Abstract
The current study evaluates the impact of high saturated fat feeding in rat model of experimental nephrotoxicity induced by gentamicin. Sprague-Dawley rats weighing 200 g were randomized into four groups; the first one received the standard rodents chow for 8 weeks and was treated as control, the second group (HFD)received an experimental high fat diet rich in palm kernel oil (40% of Calories as fat) for the same period. The third group (HFDG) was given 80 mg/kg (body weight)/day gentamicin sulphate intraperitoneally during the last 24 days of the feeding period while the fourth group was given gentamicin as above along with the standard rodents chow. Renal function was assessed through measuring serum creatinine, creatinine clearance and absolute and fractional excretion of both sodium and potassium. At the end, rats underwent a surgical procedure for blood pressure measurement. Renal function study showed a stronger nephrotoxicity for HFDG group. Hypertension was observed in HFD group while the pressure declined after gentamicin co-administration. Overall, changing the feeding behavior toward using more SAFFAs for rats injected with gentamicin promotes the progression of renal failure.
KEY WORDS: nephrotoxicity, blood pressure, hypertension, creatinine clearance, high fat diet
INTRODUCTION
Gentamicin is a widely used aminoglycoside antibiotic to treat various Gram +ve and Gram -ve infections. Its nephrotoxic action is attributed to its potential to damage the proximal convoluted tubules (PCT) and glomerular basement membrane [1]. Oxidative stress mediated damage is the main character of its nephrotoxicity [2, 3]. Substituting the elementary dietary constituents by fats, rich in saturated free fatty acids (SAFFAs), has a negative impact on the ability of the body to cope with various patho-physiological conditions [4]. In spite of that, these fats are used extensively in food industry. This is due to their physiochemical properties that give food products the besought texture [5]. SAFFAs induce their deleterious effects through several mechanisms [4]. They stimulate a specific type of immune system related receptors called TLRs (toll like receptors). TLRs are expressed in most body cells including myocytes, hepatocytes and adipocytes [6]. Their activation triggers the inflammatory pathway related to insulin resistance [6, 7]. From the other hand, SAFFAs induce visceral obesity. When the visceral adipose tissue is engorged with fats, it does not merely act as a fat depot but it also acts as an endocrine gland releases the inflammatory cytokines related to insulin resistance [8]. Disposition of the source of cellular energy from carbohydrates to fats rich in SAFFAs increases the release of free radicals during mitochondrial internal respiration. This results in higher oxidative stress [9]. Our study aimed to find the impact of replacing the essential dietary elements in food by SAFFAs on progression of renal damage in rats model of gentamicin induced nephrotoxicity.
MATERIALS AND METHODS
Animals and diet
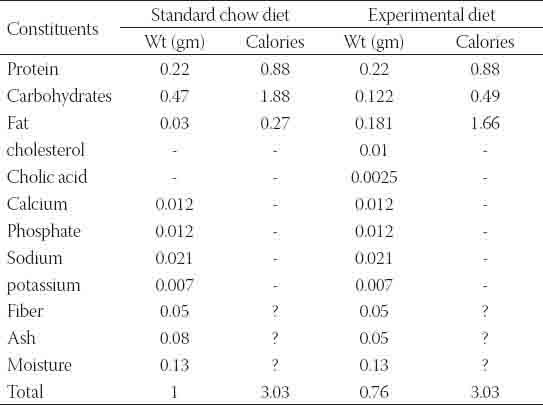
Male Sprague-Dawley rats, weighing 190±14.7 g and obtained from animal house of Universiti Sains Malaysia, were used in the study. They were housed at the animal transit room with 4 animals per cage at room temperature with 12:12-h light-dark cycle. All the procedures were performed according to guidelines of the Animal Ethics Committee, Universiti Sains Malaysia for the use of animals in research. Time dependant effect for nephrotoxicity induction and the required sample size were estimated according to a preliminary study. In which 80 mg/kg/day gentamicin sulphate was injected intraperitoneally for 30 days with a continuous monitoring of body weight and urine flow rate. Serum and urine samples were collected throughout the treatment period to assess progression of renal dysfunction. Maximum nephrotoxicity was obtained on day 16 and sustained up to the end of the treatment period (Figure 1). Then twenty four days were chosen as the treatment period to all the treatment protocols. Before starting the experiment, rats were left for habituation and fed the standard commercial rodents chow with free access to water ad libitum. Rats were divided into four groups (10 animals for each); C; received a standard rodents chow for 8 weeks, HFD; received the experimental high fat diet during the same period, HFDG; received genatmicin sulphate intraperitoneally in a dose 80 mg/kg/day along with the high fat diet during the last 24 days of the feeding period and G; received the standard rodents chow and was given gentamicin sulphate as mentioned above. The experimental diet was formulated to supply equal quantity of all nutrients as a percentage of energy with the exception of fat and carbohydrates (Table 1). It was stored in refrigerator at 4 °C after being prepared and was given daily to the animals. The main oil sources of the high fat diet were palm kernel oil and palm oil whose fatty acids profile contains lauric acid, myristic acid and palmitic acid (10). Cholesterol and cholic acid were added to induce hypercholestremia. Cholic acid suppresses cholesterol conversion to bile acids through interfering with the activity of 17-α hydroxylase enzyme [11].
FIGURE 1.
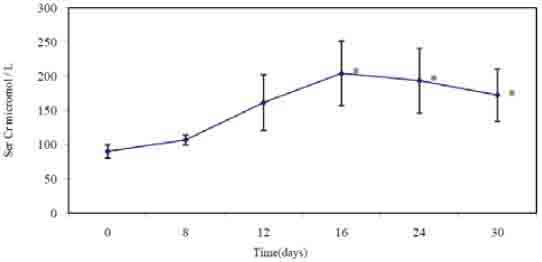
Serum creatinin in μmol/l through out the genamicin treatment during the preliminary study. * indicates statistical significance (p<0.05) as compared to day 0
TABLE 1.
Composition of the experimental diet as compared to the standard chow diet as a percent of total calories.
Animals monitoring
Body weight and food intake (corrected for spillage) were monitored continuously during the treatment period. 24 hr urine and fasting tail vein serum samples were obtained on days (34, 50 and 58) of the high fat diet feeding period which corresponds to days (0, 16 and 24) of gentamicin treatment period. At the end, they were subjected to an acute study in which the rats underwent a surgical session under anesthesia for blood pressure measurement.
Serum and urine biochemical analyses
Serum and urine samples were collected to measure creatinine, sodium and potassium concentrations. Creatinine clearance (Cr c) and both absolute and fractional excretions of the electrolytes were calculated using the conventional equation. Creatinine was measured using Jaff reaction (picric acid test) while the electrolytes were measured using the flame photometer (Jenway PFP7). 24 hr urinary protein excretion was measured using Cromassia Brilliant Blue test.
Acute study
The rats were fasted for (12–14h) with free access to drinking water before starting with the acute study. They were anaesthetized with 60 mg/kg (body weight) of sodium pentobarbital (Nembutal®, CEVA, France) and kept at 37°C to maintain body temperature. After that neck incision was performed and the right jugular vein was catheterized with polyethylene tubing (PE50, Portex limited Hythe, Kent, England) to supply fluids during the surgery (N/S was given at rate 6 ml/h). For the blood pressure measurement, the left carotid artery was cannulated with a fluid filled catheter (PE50, Portex limited). It was connected to pressure transducers (P23 ID Gould, Statham Instrument, Nottingham, UK) linked to a computerized data acquisition system (Power Lab®, ADInstrumentat, Sydney Australia). At the end of the experiment, the animals were euthanized with the lethal dose of pentobarbital sodium (200 mg/kg). Then the fat depot was collected for obesity index measurement and both kidneys were collected for measuring kidney index and to perform biochemical studies on renal homogenate and histological studies.
Renal homogenate study
One kidney from each rat was homogenized using 1 ml for each gram of kidney tissue of ice chilled 150 mM potassium chloride solution and tissue homogenizer (Homogenizer MSE, England). TBARS (thio-barbituric- acid- releasing-substance) level was measured according to the established method.
Histology study
Tissues were fixed using formalin solution. Then they were subjected to the conventional histology procedures; procession, sectioning, staining and slide mounting. Hematoxylin and eosin were used to stain the slides.
Statistical analysis
Results were expressed as mean ± s.e.m. One way ANOVA followed by Tukey test was used for statistical analysis at 95% confidence level using students pack SPSS program version 16.
RESULTS
Metabolic study
Gentamicin co-administration along with the high fat diet has reduced the weekly increment of the body weight as compared to the other groups. This was accompanied by an observable decline in calories uptake through out the gentamicin treatment period (Figures 2 and 3). Urine flow rate dropped consistently throughout the high fat diet feeding period. It was increased substantially after gentamicin injection for rats injected with the standard rodents chow and for those fed with the high fat diet (Table 2).
FIGURE 2.
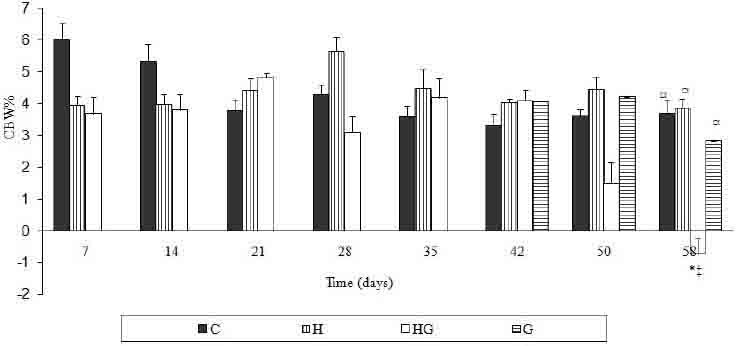
Percent of weekly change of body weight for all the treated groups throughout the treatment period. Results are expressed in mean± S.E.M. * Indicates statistical significance as compared to control (p<0.05). # indicates statistical significance as compared to H group (p<0.05). ☼ indicates statistical significance as compared to HG group (p<0.05). § indicates statistical significance as compared to G group (p<0.05).
FIGURE 3.
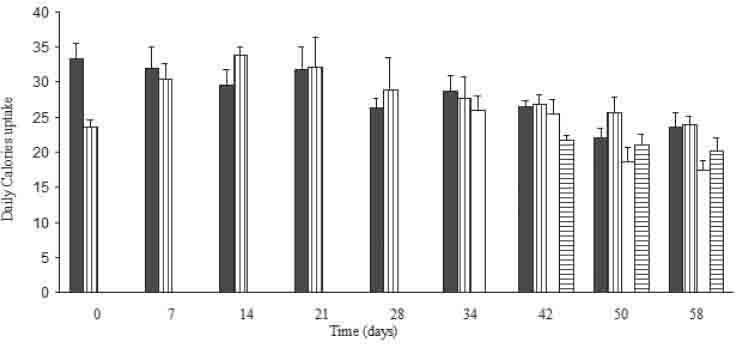
Food uptake in Kcal/day/100 g B.W for all the treated groups through out the treatment period. Results are expressed in mean± S.E.M. * Indicates statistical significance as compared to control (p<0.05). #indicates s (Nembutal tatistical significance as compared to H group (p<0.05). ☼ indicates statistical significance as compared to HG group (p<0.05). § indicates statistical significance as compared to G group (p<0.05).
TABLE 2.
Metabolic and hemodynamic parameters. The renal function study was for days 0, 16 and 24 which correspond to the 34th, 50th and the 58th days of the ad libitum a high fat diet feeding period. Results are expressed in mean± S.E.M. * Indicates statistical significance as compared to control (p<0.05). # indicates statistical significance as compared to HFD group (p<0.05). ☼ indicates statistical significance as compared to HFDG group (p<0.05). § indicates statistical significance as compared to G group (p<0.05).
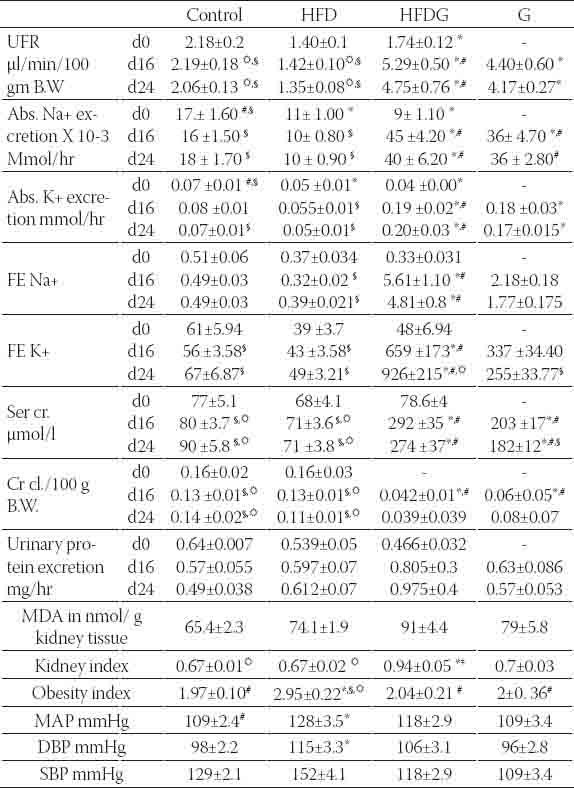
Renal function
Results of renal function study showed a higher decline in renal function for HFDG as compared to G group. This was obviously seen in results of serum creatinine and creatinine clearance. Creatinine clearance dropped to half the value of the control for G group and dropped to about the quarter for HFDG group while serum creatinine was as twice as its control for G group and thrice for HFDG group at the end of the treatment period (Table 2). Tubular function study revealed higher fractional excretion for both sodium and potassium in renal failure groups. The increase was higher for HFDG group that FE Na+ has increased by about 10 times for HFDG group as compared to control and by about four times for G group while FE K+ has multiplied by about three times the control for G group and about eighteen times for HFDG group at the end of the treatment period (Table 2). Urinary protein excretion was obviously increased for HFDG group as compared to other groups without showing any statistical significance. Moreover, it did not show any change for G group as compared to control. The results suggest higher glomerular and tubular dysfunction for HFDG group as compared to G group (Table 2). These observations were confirmed in the histology study (Table 3, Figure 4). Acute tubular necrosis of the PCT (proximal convoluted tubules) was spotted in all the slides of HFDG group. The intensity of the damage was ranging from mild to moderate to severe. While for G group, it was ranging from very mild to mild and the damage was not observable at all in some slides (Table 3, Figure 4). The slides showed also glomerular changes as characterized by glomerular congestion, mesangial hyperproliferation, glomerular membrane hyalinization and DCT (distal convoluted tubules) ectasia by protein cast. The changes were ranging from mild to severe in HFDG while they were mild or absent in G group (Table 3, Figure 4). These histological changes were accompanied by a noticeable increase of kidney index in HFDG group while it was slightly increased in G group. From the other hand, MDA (malonyldialdehyde), a biomarker of oxidative stress was elevated in the renal homogenate of all gentamicin treated groups. The increase was highly increased after gentamicin coadministration along with the high fat diet (Table 2).
TABLE 3.
Histological changes grading for the HFDG and G groups at the end of the treatment period.
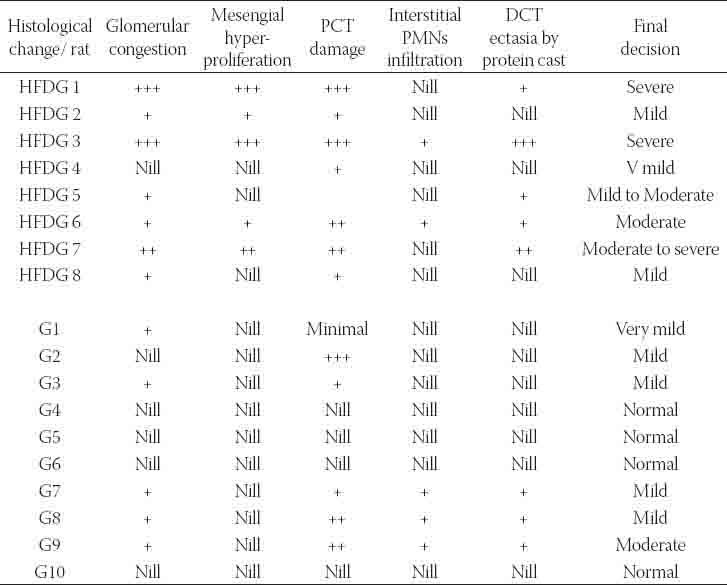
FIGURE 4.
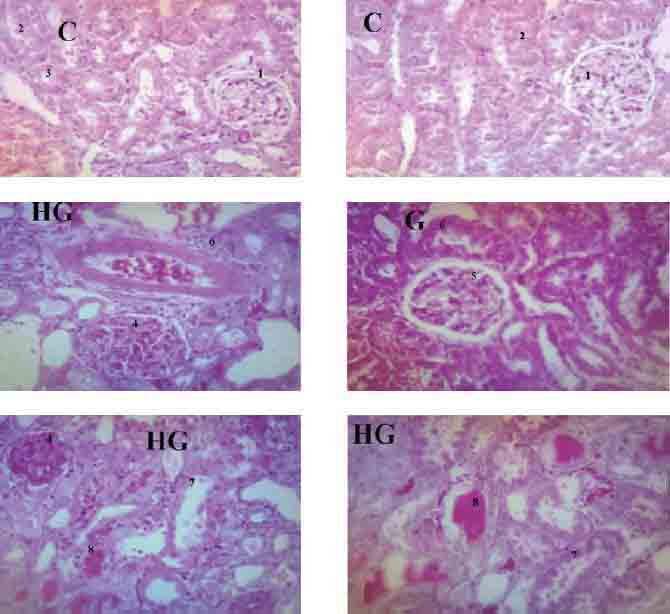
Kidney histology slides for control (C), gentamicin treated group (G) and gentamicin treated group along with high fat diet (HG). “1”, “2” &”3” represent normal glomerulus, PCT and DCT respectively. “4” represents glomerular congestion, mesangial hyperproliferation and glomerular hyalinization. “5” represents mild glomerular congestion with thickening of glomerular basement membrane. “6” represents mild PCT damage. “7” represents severe PCT damage with denuded tubular epithelial cells in the lumen. “8” represents DCT ectasia by protein cast.
Blood pressure
Unlike the other treated groups, HFD group showed significantly higher blood pressure parameters (DBP, SBP and MAP) (p < 0.05) as compared to control. Gentamicin co-administration along with high fat diet has dropped the blood pressure as compared to that in the rats with the high fat diet feeding (Table 2).
Obesity index
The obesity index was significantly higher for the group fed with the high fat diet while it was reduced after gentamicin co-administration (Table 2).
DISCUSSION
Gentamicin is a nephrotoxic drug. Our preliminary study showed maximum nephrotoxicity after 16 days of gentamicin injection, which stayed high up to the end of the treatment period. Latency time and pattern of nephrotoxicity induction rely on so many factors; as the dose, its timing, number of times the dose is given and the responsiveness of the animal to the nephrotoxic action [12]. Both glomerulonephritis (GN) and proximal convoluted tubules (PCT) tubulonephritis were the prominent outcomes of gentamicin-induced nephrotoxicity. They were more severe in the renal failure group fed with the high fat diet. Gentamicin as a cationic amino glycoside has an aptitude to bind to glomerular basement membrane glycoproteins. This results in glomerular damage characterized by glomerular congestion, leukocyte infiltration, mesangial hyper proliferation and hyalinization of the glomerular basement membrane [2, 3]. Mesangium plays a role in regulation of the glomerular filtration rate. When the glomerular membrane destruction occurs, the mesangium contract and hyperproliferate and this results in reduction of the single nephron glomerular filtration rate [13]. From the other hand, gentamicin is selectively taken by the brush border membrane of the PCT lining cells resulting in accumulation of gentamicin intracellularly. When the intracellular concentration exceeds the toxic threshold, it induces lysosomal, mitochondrial and cell membrane damage. This results in loss of the reabsorptive function and some degenerative and necrotic changes that lead to exodus of cellular constituents and sloughing of the lining cells. Consequently tubular occlusion and decrease in glomerular filtration incur [2, 3]. These changes were obviously correlated with the results of the biochemical study that showed a decline in creatinine excretion and tubular handling of electrolytes which are related to the loss of glomerular filtration and tubular reabsorptive functions respectively. The higher severity of nephrotoxicity in the rats fed with the experimental high fat diet confirms the negative impact of the SAFFAs on the ability of cells to cope with toxic effect of drugs. It was suggested that, uptake of high amount of SAFFAs create some intracellular biochemical changes characterized by increasing the preparedness of the mitochondria to unleash more free radicals after being subjected to any injury. Mechanism of gentamicin induced nephrotoxicity is closely related to oxidative stress [4]. Gentamicin interferes with the oxidative phosphorelation in the mitochondria resulting in higher oxidative stress [14]. MDA (Malonyldialdehyde) which is one of the famous biomarkers of the oxidative stress was increased in gentamicin injected groups. The increase was higher in rats which were under the experimental high fat diet feeding. This confirms the role of oxidative stress in gentamicin induced nephrotoxicity and the cooperative role of the SAFFAs for its increase. It is suggested that SAFFAs promote the inflammatory pathway. It was found that, SAFFAs activate a specific type of receptors called TLRs (toll like receptors). TLRs are immune system related receptors. They are expressed in muscles, immune cells and hepatocytes. A subtype of these receptors (TLRs13) is affected by SAFFAs. Its activation triggers a cascade of sequential reaction of intracellular pathways related to more inflammatory genes expression [4, 15]. This results in an outflow of cytokines as; interleukine-1, interleukine6 and interleukine-8 and tumor necrotic factor a (TNF-α), acute phase proteins as C-reactive proteins (CRP) and some chemo-attractant factors as MIF (Macrophage inhibitory factor) and macrophage chemotactant factor (MCP-1) [16]. From the other hand, Uptake of high amount of SAFFAs triggers engorgement of the visceral adipose tissue with fat [17]. This effect was obviously seen in results of obesity index which was significantly increased after the experimental high fat diet ingestion. Some recent studies found that, visceral adipose tissue does not merely act as fat depots. It acts also as an endocrine gland secrete the mentioned inflammatory cytokines and macrophage chemotactant factors [17]. The histology slides showed higher inflammatory response for rats injected with gentamicin along with the high fat diet (Table 4, Figures 4). Distal convoluted tubules ectasia by protein cast was one of the prominent outcomes of gentamicin induced renal failure.
It results from leakage of some low g.m.wt proteins as albumin in the tubular filtrate after glomerular membrane damage. Proteins in the tubular filtrate precipitate a specific type of glycoprotein called Tamm Horsfall protein. This protein is normally secreted by distal convoluted tubules (DCT) and precipitates when proteins exist in the filtrate [2]. Presence of protein cast was mild in some slides of renal failure rats under standard rodents chow feeding while it was higher in the renal failure rats under the experimental high fat diet feeding (Table 3, Figure 4). This also confirms the higher incidence of glomerular damage for rats, which were under the high fat diet feeding. This view is closely related to with the observed protein urea in the renal failure rats fed with the high fat diet. In spite of polyurea, serum concentrations of both sodium and potassium were slightly decreased for HFDG group without showing any statistical significance and were indifferent for the other groups. This may be due to loss of equimolar amount of water along with the lost amount of sodium and the elaborate mechanism the body uses to maintain K+ concentration constant through regulation of cellular Na+-K+ pump and renal excretion of potassium [18]. The observed decline in the weekly increment of the body weight in HFDG group can be ascribed to the stronger incidence of nephrotoxicty. Reduction of body weight in renal failure occurs due to fluid loss or the hyperuremia which triggers anorexia and cachexia [19]. Our results showed that high fat diet administration has increased the visceral obesity. Previous studies revealed that visceral fat does not merely act as a fat depot but it acts also as an endocrine gland releases inflammatory cytokines. This in turn triggers a higher inflammatory response to any injurious factor [8]. Our results showed a higher inflammatory reaction to gentamicin when it is co-administered along with the high fat diet. Although our results showed that gentamicin intervention has interfered with building up of adiposity. This may be attributed to the anorexia which accompanied the renal failure [18]. In spite of polyurea and fluid loss, results of acute study did not show any change in hemodynamic parameters for G group. This may be due to the compensatory mechanisms that regulate the pressure [20]. High fat diet produced a prominent increase in pressure parameters. Previous studies showed that ingestion of high amount of SAFFAs induces hyperinsulinemia. Hyperinsulinemia causes salts and fluid retention and encourages sympathetic outflow [21]. The fluid retention was obvious in results of tubular function assessment for the HFD group in which the values of the absolute and fractional excretion of electrolytes was dropped. Beside that SAFFAs relent purging of inflammatory cytokines which are culminated in deteriorating the endothelial function. Endothelium plays an important role in blood pressure regulation [22, 23]. Gentamicin co-administration along with high fat diet has decreased the pressure parameters as compared to HFD group. This may be attributed to the persistent polyurea that accompanied renal failure up to the end of treatment period [3]. Although in renal failure, overactivation of the renin angiotensin system is expected. Overall, co-administration of high fat diet along with gentamicin significantly intensifies the progression of gentamicin induced nephrotoxicity.
CONCLUSION
Feeding behaviour has a great impact on progression of pathophysiological conditions. Ingestion of saturated fat rich food hastens progression of diseases in a mechanism related to elevation of oxidative stress. Furthermore, ingestion of the saturated fat rich food hastens toxicity of drugs. Therefore, it is very important to monitor the feeding behaviour along with drugs administration.
ACKNOWLEDGEMENTS
School of pharmacy/Universiti Sains Malaysia is gratefully acknowledged for financial support. We also thank The Advance Medical and Dental Institute (AMDI) USM for technical support in the experiment.
DECLARATION OF INTEREST
All the authors declare that the research was carried out with the support of IPS-University Sains Malaysia. The facilities were supported by school of pharmacy/Universiti Sains Malaysia. The authors declare that conflicts of interest do not exist.
REFERENCES
- [1].Lopez-Nova JM, Quiros Y, Vicente L, Morales AL, Lopez-Hernandez FJ. New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int. 2011;79(1):33–45. doi: 10.1038/ki.2010.337. [DOI] [PubMed] [Google Scholar]
- [2].Cojocel C, Dociu N, Maita K, Sleight SD, Hook JB. Effects of aminoglycosides on glomerular permeability, tubular reabsorption, and intracellular catabolism of the cationic low-molecular-weight protein lysozyme. Toxicol Appl Pharmacol. 1983;68(1):96–109. doi: 10.1016/0041-008x(83)90358-7. [DOI] [PubMed] [Google Scholar]
- [3].Marie-Paule Mingeot-Leclercq, Paul M. Tulkens. Aminoglycosides: nephrotoxicity. Antimicrob Agents Chemother. 1999;43(5):1003–1012. doi: 10.1128/aac.43.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kennedy A, Martinez K, Chuang CC, Lapoint K, Mcintosh M. Saturated fatty acid-mediated inflammation and insulin resistance in adipose tissue: mechanisms of action and implications. J Nutr. 2009;139(1):1–4. doi: 10.3945/jn.108.098269. [DOI] [PubMed] [Google Scholar]
- [5].Wing-Kiung NG, Gibon V. Palm oil and saturated fatty acids-rich vegetable oil. In: Turchini GM, Ng WK, Tocher DR, editors. Fish Oil Replacement and Alternative Lipid Sources in Aquaculture Feeds. 5th edn. Boca Raton: CRS Press. Taylor & Francis; 2011. pp. 1–18. [Google Scholar]
- [6].Ajuwon KM, Spurlock ME. Palmitate activates the NF-kappa B transcription factor and induces IL-6 and TNF alpha expression in 3T3-L1 adipocytes. J Nutr. 2005;135(8):1841–1846. doi: 10.1093/jn/135.8.1841. [DOI] [PubMed] [Google Scholar]
- [7].Song MJ, Kim KH, Yoon JM, Kim JB. Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem Biophys Res Commun. 2006;346(8):739–745. doi: 10.1016/j.bbrc.2006.05.170. [DOI] [PubMed] [Google Scholar]
- [8].Bergman RN, Kim SP, Catalano KJ, Hsu IR. Why visceral fat is bad: mechanisms of the metabolic syndrome. Obesity. 2006;14(1):16S–19S. doi: 10.1038/oby.2006.277. [DOI] [PubMed] [Google Scholar]
- [9].Sreekumar R, Unnikrishnan J, Fu A, Nygren J, Short KR, Schimke J, Barazzoni R, Nair KS. Impact of high-fat diet and antioxidant supplement on mitochondrial functions and gene transcripts in rat muscle. Am J Physiol Endocrinol Metab. 2002;282(5):E1055–1061. doi: 10.1152/ajpendo.00554.2001. [DOI] [PubMed] [Google Scholar]
- [10].Wai-Lin Siew, Chiew-Let Chong, Yew-Ai Tan. Composition of the oil in palm kernel from Elaeis guineensis. JAOCS. 1995;72(12):1587–1589. [Google Scholar]
- [11].Beher WT, Baker GD. Inhibition of cholesterol biosynthesis by cholic acid. Am J Physiol. 1959;197:1339–1340. doi: 10.1152/ajplegacy.1959.197.6.1339. [DOI] [PubMed] [Google Scholar]
- [12].Neil E Reiner, Don D Bloxham, Leigh Thompson W. Nephrotoxicity of gentamicin and tobramycin given once daily or continuously in dogs. J Antimicrob. Chemother. 1978;4(Suppl-A):85–101. doi: 10.1093/jac/4.suppl_a.85. [DOI] [PubMed] [Google Scholar]
- [13].Lieberthal W, Nigam S K. Acute renal failure. II. Experimental models of acute renal failure: imperfect but indispensable. Am J Physiol Renal Physiol. 2000;278(1):F1–F12. doi: 10.1152/ajprenal.2000.278.1.F1. [DOI] [PubMed] [Google Scholar]
- [14].Walker L M, York JL, Imam SZ, Ali SF, Muldrew KL, Mayeux PR. Oxidative stress and reactive nitrogen species generation during renal ischemia. Toxicol Sci. 2001;63(1):143–148. doi: 10.1093/toxsci/63.1.143. [DOI] [PubMed] [Google Scholar]
- [15].Gordon GB. Saturated free fatty acid toxicity: II. Lipid accumulation, ultrastructural alterations, and toxicity in mammalian cells in culture. Exp Mol Pathol. 1977;27(2):262–276. doi: 10.1016/0014-4800(77)90035-1. [DOI] [PubMed] [Google Scholar]
- [16].Lago F, Dieguez C, Gómez-Reino J, Gualillo O. Adipokines as emerging mediators of immune response and inflammation. Nat Clin Pract Rheumatol. 2007;3(12):716–724. doi: 10.1038/ncprheum0674. [DOI] [PubMed] [Google Scholar]
- [17].Faria AN, Ribeiro Filho FF, Gouveia Ferreira SR, Zanella MT. Impact of visceral fat on blood pressure and insulin sensitivity in hypertensive obese women. Obes Res. 2000;10(12):1203–6. doi: 10.1038/oby.2002.164. [DOI] [PubMed] [Google Scholar]
- [18].Tirosh A, Garg R, Adler GK. Mineralocorticoid receptors in the metabolic syndrome. Curr Hypertens Rep. 2010;12(4):252–7. doi: 10.1007/s11906-010-0126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cheung W, Yu P.X, Little B.M, Cone R.D, Marks D.L, Mak R.H. Anorexia and cachexia in renal failure-Is Leptin the culprit?: Role of leptin and melanocortin signaling in uremia-associated cachexia. J Clin Invest. 2005;115(8):1659A–1665. doi: 10.1172/JCI22521. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ruth A Hannon, Charlotte Pooler, Coral Matsone Porth. 2nd ed. Lippincott Williams & Wilkins; 2009. Porth pathophysiology: concepts of altered health States. [Google Scholar]
- [21].Herlitz H, Widgren B, Urbanavicius V, Attval S, Persson B. Stimulatory effect of insulin on tubular sodium reabsorption in normotensive subjects with a positive family history of hypertension. Nephrol. Dial. Transplant. 1996;11(1):47–54. [PubMed] [Google Scholar]
- [22].Chait A, Kim F. Saturated fatty acids and inflammation: who pays the toll? Arterioscler Thromb Vasc Biol. 2010;30(4):692–3. doi: 10.1161/ATVBAHA.110.203984. [DOI] [PubMed] [Google Scholar]
- [23].Tentolouris C, Tousoulis D, Antoniades C, Bosinakou E, Kotsopoulou M, Trikas A, Toutouzas P, Stefanadis C. Endothelial function and proinflammatory cytokines in patients with ischemic heart disease and dilated cardiomyopathy. Int J Cardiol. 2004;94(2-3):301–5. doi: 10.1016/j.ijcard.2003.08.002. [DOI] [PubMed] [Google Scholar]


