Abstract
Microfluidic, cellular co-cultures that approximate macro-scale biology are important tools for refining the in vitro study of organ-level function and disease. In recent years, advances in technical fabrication and biological integration have provided new insights into biological phenomena, improved diagnostic measurements, and made major steps towards de novo tissue creation. Here we review applications of these technologies specific to the cardiovascular field, emphasizing three general categories of use: reductionist vascular models, tissue-engineered vascular models, and point-of-care diagnostics. With continued progress in the ability to purposefully control microscale environments, the detailed study of both primary and cultured cells may find new relevance in the general cardiovascular research community.
Keywords: Microengineering, vascular models, point-of-care diagnostics, microfluidics, three-dimensional cell culture
1. Applications of microfluidic models in cardiovascular science: an overview
As cardiovascular research increasingly emphasizes the molecular aspects of cardiovascular disease, predictive in vitro cellular models are playing greater roles in hypothesis testing and generation. Microfluidic technologies provide several relevant solutions for cardiovascular science: (i) lower cost associated with easily-available fluidic materials and smaller sample volumes and (ii) precise control over flow, including shear stress and pulsatility, factors relevant to the in vitro investigation of cardiovascular phenomena.
Besides insight into fundamental biological phenomena, clinical applications include the screening of compound libraries, circulating cell capture and point-of-care diagnostics. With the pharmaceutical industry spending an estimated US$1 billion per compound delivered to clinical application,1 there is a powerful financial motive to move away from animal models to affordable models that use human cells in predictive, in vitro culture systems. Half of all drug failures are attributed to efficacy and toxicity problems specific to human subjects and not predicted by animal models.2,3 Thus, advanced culture techniques incorporating human samples hold great interest.
An offshoot of the microelectronics industry, early microfluidic devices were fabricated in silicon and glass using expensive and rigid photolithography and etching methods. In the early 1990s, Whitesides et al.4 introduced soft lithography fabrication methods using poly (dimethylsiloxane) (PDMS) polymers, making lithography cheap and accessible to biologists. Among the many benefits of PDMS are its low toxicity, high gas permeability, simple fabrication process, and excellent optical transparency, the latter allowing direct readouts and quantitative analysis during the investigation of biophysical phenomena.
It soon became clear that the experimental use of microfluidics carried key advantages over existing 2D platforms. Microfluidic devices could be used to design reductionist models of vascular biology, such as to recapitulate fluid flow found in in vivo conditions. More recently, microfluidic devices have been used to approximate the complex physiology of 3D tissue in vitro, or what we define in this review as integrative vascular models. A popular direction in biological engineering in recent years is the development of ‘organ-on-a-chip’ systems where human cells and tissue are grown in microfluidic chips to better represent in vivo organ structure and function.5
Finally, high-throughput experiments were made possible due to the low consumption of expensive reagents and cells (10–100 µL range). These experiments could be carried out in microfluidic chips that were patterned in parallel, allowing for assays on multiple cell types, drugs, cytokines, and shear rates simultaneously using a single sample of a patient's blood. Because of the small dimensions of the devices, fluid flow is typically laminar, permitting the introduction and withdrawal of individual or combinatorial signals (e.g. growth factors and cytokines) in a systematic and well-defined manner.6
2. Scope of the review: recent progress in the generation of cardiovascular disease models
Here, we will review the recent advances of microfluidic platforms in the generation of cardiovascular models, with an emphasis on in vitro models of cardiovascular disease states and their potential impacts on therapeutic and diagnostic translation. Specifically, this review covers proof-of-concept examples in three major areas of cardiovascular research: (i) reductionist models for disease modelling in atherosclerosis, thrombosis, and other haemodynamic disorders; (ii) integrative models that incorporate more physiological features and approach tissue level physiology, and (iii) point-of-care devices for cardiovascular disease diagnosis and therapy. Our goal is not to comprehensively review the technical literature of microfluidic engineering. Rather, we focus on recent developments that have attempted to answer cardiovascular questions where research is currently hindered by the limitations of current technologies.
Likewise, the authors of this review note that no in vitro method—2D or 3D—will fully recapitulate human vascular physiology or disease. Taking into consideration factors such as cost, ethics, and technical limitations of animal experimentation, researchers moving from bench to bedside are still in search of a scientific bridge between simple cell culture and complex in vivo models. Thus, the relatively recent ability to observe cellular movement and vascular changes in real-time creates an early test bed for pharmaceutical screening and opportunities for point-of-care diagnostics.7
3. Reductionist models for disease modelling
3.1. Microfluidic studies of atherosclerosis and arterial function
The use of microfluidics in the study of atherosclerotic disease enables modelling of the biochemical and haemodynamic environments found in vulnerable or stenotic plaques. The importance of haemodynamic shear in vascular health and disease has long been a subject of interest. It is generally agreed that steady laminar flow contributes to a normal endothelial phenotype, while flow disturbances such as recirculation and separation such as that often found near arterial branches or stenoses are considered atherogenic.8 In the case of arterial stenosis, how the high-shear stenotic region may lead to disease progression is not well-understood.
Westein et al.9 engineered stenotic microchannels to study the dynamics of thrombus formation. The channels were 300 µm wide and 52 µm high, and a stenosis was incorporated by an eccentric circular indentation that obstructed up to 80% of the lumen. The glass bottom of the channels was coated with patches of von Willebrand factor (vWF) and fibrinogen to initiate thrombosis. A key feature in this model was its microfluidic nature—with Reynolds number = 0.4 in the channel and 1.2 at stenosis. The Reynolds number is a physical quantity that characterizes the relative scale of the inertial over viscous forces of the flow: at low Reynolds numbers (<10), viscous drag dominates and flow is laminar and orderly; at high Reynolds numbers (>500), flow disturbances or even turbulence may occur in branches or irregular geometries.8 Therefore, this study highlighted the effects of laminar shear per se by avoiding additive effects of flow disturbances. The differences in flow rates and channel dimensions result in the different Reynolds numbers observed between in vitro and in vivo conditions: Reynolds numbers are higher for a human carotid (2600/4300) and mouse carotid (28/44), upstream of a stenosis and at a stenosis, respectively. Nonetheless, the channels were designed and perfused such that the main fluid mechanical and geometrical parameters match in vivo conditions, namely the angle of the stenosis inlets, the wall shear rates in stenotic and non-stenotic areas, and the flow elongation rates.
Upon perfusion of whole blood, researchers observed platelet aggregation in stenotic channels with the degree of aggregation correlating with the extent of stenosis. Importantly, these effects were due to the presence of a shear gradient induced by the stenotic geometry but not the absolute magnitude of shear, because perfusion at a shear rate of 8000s−1 in straight channels—the highest shear caused by an 80% stenosis—did not lead to platelet aggregation. Further, researchers demonstrated the vWF-dependence of platelet aggregation and that blockade of glycoprotein Ibα with a monoclonal antibody eliminated the rise in platelet calcium concentrations. These data showed the involvement of vWF-glycoprotein Ibα binding in shear gradient-dependent platelet activation in this model.
In the setting of myocardial infarction (MI), post-stenotic thrombus burden is a predictor of major cardiovascular events and is caused both from embolization of thrombi from the primary stenosis in addition to in situ effects related to flow and downstream endothelial biology.10,11 To examine whether endothelial cells may be involved in post-stenotic thrombotic events, HUVECs were cultured on the bottom of the channels, replacing the vWF/fibrinogen patches. HUVECs near the post-stenotic regions were found to secrete vWF, which in turn led to platelet aggregation. One question that remains, however, is whether the shear profile of the endothelial monolayer sufficiently mimics what is expected in vivo, given that the experimental stenosis was imposed on the side wall in the microfluidic device while the endothelium was cultured on the bottom. Nonetheless, this work recapitulated the results of a translational in vivo study that also demonstrated preferential thrombus formation in the downstream region of a stenosis.12
In addition to shear stresses, haemodynamic forces in the cardiovascular system consist of pressure and stretch, and are inherently dynamic and transient. However, studies of these aspects on endothelial function have traditionally focused on a single variable while employing non-physiological conditions on others—an example being cone-plate rheometry which controls shear but subjects cells on a rigid substrate to atmospheric pressure. To address these shortcomings, Estrada et al.13 developed an in vitro model that replicates the waveforms of the shear, pressure, and stretch found in arterial regions with normal or disturbed flow characteristics. Here, they imposed pulsatile, physiological pressure, and stretch on endothelial cells by using a peristaltic pump and a compliant membrane as the substrate. Disturbed flow seen in atherosclerosis susceptible regions, such as the abdominal aorta in the infrarenal segment at the wall distal to the inferior mesenteric artery, was mimicked by allowing retrograde flow which results in ∼10-fold decrease in mean flow rate and shear stresses. Phase contrast microscopy showed that >70% of the human aortic ECs attained an ellipsoidal shape and showed good alignment in the direction of flow, compared with >90% of cells cultured under disturbed flow that instead exhibited a rounded or cuboidal shape and random orientation. Cells under disturbed flow also showed low and discontinuous levels of expression of β-catenin, indicating compromised endothelial monolayer permeability.
The above examples demonstrate several key advantages for modelling human diseases with microfluidic cell cultures, including the fabrication of highly consistent geometries on the microscale and optical transparency for quantitative microscopy. These properties are also well suited to systematic evaluation of novel therapeutics before pre-clinical studies. Researchers in the treatment of atheroma often validate drug candidates using hypercholesterolaemic mouse models—the apo-E-deficient mouse (ApoE−/−) and LDL receptor-deficient mouse (Ldlr−/−),14 which carry limitations of expense and comparatively long experimental time.15
To find commonality between simple cell culture and animal studies, Korin et al.16 designed a 3D model of vascular stenosis that mimics blood vessels with 90% lumen obstruction. They used the microfluidic system to test their shear-activated nano-therapeutics (SA-NTs), which are microparticles (1–5 µm in diameter) that release smaller poly(lactic-co-glycolic acid) (PLGA) nanoparticles (180 ± 70 nm) in response to shear at stenotic regions (Figure 1A and B). Computational fluid dynamics modelling of flow in the microfluidic device demonstrated that a shear stress of 10 dyn/cm2 upstream from the occlusion resembled fluid shear stresses in highly constricted arteries in vivo.17 Perfusion of the SA-NTs through these devices resulted in a 16-fold increase in the release of free NPs, which accumulated in the endothelial cells distal to the narrowed region (post-stenosis). The researchers demonstrated in vitro the potential for SA-NTs to treat life-threatening embolic occlusions. SA-NTs carrying nanoparticles coated with serine protease tissue plasminogen activator (tPA) shrank preformed fibrin clots (250 ± 150 μm diameter) that partially obstructed flow in the channels by 50%. Their results were validated in vivo using a mouse mesenteric injury model with ferric chloride-induced arterial thrombus and a mouse pulmonary embolism model.
Figure 1.
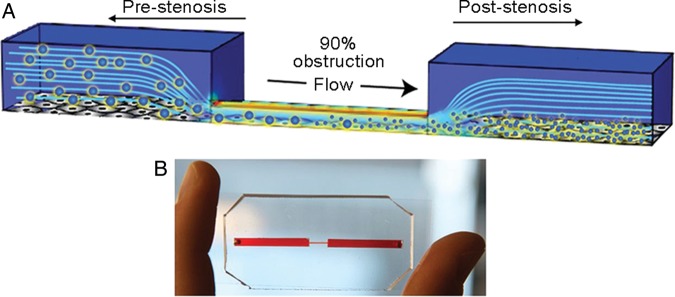
Microfluidic studies of arterial stenosis. (A) Schematic representation of a 3D microfluidic model of vascular narrowing that mimics a blood vessel with 90% lumen obstruction. (B) A photograph of the PDMS-based device that mimics vascular stenosis. Reproduced with permission from Korin et al.16.
3.2. Blood cell properties in haemodynamic disorders
Because their dimensions replicate the diameter of small blood vessels, microfluidic systems are well suited to model blood cell deformability and pathology in diseases such as reperfusion injury in stroke and MI, diabetes mellitus, and essential hypertension.18 Forsyth et al.19 studied the effects of RBC deformation on cell flow dynamics in a microfluidic device. Their microchannels had a cross-sectional height of 18 µm, which caused the cells to experience a pressure-driven flow, similar to conditions found in arteriole-sized vessels in vivo. They showed that glutaraldehyde, a non-specific fixative and stiffening agent, was more effective in increasing the rigidity of the RBCs compared with diamide, which cross-links spectrin skeletal membrane proteins in RBCs. Data from coupled high-speed camera and image-processing revealed the RBCs displayed three different types of motion due to the increased shear rate in the channel constriction: stretching, tumbling, and recoiling, all characteristic features of more rigid cells.
Instead of studying single cell behaviour, Rosenbluth et al.20 studied the population behaviour of blood cells as they traversed a 6 × 13 µm microfluidic capillary network. In sepsis and acute respiratory distress syndrome (ARDS), increased rigidity of neutrophils has been associated with retention of these cells in capillaries and subsequent tissue ischaemia.21 Inflammatory mediators such as N-formyl-methionyl-leucylphenylalanine (fMLP) and TNF-α have been shown to be responsible for this stiffening behaviour.22 Rather than due to an entire population of moderately stiffer cells, Rosenbluth et al.20 showed that the obstruction of capillaries seen in the normal inflammatory response, in sepsis, and in ARDS was caused by a small outlier population of neutrophils. Such information would not be obtainable without the use of microfluidic flow models and automated real-time imaging, analysis, and quantification.
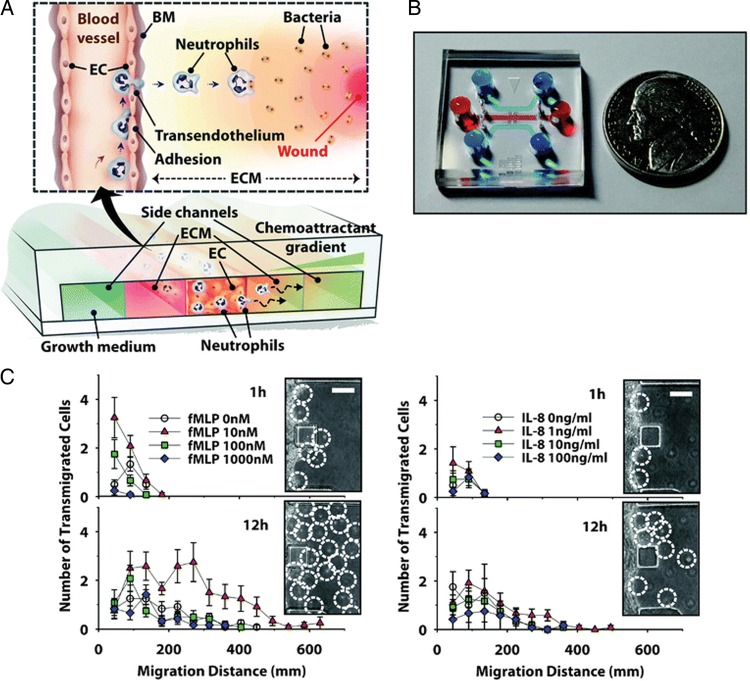
Microfluidic technology can also be used to study the transendothelial migration (TEM) of neutrophils in inflammatory states. Previous assays to study leucocyte chemotaxis, such as the Boyden chamber assay, have not been able to reconstitute the stabilized chemokine gradients needed to construct in vivo-like conditions of inflammation. Microfluidic systems, on the other hand, provide a 3D microenvironment and allow for accurate control over spatial and temporal resolution.
Microfluidic systems have been used to study leucocyte TEM, a multi-step process in the inflammatory response that involves leucocyte rolling on the activated endothelial surface, firm adhesion, extravasation, and migration through the extracellular matrix (ECM) along a concentration gradient of inflammatory molecules (Figure 2A and B). To investigate the in situ 3D inflammation response of neutrophils to precisely regulated chemoattractant gradients, Han et al.23 established a stable chemoattractant gradient of fMLP and human interleukin-8 (IL-8) across the endothelial cell monolayer and ECM (Figure 2C and D). Confluency was confirmed by permeability experiments using 10 kDa fluorescein isothiocyanate (FITC)-conjugated dextran. Using a three-channel microfluidic device, the researchers observed the contribution of specific inflammatory molecules to the TEM process and imaged the inflammatory process in a real-time manner from initiation. Interestingly, in the absence of an intact endothelial cell monolayer, only small numbers of neutrophils migrated relatively short distances through the collagen gel, even in the presence of optimum fMLP concentration gradient. In addition, the researchers showed a strong inverse correlation between 3D migration of neutrophils and mechanical stiffness of the type I collagen matrix, with migration decreasing as the stiffness of the ECM fibres increased.
Figure 2.
Microfluidic studies of inflammation and transendothelial migration (TEM). (A) Schematic representation of a 3D ‘inflammation-on-a-chip’ device. The three-channel device incorporates an EC-lined channel flanked by two ECM gel regions and a chemoattractant gradient across the device. BM, basement membrane. (B) A photograph of the device. (C) Interstitial migration of neutrophils towards fMLP and IL-8, monitored in situ by phase-contrast microscopy at 1 h and 12 h after neutrophil seeding into the EC channel. Inset: white-dotted circles indicate the position of neutrophils in the ECM region. Scale, 200 um. Error bars, ± SEM (n = 12). Reproduced with permission from Han et al.23.
4. Tissue-engineered vascular models
The aforementioned microfluidic devices contained essential components for biological applications, however, challenges to these systems exist. For example, studies on the endothelium often involve a flat 2D monolayer on the glass bottom or on a vertical side wall, primarily because standard lithographic techniques yield rectangular channels which contain sharp corners. The most common materials used in microfluidic systems, PDMS and glass, are stiffer than the natural ECM in vivo24 and are impermeable to fluid flow, precluding studies of transvascular transport, transendothelial cell migration, and angiogenesis. The lack of a natural ECM also ignores the importance of interstitial flow and pressure, gradients of growth factors, or other diffusible signalling molecules, and prevents the inclusion of mural and parenchymal cells.25 In vitro culture conditions subject endothelial cells to ambient oxygen levels (21%), which is much higher than physiological levels26 and greatly increases their turnover rate.27
Several research groups have begun to engineer tissue models that incorporate more physiological features. This approach is aimed at a more realistic modelling of the native vasculature, enabling the simultaneous measurements of multiple morphometric and physiological data, and may reveal new interactions and thereby enable mechanistic understanding. These goals challenge researchers to reconsider a variety of additional factors in fabrication, materials and culture conditions.
4.1. Perfused, functional vessels in extracellular matrices
In general, microfluidic studies are carried out using rectangular channels due to the lithographic procedures involved. Using a needle-moulding technique, however, Chrobak et al.28 were able to produce completely rounded channels. They created circular microchannels (55–120 µm in diameter) in type I collagen matrices by polymerizing the gel around a small needle, and used these channels to culture confluent endothelial tubes.28 This model represents the first engineered microvessels that could be stably perfused for days in an ECM scaffold, and reconstituted several key features of microvascular physiology. For instance, endothelial activation by the classical inflammatory agonists histamine, thrombin, or TNF-α resulted in barrier breakdown as well as the adhesion of neutrophil-like HL-60 cells.28 Introduction of the potent anti-inflammatory second messenger cyclic adenosine monophosphate induced cellular quiescence and normalized the barrier function to in vivo levels—as demonstrated by the very low permeability (∼10−7 cm/s) to albumin and dextrans (10 kDa and 40 kDa) as well as the restoration of a charged barrier which may indicate the presence of a glycocalyx.29 In response to high, physiological shear stresses (>20 dyn/cm2), these microvessels displayed a strong barrier and intact VE-cadherin junctions.30 In addition, the importance of a positive transmural pressure (defined as the pressure differential between the inside and the outside of the lumen) in maintaining a stable endothelium was revealed: at zero transmural pressure, low shear resulted in vascular collapse, whereas high shear led to endothelial sprouting into the collagen scaffold.30
In more recent work, empty (cell-free) microfluidic channels that were held at atmospheric pressure were incorporated into ECM scaffolds (fibrin), providing the first lymphatic-mimicking drainage function in perfused engineered tissue constructs.31 This study demonstrated that proper drainage of interstitial fluid is, similar to native tissues in vivo, critical in maintaining tissue fluid homeostasis especially in scaffolds of physiological hydraulic conductivities (a measure of porosity).
While single tubes can be easily produced by needle molding, interconnected networks of channels in hydrogels are best generated using lithographic techniques.32 It is worth pointing out the intrinsic merits of using ECM hydrogels here: because they are natural substrates for cell adhesion and can be remodelled by cells (as opposed to PDMS), the endothelium is capable of remodelling the channels and rounding up the sharp corners, producing channels that are still rectangular, but with curved corners.33 These properties enabled the generation of patterned, confluent microvascular networks that are entirely encased by ECM scaffolds.33,34 Zheng et al.35 designed such a perfused microvascular network in collagen scaffolds (Figure 3A) to study angiogenesis, pericyte-endothelial cell interactions, and thrombotic events during an inflammatory response. Vascular networks composed of HUVECs were allowed to mature for 7 days in growth medium before angiogenic factors (VEGF, bFGF, and PMA) were introduced for an additional 7 days, causing angiogenic sprouts to form. Co-culture with embedded human brain vascular pericytes led to two distinct phenotypes. In one case, endothelial cells sprouted into the matrix but the barrier remained intact. In the other, the endothelium retracted from the walls of microchannels, showed increased leakiness of fluorescent dextran (70 kDa), and endothelial sprouts were absent. In both cases, pericytes were found to associate with endothelial cells, although the frequency was found to be higher in the retracted case. The complex interplay between pericytes, endothelial cells, and angiogenic factors remain to be elucidated, but the system provides the basis for further studies.
Figure 3.
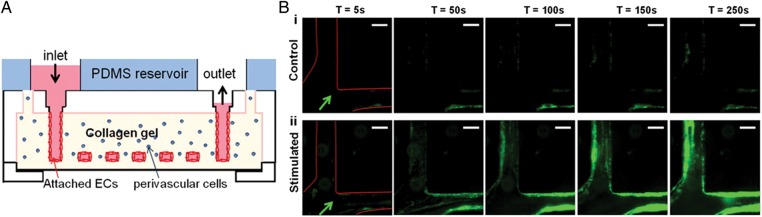
Microfluidic devices to study thrombosis. (A) Schematic representation of a collagen hydrogel scaffold embedded with perivascular cells such as human brain vascular pericytes (HBVPs) and microchannels lined with ECs. (B) Whole blood was perfused at 10 µL/min through quiescent (control) and stimulated vessels at time points of 5, 50, 100, 150, and 250 s after initiation of stimulation. The platelets are labelled green with antibodies against platelet-specific glycoprotein IIb (integrin αIIb); an arrow indicates flow direction. Scale, 100 µm. Reproduced with permission from Zheng et al.35.
The translational potential of this tissue model in studying thrombosis was then demonstrated with the perfusion of whole blood. These mature microvascular networks were treated with phorbol-12-myristate-13-acetate (PMA), which is a potent inflammatory mediator known to stimulate the release of vWF by activating protein kinase C. Inflamed vascular networks induced rapid adhesion and aggregation of platelets within minutes, which first obstructed the lumen but were then shed into the flowing blood. After 1 h, leucocytes began to attach to the endothelium and transmigrate into the collagen matrix, and thrombi became stably adhered. In addition, 3D webs of vWF-platelet fibres transversed the lumen, further perturbing blood flow (Figure 3B). These results highlight the unique advantage of in vitro tissue systems for the modelling of human disease in 3D, and also for obtaining functional measures of perfusion and immune cell trafficking.
4.2. Angiogenesis under flow conditions
In the tumour microenvironment, the dysfunctional vasculature is often dilated and leaky, leading to increased plasma extravasation into the tissue space. To incorporate the features of interstitial flow and VEGF morphogen gradients in a model of angiogenesis, Song et al.36 sandwiched a rectangular block of collagen matrix between two parallel channels lined with confluent HUVECs. Independent fluidic ports connecting the endothelial channels were used to deliver tangential fluid shear and create gradients of the vascular morphogen, vascular endothelial growth factor (VEGF). Interstitial flow was generated by a pressure gradient across the collagen block (i.e. across the endothelial layers).
In static, no-flow conditions, addition of VEGF induced endothelial morphogenesis and invasion into the collagen gel from both vascularized channels. A low but physiological shear stress (3 dyn/cm2), however, attenuated endothelial invasion and decreased cell proliferation, and this inhibition was effective even in the presence of a VEGF gradient. The addition of the pan nitric oxide synthase (NOS) inhibitor, NG-monomethyl-l-arginine monoacetate (L-NMMA), reversed the inhibitory effects of shear stress, suggesting the involvement of shear-activated NO signalling mechanisms. Imposing interstitial flow increased the rate of endothelial morphogenesis and sprout formation both with and against the direction of flow to the same extent. Interestingly, the researchers observed that VEGF gradients can lead to different sprout morphology: positive VEGF gradients—defined as invasion towards the source of VEGF—induced filopodial extensions analogous to tip cell sprouts seen in vivo, but negative gradients instead promoted sheet-like migration analogous to vessel dilation. Together, the researchers hypothesized that these mechanical signals encourage extensive sprouting toward the leaky vessels and at the same time prevent sprouting from well-perfused vessels.
4.3. Physiological oxygen tension
Given the involvement of oxidative stress in cardiovascular disease, and that the ambient oxygen levels in standard cell culture lead to oxidative damage,37,38 it is desirable to achieve physiological levels of oxygen within in vitro disease models. Endothelial cells grown at high oxygen levels have been shown to have much higher turnover rates (1–10% per day) compared with levels in vivo (∼0.1% per day).27 Similar to other attempts to create more biologically realistic models, progress is likely hindered by technical challenges. In this case, culturing in lower oxygen tension than atmospheric requires continuous flow of low-oxygen media because PDMS is gas-permeable.
Recently, Abaci et al.39 engineered a vascular model focusing on the simultaneous application of physiological oxygen (5%) and shear stress (12 dyn/cm2) on endothelial monolayers. It was found that this culture condition provided a more in vivo-like phenotype, characterized by cell alignment to the flow direction, cellular quiescence (i.e. lower proliferation), increased expression of KLF2, eNOS, VEGF, VEGF-2, and decreased expression of ANG2. Using this condition as a physiological baseline, researchers studied the vascular response of an anti-cancer drug, 5-fluorouracil (5-FU), which is cardiotoxic in a small fraction (10%) of patients but whose mechanisms were not fully understood. It was found that 5-FU had no effects on apoptosis, but led to an increase in the number and area of intercellular gaps which indicated a compromised vascular barrier. Treatment with the vasoprotective chemical resveratrol rescued this disruption in barrier integrity, and its effects were further validated in a mouse model. Remarkably, such protective effects were not observed in endothelial monolayers cultured under conventional static culture, indicating that this physiological vascular model could better mimic in vivo responses.
To further demonstrate its utility in studying vascular pathology, the team subjected the vascular model—after it had adapted to the physiological baseline—to ischaemic conditions which contained 1% oxygen and a very low shear stress (0.01 dyn/cm2). Such conditions disrupted the localization of VE-cadherin junctions and increased the formation of actin stress fibres. Genes representative of a healthy phenotype, KLF-2 and eNOS, were downregulated, while the expression of angiogenic factors VEGF and ANG2 as well as cell-proliferation index were greatly increased. Disruption in junctional integrity was not observed in static culture conditions, again indicating that this vascular model was superior in reproducing an activated state of the endothelium in response to ischaemia.
Together, by using culture conditions that more accurately resemble the in vivo microenvironment, researchers were able to reconstitute vascular physiology that simulates in vivo phenomena. With further standardization and automation, functional studies that extend beyond molecular characterization have the potential to greatly accelerate screening of compounds for predicting drug efficacy as well as unwanted side effects.
4.4. Ex vivo models for drug testing
Although ‘organ-like’ structures can be fabricated using soft lithography, there are instances where it may be favourable to investigate ex vivo samples. Ex vivo small artery studies are carried out by mounting the arteries on two wires (isometric approach), or cannulating and perfusing the arteries with glass micropipettes (isobaric approach), neither of which are scalable due to the technical challenges of preparing the vessel for investigation.
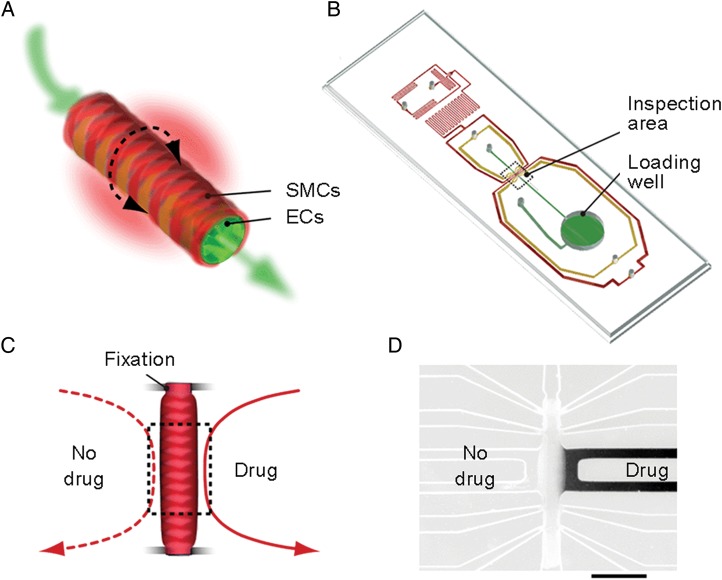
Gunther et al.40 cultured ex vivo intact mouse mesenteric artery segments in microfluidic devices to study the subtle microenvironment changes in resistance arteries that lead to a hypertensive phenotype, a major risk factor in heart disease and stroke. The arteries were reversibly immobilized for 24 h experiments and perfused and superfused using luminal and abluminal fluid streams (Figure 4A and B). To test the hypothesis that the vascular smooth muscle cells of the arterial wall behave like a syncytium, such that through communication all VSMC of the wall would contract, they exposed discrete regions of the artery wall to agonists such as phenylephrine (PE) or acetylcholine (ACh) (Figure 4C and D). Exposure to PE yielded dose-response curves similar to conventional myography, but unilateral application of PE led to asymmetric vasoconstriction that was spatially restricted to the site of stimulation. Hence, the study supports previous evidence that not all responses are conducted equally along the arterial wall,41 and that initiated events may remain highly localized in the smooth muscle layer of resistance arteries.
Figure 4.
Ex vivo arterial model. (A) Schematic representation of arterial cross-section with endothelial cells (ECs) and smooth muscle cells (SMCs). (B) Schematic representation of a microfluidic chip containing an artery loading well and artery inspection area. (C) Experimental configuration for the uneven application of an agonist (e.g. phenylephrine, PE) to two sides of the artery. (D) Fluorescence micrograph (intensity inverted) confirming that abluminally applied buffer solutions were strictly confined to the side of application. Scale bar, 500 µm. Reproduced with permission from.40.
5. Point-of-care diagnostics
5.1. Diagnostic immunoassays in point-of-care testing
Point-of-care testing (POCT) of biomarkers has gained clinical importance, fuelled by the global burden of cardiovascular disease. It is gaining particular importance in the triage and risk management of acute coronary syndromes (ACS)42 and heart failure (HF),43 as rapid diagnosis and early risk stratification are extremely important for effective treatment. These advanced lab-on-a-chip immunoassays can be used for biomarker separation with high resolution, rapid detection with high sensitivity, short analysis time, low sample and reagent consumption, low cost, and a small device fabrication footprint.44 From a public health perspective, POCT systems can significantly reduce the turnaround time required for clinical decision-making by avoiding non-essential delays such as test ordering, transporting samples to diagnostic laboratories and data reporting.45 In addition, some POCT systems are simplified to reduce the chance of operator error and use blood or saliva samples without pre-treatment or labelling.
ACS is a spectrum of presentations that comprises unstable angina and acute MI. According to the third universal definition of MI published in 2012, cardiac troponins are the primary biomarker for identifying if an MI has occurred.46 Cardiac troponin I (cTnI) is considered a ‘gold standard’ marker as it is highly specific to cardiac injury. Since any delay in diagnosis and institution of effective therapy has a detrimental impact on short- and long-term outcomes for MI patients, rapid assessment of cardiac marker elevation in whole blood is crucial when patients present with acute symptoms consistent with acute MI. In HF diagnosis, microfluidic systems that can measure B-type natriuretic peptides (BNPs) and their amino-terminal co-metabolites are crucial for the evaluation of patients in urgent care.43
Clinically available POCT systems such as the Stratus® CS Acute Care™ Diagnostic System (Siemens Healthcare Diagnostics, Inc.) and i-STAT® Analyzer (Abbott Technologies, Inc.) can provide a result within 30 min or less.47 Researchers are developing new microfluidic-based POCT technologies to improve on portability, cost, sensitivity, size, and multiplex detection, all of which may shape the future development of POCT diagnostics.
There are two commonly used methods of preparing the POCT systems for diagnostic use. The first is to insert microsphere carriers pre-coated with antibodies into microfluidic devices, and the second is to coat assay-specific antibodies into the channels of devices. In the first option, microspheres are pre-coated with antibody probes and then packed into microchannels, which provide the benefit of increasing the surface-to-volume ratio so that more of the biomarker target can be captured. Here, Ren et al.48 designed a microfluidic device to detect two acute MI-related early biomarkers, myoglobin and heart-type fatty acid binding protein (H-FABP). Magnetic microspheres were covalently conjugated with capture antibodies and inserted into the device composed of pneumatic micro-valves and a membrane mixer. The post-reaction substrate resultant was analysed using a micro-plate reader for absorbance values that correlate to the concentration of the cardiac markers. Using this set-up, the researchers detected both myoglobin and H-FABP in a buffer solution at a limit of detection (LOD) of 5 ng/mL for myoglobin and 1 ng/mL for H-FABP, which are at an equivalent or higher sensitivity compared with existing immunoassay strategies.
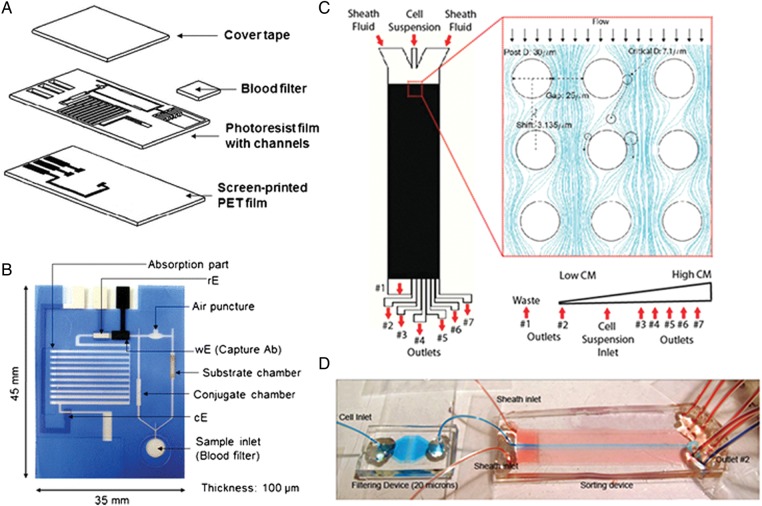
In another example of a microsphere-based immunoassay, Park et al.49 used a fully automated lab-on-a-disc device to simultaneously detect multiple protein biomarkers in serum and whole saliva. Here, a novel centrifugal microfluidic layout was used to simultaneously detect high sensitivity C-reactive protein (hsCRP), cTnI and N-terminal pro-BNP (NT-proBNP) based on a bead-based sandwich type enzyme-linked immunosorbant assay (ELISA). Three reaction chambers—initially interconnected for sample injection, incubation, and washing—were preloaded with antibody-conjugated beads against the three biomarkers, but then isolated for independent processes such as substrate incubation and final detection. Notably, the device uses the reversible actuation of multiple laser irradiated ferrowax microvalves (LIFM), allowing the channels to be reversibly closed and opened by laser irradiation for fluidic transfer. The system can be used to process up to six biomarkers per disc, but one to three units can also serve as a positive or negative control. In the study, the LOD and dynamic range using whole saliva and serum was comparable with existing ELISA assays; in addition, the use of multiple biomarkers in combination may also significantly enhance the predictive value for the risk factor.
More conventionally, antibodies are coated on microfluidic device surfaces for substrate detection. In one effort, Lee et al.50 developed an automated POCT system to detect cTnI in plasma and whole blood. The assay, which they named the surface acoustic wave (SAW) immunosensor, is based on a gold nanoparticle sandwich immunoassay packaged inside a disposable microfluidic cartridge. The targets (cTnI) were captured by AuNP-conjugated antibodies bound on the surface of the working sensor trough, while non-specific AuNP-antibody conjugates in a reference sensor acted as a positive control. The 20 min fluidic process is shorter than conventional fluorescence- or chemiluminescence-based end-point assays, and its LOD of 6.7 pg/mL cTnI using patient plasma samples is on the same scale as clinical diagnostic systems. Unlike conventional end-point assays, it also allows for real-time monitoring of events.
In another use of surface-coated POCT electrochemical immunoassay, researchers used microfluidic devices to capture hsCRP, a classic acute phase plasma protein that increases rapidly in cardiovascular disease, stroke, tissue infection, or inflammation.51 The researchers designed a microfluidic-based hsCRP test using ELISA and experimentally measured electrical current as a function of the concentration of the alkaline phosphatase (ALP)-labelled CRP antigen–antibody complex in the microfluidic chip (Figure 5A and B). The LOD of the chip was 0.1 mg/L CRP in serum samples, which is the concentration of hsCRP in a physiologically relevant range.
Figure 5.
Microfluidic point-of-care devices. (A) Schematic representation of hsCRP capture chip. (B) Photograph of chip with annotations to the various chambers and channels. Reproduced with permission from Lee et al.51 (C) Illustration of CM enrichment device with an array of 30 µm posts. CM purity increases from outlet #1 to #7. (D) Photograph of the CM enrichment device with colour dyes illustrating stable hydrodynamic focusing of the blue dye by flanking red dyes into the centre of the chamber. A filtering device upstream of the sorting chamber eliminates cell clumps. Reproduced with permission from Martinez et al.52
For clinicians, there is a pressing need to demonstrate relevance of micro-engineering techniques in cost-effective, real-life settings. One rapidly developing application is the use of microfluidic paper-based analytical devices (µPADs) in analytical research.53 Paper-based devices wick aqueous fluids using capillary force and are inexpensive, robust, portable, and can be surface modified. While paper-based diagnostics such as pregnancy tests already exist, these µPADs can be used for multiplexed assays, giving them the potential to diagnose a range of conditions using a finger-prick blood sample or urine sample. µPADs have been further multiplexed in 3D54 and incorporated with electrodes for electro-chemiluminescence (ECL) immunoassays.55 At an estimated three to five cents per assay, µPADs are best suited for application in the developing world,56 such as a paper-based test to diagnose liver failure,57 and may also be relevant for the diagnosis of acute cardiac conditions at a patient's location. One goal of cardiac diagnostics is to prevent even minor infarction; hence, rapid and accurate assessment of ACS biomarkers prior to necrosis using paper-based devices may benefit many at-risk groups and generate further interest in microfluidic technologies.
5.2. Cellular capture and enrichment for cardiovascular therapy
More commonly discussed in the context of circulating tumour cells, microfluidic devices have also been used for enrichment of cardiovascular cells [e.g. cardiomyocytes52 and endothelial progenitor cells (EPCs)]58 without the need for pre-treatment or labelling.
In the case of EPC-capture microfluidic technology, the technique holds significant promise in both progenitor cell biology research and clinical management. In particular, captured EPCs can be used for cardiovascular disease therapies, which are currently limited by the low percentage of mobilized adult peripheral blood-derived EPCs. To address this issue, Plouffe et al.58 used a microfluidic system to isolate and phenotypically identify circulating EPCs in human blood using immobilized antibodies against EPC cell-surface markers (CD34, VEGFR-2, CD31, and CD146) under low shear. The approach was specific as EPCs were preferentially captured by the immobilized antibodies compared with cellular controls (mesenchymal stem cells, vascular endothelial cells, and vascular smooth muscle cells). The applications of captured EPCs are plenty; e.g. expanded pure EPC populations can be used to construct tissue engineered heart valves and conduit arteries for children with congenital heart disease. For cell-based revascularization strategies in coronary artery disease, vascular prostheses or struts in intravascular stents can be coated with immobilized antibodies designed specifically to capture EPCs.59 This approach could promote early graft endothelialization, which in turn may reduce the risks of restenosis and stent thrombosis.
In another study, Zhang et al.52 demonstrated the enrichment of primary CMs directly from heart tissue of neonatal rats, to high purity, without any pre-treatment or labelling. Label-free separation could be achieved microfluidically using principles of deterministic lateral displacement (DLD), which makes use of repeating arrays of posts to translate objects larger than a certain critical size across fluid streamlines. Therefore, the larger CMs (11–14 µm) could be separated from a heterogeneous mixture of smaller cells such as fibroblasts (5–6 µm) in a predictable and continuous manner (Figure 5C and D). The enriched CMs were subsequently identified with anti-Troponin C antibodies. Overall, the CM yield was around 55%, as some smaller CMs were lost in the sorting process. The CMs were also shown to be functional; after 3 days of cultivation on 3D porous collagen scaffolds, they connected and formed a synchronously beating cardiac patch. An advantage of the microfluidic system designed by Zhang et al.52 is that it uses only a single pump unit and can be scaled up with parallel processing. It also requires no pre-treatment or pre-labelling, making it suitable for clinical applications or potential integration with other microfluidic units.
5.3. Conclusion and outlook
Although microfabrication technologies have provided researchers with enhanced capabilities in biological research, challenges to widespread adoption by the biological community persist, including (i) comfort level with basic fabrication techniques, (ii) standardization and reproducibility of microfluidic models, and (iii) the need for confocal and video imaging facilities and software.
Fundamentally, PDMS as a material does in fact possess several properties that are of concern to biological researchers. While we do not discourage the use of PDMS, we believe that both engineers and biologists should be aware of potential pitfalls of using any material in any given study, as other reviews have similarly discussed.60,61 For example, PDMS is very permeable to gas; coupled with the small fluid volumes in microfluidic studies, evaporation may result in significant changes in solute concentration and osmolarity. Small hydrophobic molecules may diffuse rapidly into the PDMS,62,63 while uncross-linked PDMS pre-polymers are also known to leach out.63 Hence, many companies are manufacturing devices made of other polymeric materials, but as for PDMS, further validation work is needed to confirm their suitability.
Other issues include the complexity of microfluidic set-ups, which may hinder scalability. As opposed to PDMS devices which are often produced in-house in small batches, polystyrene tissue culture plates have been standardized and widely used for decades. Many biological laboratories do not have clean room microfabrication facilities and biologists are often unfamiliar with the software used to design these devices (e.g. AutoCAD) or render simulation data (e.g. MATLAB). Quantitative control of fluid flow in microfluidic channels often requires special micro-valves, gas–liquid mixers, and pumps.64 One should also consider issues inherent in the micro scale of any material, such as the much lower volume-to-cell ratio compared with standard cell-culture plates, and the generation of excessive shear at the fluid-air interface upon the introduction of air bubbles. Given that the vasculature and the immune system are extremely sensitive to external stimuli and foreign materials, care must be taken to interpret in vitro results.
To date, there has been a great deal of interest into microfluidic technologies for applications in disease modelling and point-of-care diagnostics. The engineered integration of biophysical variables such as flow, 3D spatial cues, and gradients into cell-culture-based assays provide tools for hypothesis testing of organ level phenomena in vitro. This level of control also can contribute to unique diagnostic modalities at the point of care. Thus, we believe that in the next 5–10 years, further advancements in microfluidic technologies will lead to increased adoption of these platforms in the broad cardiovascular community. However, as with any reductionist investigation of complex biology, good scientific judgement in experimental design and educated peer review will remain critical to the longevity of findings inferred from microengineered technologies.
Funding
J.M.C. acknowledges start-up grant funding from Nanyang Technological University (NTU) and the Lee Kong Chian School of Medicine (LKCMedicine). C.L.D. acknowledges funding from the National Medical Research Council (NMRC) Clinical Scientist Award (CSA), and funding from the National University of Singapore (NUS) and National University Health System (NUHS).
Acknowledgements
The authors thank Ioannis Zervantonakis for his helpful comments on the manuscript.
Conflict of interest: none declared.
References
- 1.Adams CP, Brantner VV. Estimating the cost of new drug development: is it really 802 million? Health Aff 2006;25:420–428. [DOI] [PubMed] [Google Scholar]
- 2.Bhogal N, Balls M. Translation of new technologies: from basic research to drug discovery and development. Curr Drug Discov Technol 2008;5:250–262. [DOI] [PubMed] [Google Scholar]
- 3.DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ 2003;22:151–185. [DOI] [PubMed] [Google Scholar]
- 4.Duffy DC, McDonald JC, Schueller OJA, Whitesides GM. Rapid Prototyping of Microfluidic Systems in Poly(dimethylsiloxane). Anal Chem 1998;70:4974–4984. [DOI] [PubMed] [Google Scholar]
- 5.Esch MB, King TL, Shuler ML. The role of body-on-a-chip devices in drug and toxicity studies. Yarmush MLDJSGML, editor. Annu Rev Biomed Eng 2011;13:55–72. [DOI] [PubMed] [Google Scholar]
- 6.Whitesides GM. The origins and the future of microfluidics. Nature 2006;442:368–373. [DOI] [PubMed] [Google Scholar]
- 7.Hasenfuss G. Animal models of human cardiovascular disease, heart failure and hypertrophy. Cardiovasc Res 1998;39:60–76. [DOI] [PubMed] [Google Scholar]
- 8.Chiu J-J, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev 2011;91:327–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westein E, van der Meer AD, Kuijpers MJE, Frimat J-P, van den Berg A, Heemskerk JWM. Atherosclerotic geometries exacerbate pathological thrombus formation poststenosis in a von Willebrand factor-dependent manner. Proc Natl Acad Sci USA 2013;110:1357–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White CJ, Ramee SR, Collins TJ, Escobar AE, Karsan A, Shaw D, Jain SP, Bass TA, Heuser RR, Teirstein PS, Bonan R, Walter PD, Smalling RW. Coronary thrombi increase PTCA risk. Angioscopy as a clinical tool. Circulation 1996;93:253–258. [DOI] [PubMed] [Google Scholar]
- 11.Sianos G, Papafaklis MI, Serruys PW. Angiographic thrombus burden classification in patients with ST-segment elevation myocardial infarction treated with percutaneous coronary intervention. J Invasive Cardiol 2010;22:6B–14B. [PubMed] [Google Scholar]
- 12.Nesbitt WS, Westein E, Tovar-Lopez FJ, Tolouei E, Mitchell A, Fu J, Carberry J, Fouras A, Jackson SP. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nat Med 2009;15:665–673. [DOI] [PubMed] [Google Scholar]
- 13.Estrada R, Giridharan GA, Nguyen M. Microfluidic endothelial cell culture model to replicate disturbed flow conditions seen in atherosclerosis susceptible regions. Biomicrofluidics 2011;5:032006–032006–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russell JC, Proctor SD. Small animal models of cardiovascular disease: tools for the study of the roles of metabolic syndrome, dyslipidemia, and atherosclerosis. Cardiovasc Pathol 2006;15:318–330. [DOI] [PubMed] [Google Scholar]
- 15.Truskey GA. Endothelial cell vascular smooth muscle cell co-culture assay for high throughput screening assays for discovery of anti-angiogenesis agents and other therapeutic molecules. Int J High Throughput Screen 2010;171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korin N, Kanapathipillai M, Matthews BD, Crescente M, Brill A, Mammoto T, Ghosh K, Jurek S, Bencherif SA, Bhatta D, Coskun AU, Feldman CL, Wagner DD, Ingber DE. Shear-activated nanotherapeutics for drug targeting to obstructed blood vessels. Science 2012;337:738–742. [DOI] [PubMed] [Google Scholar]
- 17.Strony J, Beaudoin A, Brands D, Adelman B. Analysis of shear stress and hemodynamic factors in a model of coronary artery stenosis and thrombosis. Am J Physiol 1993;265:H1787–H1796. [DOI] [PubMed] [Google Scholar]
- 18.Wong KHK, Chan JM, Kamm RD, Tien J. Microfluidic models of vascular functions. Annu Rev Biomed Eng 2012;14:205–230. [DOI] [PubMed] [Google Scholar]
- 19.Forsyth AM, Wan J, Ristenpart WD, Stone HA. The dynamic behavior of chemically “stiffened” red blood cells in microchannel flows. Microvasc Res 2010;80:37–43. [DOI] [PubMed] [Google Scholar]
- 20.Rosenbluth MJ, Lam WA, Fletcher DA. Analyzing cell mechanics in hematologic diseases with microfluidic biophysical flow cytometry. Lab Chip 2008;8:1062–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishino M, Tanaka H, Ogura H, Inoue Y, Koh T, Fujita K, Sugimoto H. Serial changes in leukocyte deformability and whole blood rheology in patients with sepsis or trauma. J Trauma 2005;59:1425–1431. [DOI] [PubMed] [Google Scholar]
- 22.Olson TS, Singbartl K, Ley K. L-selectin is required for fMLP- but not C5a-induced margination of neutrophils in pulmonary circulation. Am J Physiol Regul Integr Comp Physiol 2002;282:R1245–R1252. [DOI] [PubMed] [Google Scholar]
- 23.Han S, Yan J-J, Shin Y, Jeon JJ, Won J, Eun Jeong H, Kamm RD, Kim Y-J, Chung S. A versatile assay for monitoring in vivo-like transendothelial migration of neutrophils. Lab Chip 2012;12:3861–3865. [DOI] [PubMed] [Google Scholar]
- 24.Gilbert PM, Havenstrite KL, Magnusson KEG, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 2010;329:1078–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol 2006;7:211–224. [DOI] [PubMed] [Google Scholar]
- 26.Tsai AG, Johnson PC, Intaglietta M. Oxygen gradients in the microcirculation. Physiol Rev 2003;83:933–963. [DOI] [PubMed] [Google Scholar]
- 27.Gimbrone MA, Cotran RS, Folkman J. Human vascular endothelial cells in culture. Growth and DNA synthesis. J Cell Biol 1974;60:673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chrobak KM, Potter DR, Tien J. Formation of perfused, functional microvascular tubes in vitro. Microvasc Res 2006;71:185–196. [DOI] [PubMed] [Google Scholar]
- 29.Wong KHK, Truslow JG, Tien J. The role of cyclic AMP in normalizing the function of engineered human blood microvessels in microfluidic collagen gels. Biomaterials 2010;31:4706–4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Price GM, Wong KHK, Truslow JG, Leung AD, Acharya C, Tien J. Effect of mechanical factors on the function of engineered human blood microvessels in microfluidic collagen gels. Biomaterials 2010;31:6182–6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong KHK, Truslow JG, Khankhel AH, Chan KLS, Tien J. Artificial lymphatic drainage systems for vascularized microfluidic scaffolds. J Biomed Mater Res A 2013;101:2181–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cabodi M, Choi NW, Gleghorn JP, Lee CSD, Bonassar LJ, Stroock AD. A Microfluidic Biomaterial. J Am Chem Soc 2005;127:13788–13789. [DOI] [PubMed] [Google Scholar]
- 33.Golden AP, Tien J. Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab Chip 2007;7:720–725. [DOI] [PubMed] [Google Scholar]
- 34.Price GM, Chu KK, Truslow JG, Tang-Schomer MD, Golden AP, Mertz J, Tien J. Bonding of macromolecular hydrogels using perturbants. J Am Chem Soc. 2008;130:6664–6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng Y, Chen J, Craven M, Won Choi N, Totorica S, Diaz-Santana A, Kermani P, Hempstead B, Fischbach-Teschl C, López JA, Stroock AD. In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci USA 2012;109:9342–9347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song JW, Munn LL. Fluid forces control endothelial sprouting. Proc Natl Acad Sci USA 2011;108:15342–15347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Packer L, Fuehr K. Low oxygen concentration extends the lifespan of cultured human diploid cells. Nature 1977;267:423–425. [DOI] [PubMed] [Google Scholar]
- 38.Chen Q, Fischer A, Reagan JD, Yan LJ, Ames BN. Oxidative DNA damage and senescence of human diploid fibroblast cells. Proc Natl Acad Sci USA 1995;92:4337–4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abaci HE, Shen Y-I, Tan S, Gerecht S. Recapitulating physiological and pathological shear stress and oxygen to model vasculature in health and disease. Sci Rep 2014;4:4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Günther A, Yasotharan S, Vagaon A, Lochovsky C, Pinto S, Yang J, Lau C, Voigtlaender-Bolz J, Bolz S-S. A microfluidic platform for probing small artery structure and function. Lab Chip 2010;10:2341–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Welsh DG, Segal SS. Endothelial and smooth muscle cell conduction in arterioles controlling blood flow. Am J Physiol 1998;274:H178–H186. [DOI] [PubMed] [Google Scholar]
- 42.Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined—a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol 2000;21:1502–1513. [DOI] [PubMed] [Google Scholar]
- 43.Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, Konstam MA, Mancini DM, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 Focused Update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults. J Am Coll Cardiol 2009;53:1343–1382. [DOI] [PubMed] [Google Scholar]
- 44.DeMello AJ. Control and detection of chemical reactions in microfluidic systems. Nature 2006;442:394–402. [DOI] [PubMed] [Google Scholar]
- 45.Yager P, Edwards T, Fu E, Helton K, Nelson K, Tam MR, Weigl BH. Microfluidic diagnostic technologies for global public health. Nature 2006;442:412–418. [DOI] [PubMed] [Google Scholar]
- 46.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD. Third universal definition of myocardial infarction. Circ 2012;126:2020–2035. [DOI] [PubMed] [Google Scholar]
- 47.Chan CPY, Mak WC, Cheung KY, Sin KK, Yu CM, Rainer TH, Renneberg R. Evidence-based point-of-care diagnostics: current status and emerging technologies. Annu Rev Anal Chem 2013;191–211. [DOI] [PubMed] [Google Scholar]
- 48.Ren L, Wang J-C, Liu W, Tu Q, Liu R, Wang X, Xu J, Wang Y, Zhang Y, Li L, Wang J. An enzymatic immunoassay microfluidics integrated with membrane valves for microsphere retention and reagent mixing. Biosens Bioelectron 2012;35:147–154. [DOI] [PubMed] [Google Scholar]
- 49.Park J, Sunkara V, Kim T-H, Hwang H, Cho Y-K. Lab-on-a-disc for fully integrated multiplex immunoassays. Anal Chem 2012;84:2133–2140. [DOI] [PubMed] [Google Scholar]
- 50.Lee W, Jung J, Hahn YK, Kim SK, Lee Y, Lee J, Lee T-H, Park J-Y, Seo H, Lee JN, Oh JH, Choi Y-S, Lee SS. A centrifugally actuated point-of-care testing system for the surface acoustic wave immunosensing of cardiac troponin I. Analyst 2013;138:2558–2566. [DOI] [PubMed] [Google Scholar]
- 51.Lee G, Park I, Kwon K, Kwon T, Seo J, Chang W-J, Nam H, Cha G, Choi M, Yoon D, Lee S. Electrochemical detection of high-sensitivity CRP inside a microfluidic device by numerical and experimental studies. Biomed Microdevices 2012;14:375–384. [DOI] [PubMed] [Google Scholar]
- 52.Zhang B, Green JV, Murthy SK, Radisic M. Label-free enrichment of functional cardiomyocytes using microfluidic deterministic lateral flow displacement. PLoS One 2012;7:e37619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martinez AW, Phillips ST, Butte MJ, Whitesides GM. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew Chemie Int Ed 2007;46:1318–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martinez AW, Phillips ST, Whitesides GM. Three-dimensional microfluidic devices fabricated in layered paper and tape. Proc Natl Acad Sci USA 2008;105:19606–19611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ge L, Yan J, Song X, Yan M, Ge S, Yu J. Three-dimensional paper-based electrochemiluminescence immunodevice for multiplexed measurement of biomarkers and point-of-care testing. Biomaterials 2012;33:1024–1031. [DOI] [PubMed] [Google Scholar]
- 56.Martinez AW, Phillips ST, Whitesides GM, Carrilho E. Diagnostics for the developing world: microfluidic paper-based analytical devices. Anal Chem 2010;82:3–10. [DOI] [PubMed] [Google Scholar]
- 57.Vella SJ, Beattie P, Cademartiri R, Laromaine A, Martinez AW, Phillips ST, Mirica KA, Whitesides GM. Measuring markers of liver function using a micropatterned paper device designed for blood from a fingerstick. Anal Chem 2012;84:2883–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Plouffe BD, Kniazeva T, Mayer JE, Murthy SK, Sales VL. Development of microfluidics as endothelial progenitor cell capture technology for cardiovascular tissue engineering and diagnostic medicine. FASEB J 2009;23:3309–3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ward MR, Stewart DJ, Kutryk MJB. Endothelial progenitor cell therapy for the treatment of coronary disease, acute MI, and pulmonary arterial hypertension: current perspectives. Catheter Cardiovasc Interv 2007;70:983–998. [DOI] [PubMed] [Google Scholar]
- 60.Berthier E, Young EWK, Beebe D. Engineers are from PDMS-land, Biologists are from Polystyrenia. Lab Chip 2012;12:1224–1237. [DOI] [PubMed] [Google Scholar]
- 61.Halldorsson S, Lucumi E, Gómez-Sjöberg R, Fleming RMT. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens Bioelectron 2014;63C:218–231. [DOI] [PubMed] [Google Scholar]
- 62.Toepke MW, Beebe DJ. PDMS absorption of small molecules and consequences in microfluidic applications. Lab Chip 2006;6:1484–1486. [DOI] [PubMed] [Google Scholar]
- 63.Regehr KJ, Domenech M, Koepsel JT, Carver KC, Ellison-Zelski SJ, Murphy WL, Schuler LA, Alarid ET, Beebe DJ. Biological implications of polydimethylsiloxane-based microfluidic cell culture. Lab Chip 2009;9:2132–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beebe DJ, Mensing GA, Walker GM. Physics and applications of microfluidics in biology. Annu Rev Biomed Eng 2002;4:261–286. [DOI] [PubMed] [Google Scholar]