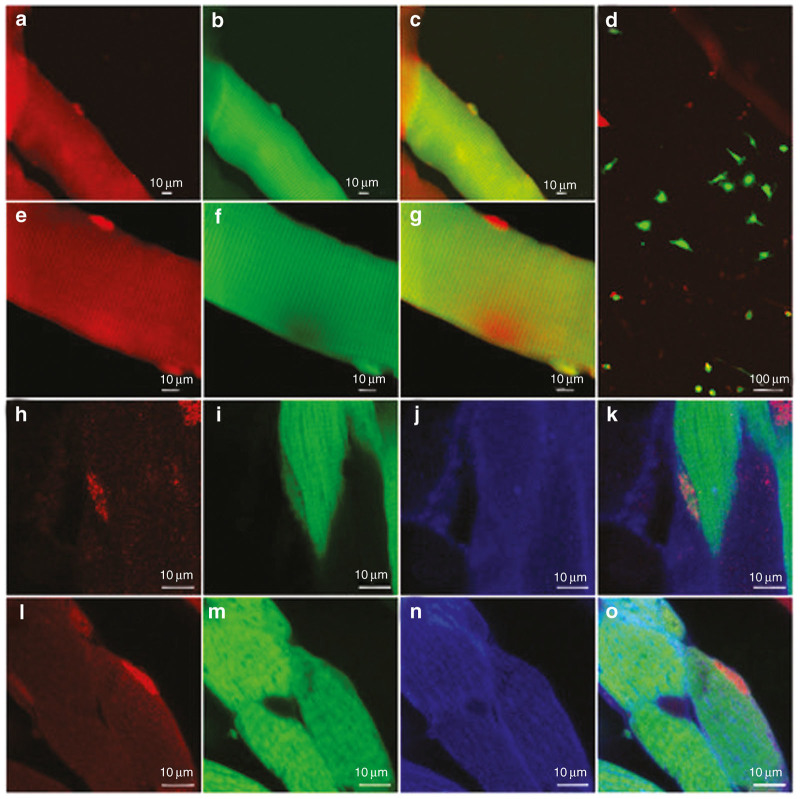
Satellite cell transduction was confirmed in vitro by the myofiber isolation assay and satellite cell migration assay. In the myofiber isolation, fibers were harvested from E14 injected Myf5-nLacZHet animals. Individual myofibers were stained for β-galatosidase in red. β−-galactosidase positive, GFP positive satellite cells can be observed on the edge of a myofiber (Figure 2a–c). Figure 2e–g demonstrates a transduced and non-transduced satellite cell on the edge of the same myofiber in a single high powered field. Satellite cell transduction was also confirmed by the satellite cell migration assay where GFP positive cells (satellite cells) can be observed migrating off of a transduced fiber in culture (Figure 2d). The fiber in the image is GFP negative as the fiber dies and loses GFP expression during the assay.
Figure 2.
Myofibers were isolated from the legs of Myf5-nlacZHet mice injected at E14 with AAV 9 carrying GFP as a reporter and stained with β-galactosidase (βgal) antibody. βgal antibody positive (red), GFP positive cells were observed on the edge of transduced myofibers. (a–c) Transduced GFP positive and untransduced GFP negative satellite cells that are (e–g) βgal positive satellite cells (red) on the edge of a transduced myofiber. Satellite cells migrate off of a transduced myofiber during satellite cell migration assay (fiber loses GFP after dying in the assay). (d) Confocal microscopy of transduced satellite cells in tibialis anterior muscle imaged at 100× of P30 mice following (h–k) E14 and (l–o) E16 injection. βgal in red. GFP in green. β-2-Laminin in blue.
Satellite cell transduction was confirmed in vivo by confocal microscopy and immunohistochemistry for GFP and β-galactosidase, at P30 following injection at E14 (Figure 2h–k) and E16 (Figure 2l–o) in Myf5-nLacZHet animals (satellite cells express a nuclear localized β-galactosidase stained in red). These cells sit underneath the basal lamina (stained for β-2-Laminin in blue) at the edge of the myofiber in the position of satellite cells. The sensitivity and specificity of our immunohistochemistry are highlighted where a GFP negative and positive satellite cell can be appreciated on a GFP positive myofiber (Supplementary Figure S1). Images were taken using a 100× objective with 1 micron optical sections, which is necessary to distinguish the cytoplasm of the satellite cell from the GFP in any transduced muscle fiber.
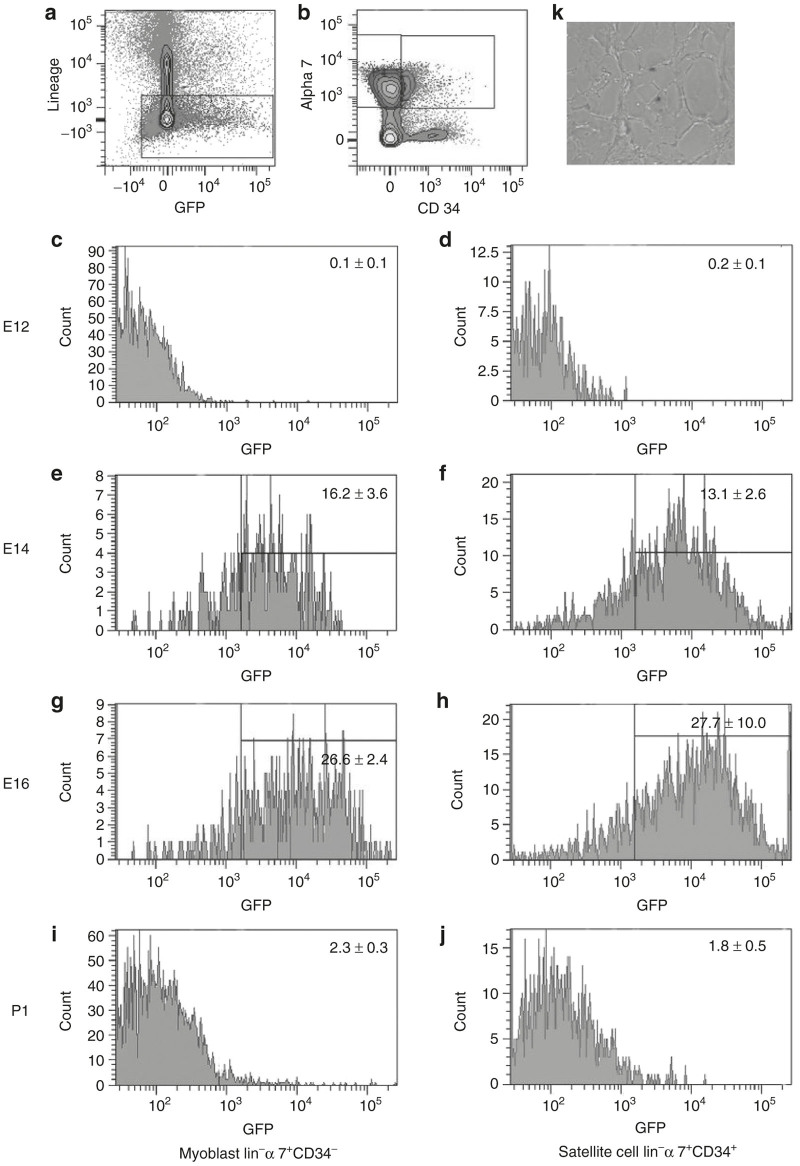
GFP expression in satellite cells was quantified by FACS analysis of myoblast preparations of 14 day old pups following injections at E12, E14, E16, and P1. Satellite cells were defined as lin− (Figure 3a), α 7 integrin+, CD34+ (Figure 3b).9 These cells from a Myf5-nLacZHet animal, display engraftment and expansion following injection into irradiated Rag mouse legs following notexin injury.9 By FACS analysis injections yielded satellite cell transduction efficiencies of: 0.2 ± 0.1% (E12), 13.1 ± 2.6% (E14), 27.7 ± 10.0% (E16), and 1.8 ± 0.5% (P1) (Figure 3d,f,h,j). The myoblast compartment, defined as lin−, α 7 integrin+, CD34−, showed comparable transduction efficiency (Figure 3c,e,g,i). Sorted GFP positive satellite cells harvested from a Myf5-nLacZHet mouse injected with AAV 9 at E14 were injected into the tibialis anterior (TA) of irradiated adult Rag mice. After engraftment and subsequent notexin injury, the muscles were harvested and examined for GFP and β-galactosidase (using β-galactosidase antibody and X-gal). Figure 3k shows β-galactosidase positive (blue) satellite cells that engraft along the edge of a myofiber following notexin injury of muscles injected with transduced, FACS sorted lin− α 7+ integrin+ CD34+ cells. However, no GFP expression was found in any muscle fibers following notexin injury in transduced satellite cell recipient mice.
Figure 3.
FACS analysis. Myf5nlacZ/+ mice underwent Intravascular injection with AAV 9 carrying GFP as a reporter gene at E12, E14, E16, and P1. Following myoblast prep of injected legs, cells were analyzed by FACS. Lineage negative cells were gated upon (a). These cells were analyzed for α 7 integrin (y axis) and CD34 (x axis) (b) and revealed two peaks: lineage negative, α 7 positive, and CD34 positive satellite cells and lineage negative, α 7 positive and CD34 negative myoblasts. Animals injected at E12, E14, E16, and P1 were analyzed for GFP positive satellite cells and myoblasts. (c,d) E12 injected myoblasts and satellite cells revealed 0.1 ± 0.1 and 0.2 ± 0.1% GFP transduction respectively. (e,f) E14 injected myoblasts and satellite cells revealed 16.2 ± 3.6 and 13.1 ± 2.6% GFP transduction respectively. (g,h) E16 injected myoblasts and satellite cells revealed 26.6 ± 2.4 and 27.7 ± 10.0% GFP transduction respectively. (i,j) P1 injected myoblasts and satellite cells revealed 2.3 ± 0.3 and 1.8 ± 0.5% GFP transduction respectively. (f) The GFP positive Satellite cells were injected into irradiated Rag mice and following engraftment, injury with notexin and healing failed to show GFP positive muscle fibers but (k) X-gal (blue) cells could be found in the satellite cell position.
GFP expression in other organs
Table 2 describes transgene expression in other tissues following intravascular injection imaged by fluorescent stereomicroscopy. In summary brain, and liver show expression throughout the gestational injection time-points tested. GFP positive cells in the thymus and lung become evident following E16 and P1 injection but are endothelial cells. Adrenal tissue and skin show expression following E16 and P1 injection. The splenic capsule had GFP positive cells but the splenic tissue never expressed GFP. The bowel and kidney were devoid of GFP expression following all gestational injection time-points.
Table 2. Expression in other tissues following E12, E14, E16, and P1 injection (N = 3 for each gestational age).
| Organ | E12 | E14 | E16 | P1 |
|---|---|---|---|---|
| Brain | + | + | + | + |
| Liver | + | + | + | + |
| Thymus | − | − | + | + |
| Lungs | − | − | Endothelium | Endothelium |
| Adrenal | − | − | + | + |
| Skin | − | − | + | + |
| Spleen | − | − | − | − |
| Kidney | − | − | − | − |
| Bowel | − | − | − | − |
Discussion
Perhaps the most compelling rationale for prenatal versus postnatal gene therapy is the developmental accessibility of stem cell populations. In previous studies, we have shown that the accessibility of different stem cell populations is dependent upon the timing (developmental stage) and mode of administration of the vector. For instance, stem cells of the eye, skin, and the central nervous system (ectoderm and neuroectoderm can be targeted by appropriately timed intra-amniotic injection,3,4,6,19 whereas stem cells of the liver, are most effectively transduced by intravascular injection at the time of liver anlage formation (E10).20 We hypothesized that the muscle satellite cell could be similarly targeted in utero. We have previously described transduction of satellite cells in the distribution of injection after intramuscular injection of lentiviral vectors at E14.5 However, in order to transduce the muscle compartment as a whole, systemic delivery is required. We have more recently developed and/or utilized methods for intravascular delivery of vector from E10 forward in the murine model but have seen very limited transduction of muscle with intravascular delivery of lentiviral vector (unpublished data).20,21 We chose AAV9 in the current study because of its known high muscle tropism and transduction efficiency with systemic administration and because of the high titers that can be generated and therefore the large number of vector particles that could be delivered by the intravascular route.16,18,22–24 With specific regard to AAV 9, in mice and dogs, it appears that compared to injections in adults, neonatal injection results in greater expression and less immune inhibition of transduction. In fact, in neonatal mice and dogs, IV injection of 2 × 1011 vp/g of AAV-9 results in broad transduction of cardiac and skeletal muscle expression without the need for immunosuppression.22,25,26 The overall success of our injections speaks to both the immunogenic profile of AAV-9 and the relatively naïve immune system of the prenatal and neonatal host. While the vector is important, earlier injection appears to be most efficient.
Due to the small size of the fetus and the relatively smaller muscle mass of the fetus, our injections can therefore be achieved using smaller volumes of vector. The dose described for neonatal mice and dogs is comparable to our injections at E12 and P1 (2.46 × 1011 and 3.59 × 1011 vp/g respectively). Injection at E12 is limited by the size of the fetal heart; in our experience only about 1 microliter can be given. Our doses at E14 and E16 were 1.72 × 1012 and 1.56 × 1012 respectively, which is at least three times greater than comparable postnatal studies. These doses do not take into account the placental circulation volume and it is notable that blood flow to muscle may change over gestation and into post-natal life. It should also be noted that the E12 injections are intracardiac, the E14 and 16 injections are into the hepatic circulation through the vitelline vein and the P1 injection are venous into postnatal circulations; although we consider all of these injections to be intravascular, there may be a difference in biodistribution of the vector.
The timing of injection was based on the premise that the best time to target satellite cells would be during their migration. In mice, the migration of nascent satellite cells, occurs from E14 until the early postnatal period.11–15 We therefore chose time points for vector administration spanning that interval. Our data confirms that systemic injection of AAV9 during satellite cell migration at E14-E16 resulted in efficient satellite cell transgene expression; whereas, injection before and after that interval resulted in very limited satellite cell transgene expression. This is despite impressive and widespread muscle expression of the marker gene at all time-points studied.
We also noted developmentally dependent differences in the type of muscle cells expressing GFP. Other groups have shown that AAV-9 injection preferentially transduced fast twitch muscle fibers.22 Our injections of postnatal mice injected at P1 did not appear to favor either fiber type, as >90% of fibers were transduced. In contrast, injection before satellite cell migration at E12 resulted in slow twitch muscle fiber transgene expression without expression in satellite cells. In fetal development, myoblast migrants from the developing somite give rise to slow myofibers; providing a likely explanation for our observation.27 Our results therefore provide further indirect support for the belief that early myoblasts do not give rise to satellite cells.13 An alternate explanation could be that the muscle fiber tropism observed is based on ontogenic differences in AAV9 receptor expression. LamR was recently identified as an AAV-9 receptor and is enriched in slow twitch muscle fibers.25,28 Developmental differences in LamR expression could contribute to the patterns of muscle expression observed. Differences in receptor profile may also account for the developmental differences in cardiac muscle expression that we observed. Early (E12 and E14) injection resulted in poor cardiac muscle expression while later (E16 and P1) injection results in nearly 100% transduction of cardiac myofibers. Our postnatal findings are similar to those reported by other groups using AAV-9 in mice.22,29 These effects are not dose related in our experiment as a much higher relative dose of AAV-9 was administered at E14 than at P1 where much higher muscle expression was observed.
Our most robust results for satellite cell transduction were following injection at E16, during satellite cell migration from the somite to their niches in muscle,11–13 resulting in 27.7 ± 10.0% satellite cell GFP expression. For assessment and confirmation of satellite cell transduction we utilized several methodologies including FACS analysis, histology at 10 and 30 days of age, satellite cell migration assay and myofiber isolation. Despite all of these methods confirming high rates of satellite cell GFP expression, when sorted GFP positive satellite cells were injected into irradiated muscle of Rag mice that were subsequently notexin injured, no GFP positive cells were seen participating in muscle regeneration. The satellite cell transplants were technically successful, in that X-gal positive donor satellite cells could be found in secondary transplant hosts, however GFP expression was lost. Following transplantation and re-expansion, these cells undergo a dramatic expansion; single sorted satellite cells undergo 14–17 doublings resulting in 21,000–84,000 cells. It is likely that there is loss of episomal AAV DNA during such an expansion.30 A more compelling explanation for the loss of GFP expression following secondary transplantation and notexin injury is the inflammatory response to the notexin injury itself. Inflammation has been shown in a mouse model to result in the loss of AAV mediated transgene expression31 and results in a loss of expression in dogs and humans.25,32–34
Currently, satellite cells can be extracted from muscles, manipulated ex vivo and injected back into a recipient.35 Any ex vivo manipulation of these cells may cause them to behave differently than native satellite cells. In utero injection of AAV would allow for rapid screening of the effects of gene products on satellite cells in their native location during development without ex vivo manipulation.
While our results provide “proof in principle” support for the potential efficacy of in utero gene therapy for the treatment of muscle disorders, there are many translational issues that remain. From a somite developmental perspective, E16, where we see our most robust results, corresponds to 10 weeks gestation in the human fetus. This is within the diagnostic window for prenatal diagnosis by early (7–8 weeks) chorionic villous sampling and perhaps in the future by maternal blood sampling for fetal free DNA. However, intravenous administration of vector at that gestational age would be challenging and, at the present time, limited to intra-cardiac injection or perhaps micro-fetoscopic approaches, the feasibility and safety of which would need to be demonstrated.2,36–38 Of equal concern are problems with scale up of this murine study to larger animals and humans, which have been well documented in many vector based strategies. However, the prenatal approach may require less scale up, due to the very small size of the human fetus at this developmental time point. Finally, AAV does not integrate at high frequency, and the likelihood of loss of episomal transgene after early gestational transduction, even in a very minimally proliferative tissue such as muscle, would be high in the context of larger, longer-lived species. Safe approaches utilizing integrating viral vectors or non-viral vectors and gene editing approaches may need to be developed to truly take advantage of the greater efficacy of prenatal delivery.
Materials and Methods
Mice
Balb/c mice (Jackson Laboratories, Bar Harbor, ME), Rag mice and Myf5-nLacZHet mice (a kind gift from MA Rudnicki, University of Ottawa, Ottawa, Ontario, Canada)39 used in this study were mated in our breeding colony. To achieve accurate time-dating, mice were mated at night and separated in the morning (E0). Mice were then palpated at E12 to E16 for pregnancy. Myf5-nLacZHet knock in mice have the gene for nuclear localized β-galactosidase gene (nLacZ) inserted into the locus of the myogenic transcription factor Myf5 gene. The expression of Myf5 is characteristic of activated satellite cells. This construct results in nuclear expression of β-galactosidase restricted to satellite cells. Pups were genotyped postnatally by PCR.
The experimental protocols were approved by the Institutional Animal Care and Use Committee at The Children’s Hospital of Philadelphia and followed guidelines set forth in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Secondary transplants were performed in irradiated Rag mice.
Preparation of AAV-9
AAV serotype-9 with a titer of 4.67 × 1013 infectious units/ml was produced and provided by the University of Pennsylvania Vector Core. The vector contained enhanced green fluorescent protein (GFP) gene under the transcriptional control of the cytomegalovirus (CMV) promoter. A pseudotyping strategy was used to produce AAV vector packaged with AAV9 capsid proteins. Recombinant AAV genomes equipped with AAV2 inverted terminal repeats (ITRs) were packaged by transfection of 293 cells as previously described.40
In utero and postnatal administration of AAV vectors
Time-dated pregnant Myf5-nLacZHet mice at postcoital days 12, 14, and 16 (E12,14 and 16) were anesthetized with inhaled isoflurane. Laparotomy was performed and vector was injected through the vitelline vein as previously described.21 E14 fetuses (190 mg) received 10 µl and E16 fetuses (450 mg) received 15 µl to account for the growth of the fetus. P1 mice (1,300 mg) were injected with 10 µl of vector through the facial vein. E12 mice (270 mg) received an intracardiac injection of 1 µl as previously described using ultrasound (Vevo 660, VisualSonics, Toronto, Ontario, Canada) guidance;20 E12 is too early for vitelline vein injection and 1 µl is the maximal injectable amount using this route.
Confocal and stereoscopic microscopy
Histological sections and myofibers were analyzed using a confocal microscope (Zeiss LSM 510, Munich, Germany). Injected mice were euthanized and examined for reporter eGFP expression under a stereoscopic fluorescent microscope (MZ16FA; Leica, Heerburg, Switzerland). The GFP positive and negative myofibers were counted on confocal images to estimate frequency of myofiber expression.
Immunohistochemistry
Skeletal muscles and hearts were fixed in 4% Formalin in phosphate-buffered-saline (PBS) for 16 hours and cryopreserved in 20% sucrose for 24 hours. Tissues were frozen in TissueTek OCT-embedding medium (Miles, Elkhart, IN) and sectioned using a cryostat (Leica, Heerburg, Switzerland). 15 micron sections were blocked in 20% normal goat serum (NGS) in PBS with 0.3% Triton X-100 and stained with primary and secondary antibodies in 5% NGS in PBS with 0.1% Triton X-100. Antibodies and concentrations were chicken anti GFP (Abcam 1:1,000), rabbit anti β-galactosidease (Invitrogen 1:1,000), and rat anti β2-Laminin (1:1,000). Secondary antibodies were Molecular probes 1:1,000 (Alexa Fluor 488 anti-chicken, Alexa Fluor 594 anti-rabbit, Alexa Fluor 633 anti-rat).
Myofiber isolation and satellite cell migration assay
Culture plates were incubated with growth media (High glucose DMEM, 10% horse serum, penicillin G (100 U/ml) and streptomycin (100 µg/ml)) for at least 30 minutes before adding fibers. 24-well plates and chamber slides were coated with 10% Matrigel(BD Biosciences #354230) and incubated at 37 °C. Myf5-nLacZHet mice that underwent injection at E14 with AAV 2/9 cmv EGFP were euthanized 14 days post-natally. Lower limb muscles were dissected, rinsed in PBS and placed in digestion media (high glucose DMEM, penicillin G (100 U/ml) and streptomycin (100 µg/ml, 10% collagenase I (Worthington, MIE4816)). While in the digestion media, the muscles sat in a 37 °C water bath for 1 hour; they were then shaken for 30 minutes and rinsed in growth media. This procedure was repeated three times. While in warm growth media fibers were separated away from other tissue using a Pasteur pipet tip. Fibers were rinsed in growth media before placing each fiber into each well of a 24-well Matrigel coated plate for satellite cell migration assay or chamber slides for myofiber isolation. For the myofiber isolation assay, fibers were given 24 hours in culture to adhere to the chamber slides. Fibers were gently rinsed, fixed, stained and examined as above. For the satellite cell migration assay, FGF (Promega 25 µg/ml) was added to the media. After 3–5 days, satellite cells crawled off of the myofibers. Cells were imaged by confocal microscopy.
FACS analysis
After euthanasia, muscle from P10 mice was harvested for satellite cell sorting. Limb and axial muscles were removed from euthanized animals and placed in PBS on ice. Muscles were minced and mixed with 800 µl of Collagenase I: 616 µl/ml (Sigma #C0130)/Dispase II: 2.4 µ/ml (Roche # 04942078001)/CaCl2: 2.5 mmol/l (Sigma)/HBSS Enzyme mix solution(Gibco #14175). The digested tissue was filtered through a 70 μm nylon mesh in a sterile funnel. Cells were centrifuged, re-suspended in 2–4 ml of HBSS/FBS: 4% (Hyclone # SH3007.03)/Hepes Buffer 1 mol/l–10 mmol/l (Gibco#15630), filtered again, counted, and exposed to antibodies. Antibodies and reagents utilized for satellite cell analysis and sorting were: anti-Mouse/Human CD45R: PECY7 conjugated (Clone # RA3-6B2, EBioscience #25-0452-82); anti-Mouse Gr-1 (Ly-6G): PECY7 conjugated (Clone # RB6-8C5, EBioscience #25-5931-82); anti-Mouse TER-119 (Ly-76): PECY7 conjugated (Clone # TER-119, EBioscience #25-5921-82); anti-Mouse CD5 (Ly-1): PECY7 conjugated (Clone # 53–7.3, EBioscience #25-0051-81); anti-Mouse Sca-I (Ly-6A/E): PECY7 conjugated (Clone #D7, EBioscience #25-5981-81); anti-Mouse CD34: Alexa 700 conjugated (Clone # RAM34, EBioscience # 56-0341-82); anti-Mouse α 7 Integrin: Biotin Conjugated (Clone #CA5.5, Sierra Bioscience); and streptavidin 750 (Invitrogen # S21008). Nonspecific Fc receptor binding was blocked by the mAb against mouse Fc receptor 2.4G2. Conjugated mAbs with irrelevant specificities served as negative controls. Propidium iodide staining was used to exclude dead cells. Analytical flow cytometry was performed on a FACSCalibur (BD Biosciences, San Jose, CA) flow cytometer/cell sorter was performed with a FACSAria cell sorter (BD Biosciences).
Secondary transplant and notexin injury of injected muscle
Thousand sorted GFP positive satellite cells harvested from a Myf5-nLacZHet mouse injected with AAV 9 at E14 were injected into the tibialis anterior (TA) of irradiated (18Gy total body radiation 3 days before transplantation) adult Rag mice. After 3 weeks, the transplanted TA’s were injured using notexin injection as previously described.41 Three weeks after notexin injection, muscles were harvested and examined for GFP and β-galactosidase (using β-galactosidase antibody and X-gal).42
Acknowledgments
This study was supported in part by the Ruth and Tristram C Colket Jr. Chair in Pediatric Surgery (A.W.F.) and by the Children’s Hospital of Philadelphia Institutional Development Funds (T.R.B.). We thank Carol Schneider and Jonathan Stitelman for their support and assistance. This work was done at The Children’s Center for Fetal Research, The Children’s Hospital of Philadelphia, Philadelphia, PA.
The authors declare no conflict of interest.
References
- Bouchard S, MacKenzie TC, Radu AP, Hayashi S, Peranteau WH, Chirmule N. Long-term transgene expression in cardiac and skeletal muscle following fetal administration of adenoviral or adeno-associated viral vectors in mice. J Gene Med. 2003;5:941–950. doi: 10.1002/jgm.421. [DOI] [PubMed] [Google Scholar]
- Davey MG, Flake AW. Genetic therapy for the fetus: a once in a lifetime opportunity. Hum Gene Ther. 2011;22:383–385. doi: 10.1089/hum.2011.3160. [DOI] [PubMed] [Google Scholar]
- Endo M, Henriques-Coelho T, Zoltick PW, Stitelman DH, Peranteau WH, Radu A. The developmental stage determines the distribution and duration of gene expression after early intra-amniotic gene transfer using lentiviral vectors. Gene Ther. 2010;17:61–71. doi: 10.1038/gt.2009.115. [DOI] [PubMed] [Google Scholar]
- Endo M, Zoltick PW, Peranteau WH, Radu A, Muvarak N, Ito M. Efficient in vivo targeting of epidermal stem cells by early gestational intraamniotic injection of lentiviral vector driven by the keratin 5 promoter. Mol Ther. 2008;16:131–137. doi: 10.1038/sj.mt.6300332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie TC, Kobinger GP, Louboutin JP, Radu A, Javazon EH, Sena-Esteves M. Transduction of satellite cells after prenatal intramuscular administration of lentiviral vectors. J Gene Med. 2005;7:50–58. doi: 10.1002/jgm.649. [DOI] [PubMed] [Google Scholar]
- Stitelman DH, Endo M, Bora A, Muvarak N, Zoltick PW, Flake AW. Robust in vivo transduction of nervous system and neural stem cells by early gestational intra amniotic gene transfer using lentiviral vector. Mol Ther. 2010;18:1615–1623. doi: 10.1038/mt.2010.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chargé SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456:502–506. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop L, Morgan JE, Partridge TA. Evidence for a myogenic stem cell that is exhausted in dystrophic muscle. J Cell Sci. 2000;113 (Pt 12):2299–2308. doi: 10.1242/jcs.113.12.2299. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Stockdale FE. Temporal appearance of satellite cells during myogenesis. Dev Biol. 1992;153:217–226. doi: 10.1016/0012-1606(92)90107-r. [DOI] [PubMed] [Google Scholar]
- Gros J, Manceau M, Thomé V, Marcelle C. A common somitic origin for embryonic muscle progenitors and satellite cells. Nature. 2005;435:954–958. doi: 10.1038/nature03572. [DOI] [PubMed] [Google Scholar]
- Hartley RS, Bandman E, Yablonka-Reuveni Z. Skeletal muscle satellite cells appear during late chicken embryogenesis. Dev Biol. 1992;153:206–216. doi: 10.1016/0012-1606(92)90106-q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang S, Rudnicki MA. The emerging biology of satellite cells and their therapeutic potential. Trends Mol Med. 2008;14:82–91. doi: 10.1016/j.molmed.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S. Skeletal muscle stem cells in developmental versus regenerative myogenesis. J Intern Med. 2009;266:372–389. doi: 10.1111/j.1365-2796.2009.02158.x. [DOI] [PubMed] [Google Scholar]
- Inagaki K, Fuess S, Storm TA, Gibson GA, Mctiernan CF, Kay MA. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther. 2006;14:45–53. doi: 10.1016/j.ymthe.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X. Clades of Adeno-associated viruses are widely disseminated in human tissues. J Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacak CA, Mah CS, Thattaliyath BD, Conlon TJ, Lewis MA, Cloutier DE. Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ Res. 2006;99:e3–e9. doi: 10.1161/01.RES.0000237661.18885.f6. [DOI] [PubMed] [Google Scholar]
- Endo M, Zoltick PW, Chung DC, Bennett J, Radu A, Muvarak N. Gene transfer to ocular stem cells by early gestational intraamniotic injection of lentiviral vector. Mol Ther. 2007;15:579–587. doi: 10.1038/sj.mt.6300092. [DOI] [PubMed] [Google Scholar]
- Roybal JL, Endo M, Radu A, Gray L, Todorow CA, Zoltick PW. Early gestational gene transfer with targeted ATP7B expression in the liver improves phenotype in a murine model of Wilson’s disease. Gene Ther. 2012;19:1085–1094. doi: 10.1038/gt.2011.186. [DOI] [PubMed] [Google Scholar]
- Merianos DJ, Tiblad E, Santore MT, Todorow CA, Laje P, Endo M. Maternal alloantibodies induce a postnatal immune response that limits engraftment following in utero hematopoietic cell transplantation in mice. J Clin Invest. 2009;119:2590–2600. doi: 10.1172/JCI38979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostick B, Ghosh A, Yue Y, Long C, Duan D. Systemic AAV-9 transduction in mice is influenced by animal age but not by the route of administration. Gene Ther. 2007;14:1605–1609. doi: 10.1038/sj.gt.3303029. [DOI] [PubMed] [Google Scholar]
- Manno CS, Chew AJ, Hutchison S, Larson PJ, Herzog RW, Arruda VR. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101:2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- Wang L, Louboutin JP, Bell P, Greig JA, Li Y, Wu D. Muscle-directed gene therapy for hemophilia B with more efficient and less immunogenic AAV vectors. J Thromb Haemost. 2011;9:2009–2019. doi: 10.1111/j.1538-7836.2011.04491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y, Ghosh A, Long C, Bostick B, Smith BF, Kornegay JN. A single intravenous injection of adeno-associated virus serotype-9 leads to whole body skeletal muscle transduction in dogs. Mol Ther. 2008;16:1944–1952. doi: 10.1038/mt.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Yue Y, Long C, Bostick B, Duan D. Efficient whole-body transduction with trans-splicing adeno-associated viral vectors. Mol Ther. 2007;15:750–755. doi: 10.1038/sj.mt.6300081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Swearingen J, Lance-Jones C. Slow and fast muscle fibers are preferentially derived from myoblasts migrating into the chick limb bud at different developmental times. Dev Biol. 1995;170:321–337. doi: 10.1006/dbio.1995.1218. [DOI] [PubMed] [Google Scholar]
- Akache B, Grimm D, Pandey K, Yant SR, Xu H, Kay MA. The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. J Virol. 2006;80:9831–9836. doi: 10.1128/JVI.00878-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Wakimoto H, Seidman JG, Seidman CE. Allele-specific silencing of mutant Myh6 transcripts in mice suppresses hypertrophic cardiomyopathy. Science. 2013;342:111–114. doi: 10.1126/science.1236921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D, Sharma P, Yang J, Yue Y, Dudus L, Zhang Y. Circular intermediates of recombinant adeno-associated virus have defined structural characteristics responsible for long-term episomal persistence in muscle tissue. J Virol. 1998;72:8568–8577. doi: 10.1128/jvi.72.11.8568-8577.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breous E, Somanathan S, Bell P, Wilson JM. Inflammation promotes the loss of adeno-associated virus-mediated transgene expression in mouse liver. Gastroenterology. 2011;141:348–57, 357.e1. doi: 10.1053/j.gastro.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly M, Kohn DB, Bartlett J, Benson J, Brooks PJ, Byrne BJ. Gene therapy for rare diseases: summary of a National Institutes of Health workshop, September 13, 2012. Hum Gene Ther. 2013;24:355–362. doi: 10.1089/hum.2013.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F, High KA. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood. 2013;122:23–36. doi: 10.1182/blood-2013-01-306647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Péault B, Rudnicki M, Torrente Y, Cossu G, Tremblay JP, Partridge T. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol Ther. 2007;15:867–877. doi: 10.1038/mt.sj.6300145. [DOI] [PubMed] [Google Scholar]
- Fan HC, Gu W, Wang J, Blumenfeld YJ, El-Sayed YY, Quake SR. Non-invasive prenatal measurement of the fetal genome. Nature. 2012;487:320–324. doi: 10.1038/nature11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfirevic Z, Sundberg K, Brigham S. Amniocentesis and chorionic villus sampling for prenatal diagnosis. Cochrane Database Syst Rev. 2003;2003:CD003252. doi: 10.1002/14651858.CD003252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oepkes D, Adama van Scheltema P. Intrauterine fetal transfusions in the management of fetal anemia and fetal thrombocytopenia. Semin Fetal Neonatal Med. 2007;12:432–438. doi: 10.1016/j.siny.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Hadchouel J, Tajbakhsh S, Primig M, Chang TH, Daubas P, Rocancourt D. Modular long-range regulation of Myf5 reveals unexpected heterogeneity between skeletal muscles in the mouse embryo. Development. 2000;127:4455–4467. doi: 10.1242/dev.127.20.4455. [DOI] [PubMed] [Google Scholar]
- Limberis MP, Wilson JM. Adeno-associated virus serotype 9 vectors transduce murine alveolar and nasal epithelia and can be readministered. Proc Natl Acad Sci USA. 2006;103:12993–12998. doi: 10.1073/pnas.0601433103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JG, Morgan JE. Muscle precursor cells injected into irradiated mdx mouse muscle persist after serial injury. Muscle Nerve. 1999;22:174–185. doi: 10.1002/(sici)1097-4598(199902)22:2<174::aid-mus5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.