Abstract
A new, simple immunochromatographic assay for rapid identification of Mycobacterium tuberculosis complex in liquid cultures has been developed. The principle of the assay is binding of the Mycobacterium tuberculosis complex specific antigen to the monoclonal antibody conjugated on the test strip. The aim of this study is evaluation of the performance of immunochromatographic assay in identification of Mycobacterium tuberculosis complex in primary positive liquid cultures of BacT/Alert automated system.
A total of 159 primary positive liquid cultures were tested using the immunochromatographic assay (BD MGIT TBc ID) and the conventional subculture, followed by identification using biochemical tests.
Of 159 positive liquid cultures, using the conventional method, Mycobacterium tuberculos is was identified in 119 (74.8%), nontuberculous mycobacteria were found in 4 (2.5%), 14 (8.8%) cultures were contaminated and 22 (13.8%) cultures were found to be negative. Using the immunochromatographic assay, Mycobacterium tuberculosis complex was detected in 118 (74.2%) liquid cultures, and 41 (25.8%) tests were negative. Sensitivity, specificity, positive and negative predictive values of the test were 98.3%; 97.5%; 99.15%; 95.12%, respectively. The value of kappa test was 0.950, and McNemar test was 1.00.
The immunochromatographic assay is a simple and rapid test which represents a suitable alternative to the conventional subculture method for the primary identification of Mycobacterium tuberculosis complex in liquid cultures of BacT/Alert automated system.
KEY WORDS: Mycobacterium tuberculosis complex, identification, immunochromatographic assay
INTRODUCTION
A rapid isolation and identification of mycobacteria in the clinical samples is essential for the effective treatment of the disease [1]. Introduction of the liquid culture automated systems has significantly shortened the cultivation time of mycobacteria [2]. Liquid cultures positive for acid-fast bacilli (AFB) indicate the presence of mycobacteria, requiring discrimination between Mycobacterium tuberculosis complex and nontuberculous mycobacteria (NTM) [2, 3]. Identification of Mycobacterium tuberculosis complex in liquid cultures is usually performed on subcultures of mycobacteria on solid media using biochemical tests [3, 4]. The identification process which requires several weeks increases the turnaround time for reporting positive results, diminishing the value of liquid cultures [2]. The nucleic acid amplification and other molecular techniques contribute to a faster identification of Mycobacterium tuberculosis, but in addition to great financial expenses, also require specific equipment and highly trained technicians [3, 5-7]. A novel, immunochromatographic assay for rapid identification of Mycobacterium tuberculosis complex has been designed, based on the specific MPT64 antigen binding to monoclonal antibodies [1, 3, 5-7]. The MPT64 antigen is a 24kDa secretory protein, highly specific to Mycobacterium tuberculosis, Mycobacterium africanum, Mycobacterium bovis and some substrains of Mycobacterium bovis bacilli Calmette-Guerin (BCG) [5, 8, 9]. The MPT64 immunochromatographic assay has been confirmed to be a useful tool for identification of Mycobacterium tuberculosis complex in liquid cultures of the BACTEC MGIT 960 system [3, 5, 7, 10]. Except the BACTEC MGIT 960 system, other liquid culture automated systems for mycobacterial detection have been applied including the BacT/Alert system 3D (formerly MB/Bact) [2]. The study objective was to evaluate the performance of immunochromatographic assay in identification of Mycobacterium tuberculosis complex in primary positive liquid cultures of BacT/Alert automated system.
MATERIALS AND METHODS
Samples
The study included a total of 669 clinical respiratory samples collected in the 12 month period. All the samples were sputum samples, submitted to the mycobacterial testing by the automated BacT/Alert system (BacT/Alert 3D, Biomerieux, France) at the Center for Microbiology, Immunology and Virusology of the Institute for Pulmomary Diseases of Vojvodina, Sremska Kamenica, Serbia.
Procedure
The samples were conventionally treated with N-acetyl- L-cysteine-NaOH (NALC-NaOH) [11]. The processed samples were inoculated into the BacT/Alert MP culture bottle as per manufacturer’s instrutions. The instrument indicated 159 primary positive cultures. The positive liquid cultures were subcultured in the solid Löwenstein-Jensen medium and incubated at 37 °C. Sufficient growth has been observed for every 2-3 days. Macroscopic inspection and acid-fast smear (Ziehl-Nielsen) preparation of the detected growth was performed. AFB positive cultures were identified using conventional biochemical testing (niacin accumulation, nitrate reduction, Tween 80 hydrolysis, arylsulfatase test) [12]. All positive liquid cultures were also identified using the immunochromatographic assay (BD MGIT TBc ID, Beckton Dickinson Diagnostic, Sparks, USA). Antigen MPT64 secreted from Mycobacterium tuberculosis complex cells binds to the antiMPT64 monoclonal antibody conjugated on the test strip, visualized as a clearly visible line. The testing was performed according to the manufacturer’s instructions. The sample well is filled with 100 μl of the positive liquid culture and the result is read in 15 minutes. A clearly visible pink to red line emerging along with the control line indicates a positive result (Figure 1).
FIGURE 1.

BD MGIT TBc identification test [13]
Statistical analysis
The statistical analysis of the results was performed using the Statistical Package for the Social Sciences (SPSS), version 14. Sensitivity, specificity, positive and negative predictive values of the examined diagnostic test were determinated. The correlation of the results of the two diagnostic methods was evaluated using the Cohen’s kappa (k) test and McNemar asymmetry test.
RESULTS
Of 159 primary positive liquid cultures, Mycobacterium tuberculosis was identified by biochemical tests in 119 (74.8%) isolates. Nontuberculous mycobacteria were found in 4 (2.5%) isolates, 2 Mycobacterium xenopi and Mycobacterium fortuitum and Mycobacterium avium in one culture respectively (Figure 2). No bacterial growth was found in 22 (13.8%) cultures. Contamination was registered in 14 (8.8%) cultures. A comparative analysis of liquid cultures by the immunochromatographic assay showed 118 (74.2%) Mycobacterium tuberculosis complex-positive cultures while 41 (25.8%) tests were negative (Figure 3). Of 119 Mycobacterium tuberculosis isolates, the test was positive in 117 (73.6%) and negative in 2 (1.3%) cultures. Of 14 contaminated cultures, in 13 (8.2%) cultures the test was negative. One (0.6%) contaminated culture had a positive test result (Table 1). Sensitivity of the test was 98.3%, and specificity equaled 97.5%. Positive and negative predictive values were 99.15% and 95.12% respectively, while the overall accuracy of the test amounted to 98.1% (Table 2).
FIGURE 2.
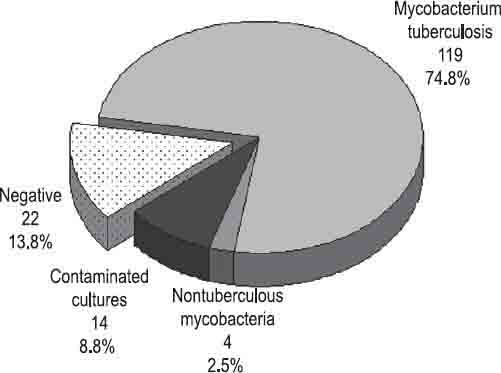
Positive liquid cultures identified by biochemical tests
FIGURE 3.
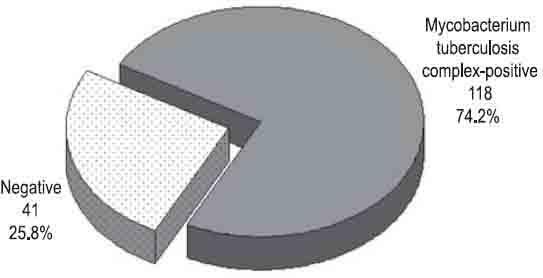
Positive liquid cultures tested by the immunochromatographic assay
TABLE 1.
Identification of mycobacteria by conventional biochemical tests and by immunochromatographic test (BD MGIT TBc ID)
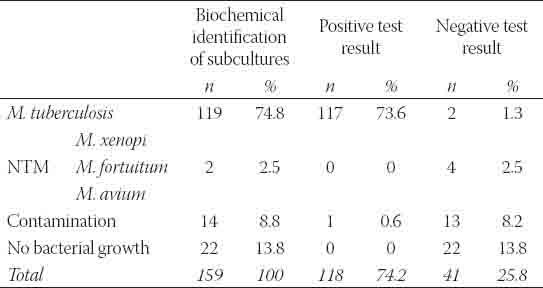
TABLE 2.
Immunochromatographic test features (BD MGIT TBc ID)

In our study, the kappa test value was 0.950, suggesting a very high congruence (0.81-1.00) of the conventional subculture identification using the conventional biochemical tests and the rapid immunochromatographic test. The congruence of the obtained results was also confirmed by the McNemar test, having the value of 1.00.
DISCUSSION
We evaluated the utility of the immunochromatographic test Tbc ID (Becton Dickinson, Sparks, MD) to identify Mycobacterium tuberculosis complex in the primary positive liquid cultures of the BacT/Alert system. Although the use of liquid cultures significantly reduces the turnaround time for culture of mycobacteria, the problem of rapid and accurate identification still persists, in particular due to increasingly frequent isolation of NTM in clinical samples. Applying the novel immunochromatographic test, the test results are produced in 15 minutes [1, 3, 5-7]. Three immunochromatographic tests for a rapid identification of Mycobacterium tuberculosis complex based on the MPT64 antigen detection are available commercially: Capilia test (Tauns Laboratories, Numatz, Japan), SD Bioline TB Ag MPT64 Rapid test (Standard Diagnostics, Inc, Kyonggi-do, Korea), and BD MGIT TBc ID test (BD Diagnostic Systems, Sparks, MD, USA). All the three tests provide similar results [1, 3, 5, 8, 6, 14, 15]. This study confirms the previous results reported. The negative test results were obtained in all the cultures with no bacterial growth registered. In all the cultures with identified nontuberculous mycobacteria, Mycobacterium xenopi, Mycobacterium fortuitum, Mycobacterium avium, the test results were negative. The immunochromatographic test is an important and reliable tool in differentiation between NTM and Mycobacterium tuberculosis complex [1, 4, 8, 14, 16]. Only occasional false positive results have been reported showing cross-reaction with nontuberculous mycobacteria, for example one Mycobacterium flavescens strain and one Mycobacterium marinum strain tested by the immunochromatographic test (MPB64-ICA) [9], also one Mycobacterium avium-intracellulare complex strain and one Mycobacterium chelonae strain tested by the Capilia test [15]. Contaminated cultures represent a unique problem for Mycobacterium tuberculosis identification. Due to a faster bacterial and fungal growth as compared to Mycobacterium tuberculosis, it is often difficult to register AFB in acid-fast smears because of their small count. The absence of AFB in acid-fast smears does not exclude with certainty their presence in the liquid culture. The presence of AFB was confirmed by inoculation onto the solid Löwenstein-Jensen medium and biochemical examination of bacterial growth. As this is a long-lasting procedure, all contaminated liquid cultures were also tested by the rapid immunochromatographic test, although it has been recommended by the manufacturer to test only the liquid cultures with a dominant AFB presence in order to avoid false positive findings. In our study, we obtained one false-positive and two initially false-negative results. The false-positive culture was AFB smear-negative. The false-positive finding may be explained by the growth and metabolism of the non-AFB microorganisms found in the sample [5], reported as a possible cause in the test instructions, although the single immunochromatographic testing of diverse bacterial and fungal strains failed to confirm a false-positive cross-reaction [1, 4]. The false negative finding may be due to a small AFB count, i.e. a small MPT64 antigen quantity, so it is recommended to perform additional testing of contaminated AFB-positive cultures in 24-hour time [4, 6]. Having performed additional testing of contaminated cultures, two positive test results were obtained, confirmed also by biochemical testing. The test specificity in Mycobacterium tuberculosis complex identification from liquid cultures ranges from 97.9% [15] to 100% [3, 4, 6, 10]. The test specificity of 97.5% was achieved in our study due to inclusion of the contaminated cultures in the testing. If all AFB smearnegative contaminated cultures had been considered negative, 100% specificity would have been achieved [5]. The false-negative test result was obtained in two primary positive liquid cultures. The positive liquid cultures with the negative test results, after conventional testing of the growth on the solid Löwenstein-Jensen medium, were also tested in the National Laboratory for Tuberculosis in Belgrade and confirmed the presence of Mycobacterium tuberculosis by DNA hybridization (GenoTypeʀMTBC, Hain, Germany). False-negative findings may be due to diverse mutations (nucleotide deletion, point mutations and IS6161 insertion mutation at 501 nucleotide position) in the mpt64 gene [16]. Also, the count of mycobacteria has to be >105/ml of the sample [1], as false-negative findings may be due to a small inoculum. The sensitivity of the test was 98.3% in our study, correlating with the sensitivity range from 97% [6] to 99.6% [14], reported by other authors.
CONCLUSION
The immunochromatographic assay (BD MGIT TBc ID) is a simple and rapid test which represents a suitable alternative to the conventional methods for the primary identification of Mycobacterium tuberculosis complex in liquid cultures of BacT/Alert automated system. The high specificity in discrimination of Mycobacterium tuberculosis complex from nontuberculous mycobacteria in liquid cultures is particularly recognized.
DECLARATION OF INTEREST
Authors declare no conflict of interest.
REFERENCES
- [1].Park MY, Kim YJ, Hwang SH, Kim HH, Lee EY, Jeong SH, et al. Evaluation of an immunochromatographic assay kit for rapid identification of Mycobaterium tuberculosis complex in clinical isolates. J Clin Microbiol. 2009;47(2):481–484. doi: 10.1128/JCM.01253-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dinnes J, Deeks J, Kunst H, Gibson A, Cummins E, Waugh N, et al. A systematic review of rapid diagnostic tests for the detection of tuberculosis infection. Health Technol Assess. 2007;11(3):1–196. doi: 10.3310/hta11030. [DOI] [PubMed] [Google Scholar]
- [3].Martin A, Bombeeck D, Fissette K, de Rijk P, Hernandez-Neuta I, Del Portillo P, et al. Evaluation of the BD MGIT TBc Identification Test (TBc ID), a rapid chromatographic immunoassay for the detection of Mycobacterium tuberculosis complex from liquid culture. J Microbiol Methods. 2011;84(2):255–257. doi: 10.1016/j.mimet.2010.12.003. [DOI] [PubMed] [Google Scholar]
- [4].Marzouk M, Kahla IB, Hannachi N, Ferjeni A, Salma WB, Ghezal S, et al. Evaluation of an immunochromatographic assay for rapid identification of Mycobaterium tuberculosis complex in clinical isolates. Diagn Microbiol Infect Dis. 2011;69:396–399. doi: 10.1016/j.diagmicrobio.2010.11.009. [DOI] [PubMed] [Google Scholar]
- [5].Gaillard T, Fabre M, Martinaud C, Vong R, Brisou P, Soler C. Assessment of the SD Bioline Ag MPT64 Rapid™ and the MGIT™ TBc identification tests for the diagnosis of tuberculosis. Diagn Microbiol Infect Dis. 2011;70:154–156. doi: 10.1016/j.diagmicrobio.2010.12.011. [DOI] [PubMed] [Google Scholar]
- [6].Ismail NA, Baba K, Pombo D, Hoosen AA. Use of an immunochromatographic kit for the rapid detection of Mycobaterium tuberculosis from broth cultures. Int J Tuberc Lung Dis. 2009;13:1045–1047. [PubMed] [Google Scholar]
- [7].Garcia-Martos P, Garcia-Agudo L, Rodriguez-Jimenez MJ, Rodriguez-Iglesias M. Rapid identification of Mycobacterium tuberculosis complex from broth cultures by immunochromatographic assay. Rev Esp Quimioter. 2010;23(4):206–209. [PubMed] [Google Scholar]
- [8].Hasegawa N, Miura T, Ishii K, Yamaguchi K, Lindner TH, Merritt S, et al. New simple and rapid test for culture confirmation of Mycobacterium tuberculosis complex a multicenter study. J Clin Microbiol. 2002;40(3):908–912. doi: 10.1128/JCM.40.3.908-912.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Abe C, Hirano K, Tomiyama T. Simple and rapid identification of the Mycobacterium tuberculosis complex by immunochromatographic assay using anti-MPB64 monoclonal antibodies. J Clin Microbiol. 1999;37:3693–3697. doi: 10.1128/jcm.37.11.3693-3697.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yu MC, Chen HY, Wu MH, Huang WL, Kuo YM, Yu FL, et al. Evaluation of the rapid MGIT TBc identification test for culture confirmation of Mycobacterium tuberculosis complex strain detection. J Clin Microbiol. 2011;49(3):802–807. doi: 10.1128/JCM.02243-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kubica GP, Dye WE, Cohn ML, Middlebrook G. Sputum digestion and decontamination with N-acetyl-L-cystein-sodium hydroxide for culture of mycobacteria. Am Rev Respir Dis. 1963;87:775–779. doi: 10.1164/arrd.1963.87.5.775. [DOI] [PubMed] [Google Scholar]
- [12].Lee LV. Conventional biochemicals. In: Isenberg HD, editor. Clinical microbiology procedures handbook. 2nd edn. Vol. 2. Washington DC: ASM Press; 2004. pp. 7.6.1–12. [Google Scholar]
- [13].Erembodegem (Belgium): Becton Dickinson International, Branch of Becton, Dickinson B.V; 2011. Oct 12, [accessed 22.09.2011]. BD [Internet] [Image], BD MGIT™ TBc Identification Test.. Available from: http://www.bd.com/contentmanager/b_article.asp?Item_ID=24198&ContentType_ID=2&BusinessCode=20065&d=home &s=ema&dTitle=Eastern+Europe%2C+Middle+East+%26amp%3B +Africa&dc=ema&dcTitle=Eastern+Europe%2C+Middle+East+%2 6amp%3B+Africa . [Google Scholar]
- [14].Ngamlert K, Sinthuwattanawibool C, McCarthy KD, Sohn H, Starks A, Kanjanamongkolsiri P, et al. Diagnostic performance and costs of Capilia TB for Mycobacterium tuberculosis complex identification from broth-based culture in Bangkok, Thailand. Trop Med Int Health. 2009;14(7):748–53. doi: 10.1111/j.1365-3156.2009.02284.x. [DOI] [PubMed] [Google Scholar]
- [15].Wang JY, Lee LN, Lai HC, Hsu HL, Jan IS, Yu CJ, et al. Performance assessment of the Capilia TB assay and the BD ProbeTec ET system for rapid culture confirmation of Mycobacterium tuberculosis. Diagn Microbiol Infect Dis. 2007;59:395–399. doi: 10.1016/j.diagmicrobio.2007.06.010. [DOI] [PubMed] [Google Scholar]
- [16].Hirano K, Aono A, Takahashi M, Abe C. Mutations including IS6110 insertion in the gene encoding the MPB64 protein of Capilia TB-negative Mycobacterium tuberculosis isolates. J Clin Microbiol. 2004;42:390–392. doi: 10.1128/JCM.42.1.390-392.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


