Abstract
The ductal intraepithelial neoplasia (DIN) classification which proposes new approaches to the diagnosis, terminology and differential diagnosis of intraductal proliferative lesions of the breast was applied to a series of female patients comprising C-erbB2 oncogene expression which may serve as an adjunct to the morphology by immunohistochemistry. The study was performed using the data of 94 patients. There was no difficulty encountered in the diagnosis of intraductal hyperplasia (IDH). In patients with Atypical Ductal Hyperplasia (AIDH), the diagnosis could be made by using the 2-mm rule of the DIN classification in patients who exhibited cytologic and structural characteristics of Ductal Carcinoma in Situ (DCIS) alone or in conjunction with classical IDH patterns. However, in lesions that mimicked classical IDH patterns despite displaying cytological features of in situ carcinomas, the experience and view point of the pathologist played a more prominent role. When the DIN classification criteria were applied to grade DCIS lesions, although the system was found to be practical, it did not provide adequate differentiation in intermediate grade (grade II-DIN 2) patients and further improvement was considered desirable. Fourty-five cases (47.8%) IDH, 19 (20.2%) AIDH, and 30 (31.9%) were DCIS. There were statistically significant differences in the levels of c-erbB2 oncogene expression between IDH, AIDH and DCIS lesions (p<0.001). In DCISs, grade, cell size, pleomorphic nuclear atypia showed statistically significant associations with c-erbB2 oncogene expression. These results suggest that c-erbB2 oncogene expression is a valuable marker in the differential diagnosis and prognostic evaluation of patients with intraductal proliferative lesions.
KEY WORDS: breast, proliferative lesions, C-Erb-b2
INTRODUCTION
Breast carcinoma is the most common malignant tumor in women and is one of the leading causes of cancer-related mortality. Studies show that intraductal epithelial proliferations are the most important risk factors [1-3]. In the classification of these lesions revealed three main groups-intraductal hyperplasia (IDH), atypical intraductal hyperplasia (AIDH) and ductal carcinoma in situ (DCIS) have been proposed. Unfortunately, there are major differences of opinion between pathologists on the differential diagnosis of these lesions that require very different treatment approaches. Also, studies are underway to identify biomarkers that will serve as adjuncts to the morphological evaluation. However, there is no gold standard to differentiate atypical ductal hyperplasia from ductal carcinoma in situ and confirm the diagnosis. The c-erbB2 oncogene that carries a poor prognosis in breast cancer is expressed at different levels in the various lesions in the spectrum of intraductal epithelial proliferations [4-6]. The aims of this study were to apply the DIN classification system to our patients, analyze the different approaches proposed by this system for the morphological differentiation of ductal proliferative lesions, to investigate the expression levels of c-erbB2 oncogene in three main lesion groups and to determine whether this marker can serve as an adjunct to morphological evaluation.
MATERIALS AND METHODS
Samples
This study initially included 124 female patients who were diagnosed at the Department of Pathology of Cerrahpaşa Medical Faculty between 1995 and 2000. The histologic slides of each patient were reevaluated and the accuracy of the initial diagnoses were checked. Patients with a previous diagnosis of histologically proven invasive carcinoma and those with concomitant invasive carcinoma were not included in this study. The clinical data on the patients were obtained from the pathology reports. In 7 patients, the available blocks were inadequate for a reevaluation, in 11 patients, comparing the reports and reexamining the findings of the blocks revealed inadequate information. In 12 patients, immunohistochemical staining failed in all three attempts and this was ascribed to inadequate fixation. Consequently, the study was performed on the data of the remaining 94 patients. For the 45 IDH, 19 AIDH and 30 DCIS patients, 2-3 micron sections were prepared from the paraffin blocks and stained with hematoxylin eosin. These were used for immunohistochemistry using the anti-c-erbB2 antibody (c-erb B2/Her-2/neu oncoprotein Ab-17 Clone e2-4001 +3B5. Neomarkers, mc. Biogen). All patients in this study were carefully evaluated according to the terminology and histopathologic criteria proposed by the DIN classification system. In lesions that showed patterns characteristic of in situ carcinoma, the diameters of the ducts that exhibited this characteristic were measured with a microscope (Nikon model eciipse E-400). In DCIS lesions, the presence and extent of necrosis as well as degree of pleomorphic nuclear atypia were graded according to the DIN classification system. The patterns were also evaluated: comedo, solid, micropapillary, cribriform or clinging. The areas of the lesions in the sections were measured with a microscope. Tumor cell size was also taken as a parameter: small (smaller than 3 times the size of a lymphocyte nucleus), medium size (between 3 and 5 times) and large size (larger than 5 times).
Procedures
For the evaluation oncogene expression, 2-micron paraffin sections were taken on APTES-coated slides. A previously evaluated DCIS lesion with known strong staining for c-erbB2 was used as a positive control. After deparaffinization and rehydration, the sections were incubated in 3% hydrogen peroxide for 15 minutes to inhibit the endogenous peroxidase activity. After washing in distilled water, they were processed in a microwave oven while immersed in citrate buffer (1/10 dilution in distilled water) for 20 minutes. After 20 minutes of waiting at room temperature, the cooled sections were washed in distilled water and taken into phosphate-buffered saline (PBS). The primary antibody (1/500 dilution) was applied for 30 minutes at 25°C. Then they were incubated for 10 minutes each in link, label and AEC chromogen. Finally, they were stained with Mayer hematoxylin and covered with glycerin gel. The antigen recognized by the c-erbB2 antibody is located in the cytoplasmic membrane; therefore, granular or diffuse cytoplasmic staining was ignored and the intensity of the cytoplasmic membrane staining was evaluated. The intensity of the staining was graded as absent (0), mild (1+), moderate (2+) and strong (3+).
Statistical analysis
Statistical analysis was carried out using the SPSS package. The results of immunohistochemistry were evaluated by using the Kruskall-Wallis test, Spearman correlation analysis and the Dunn test.
RESULTS
A total of 45 women with intraductal hyperplasia were included in this study. Age range was 16-75 (median 42.6). The specimen was an excisional biopsy in 43 patients, quadrantectomy material in 1 patientand a trucut biopsy in one. The degree of epithelial proliferation in the ducts was graded as mild (3-5 layers of epithelial cells on the basal membrane), moderate (proliferations that formed balls” towards and tend to transverse the lumen) and severe (epithelial proliferations that formed bridges and filled the lumen). The majority of the patients (54.1%) had mild epithelial proliferation, 26.6 % had moderate and 19.3% had severe proliferation. All patients were classified as DINIa. The age distributions of the patients with mild, moderate and severe epithelial proliferation were similar. Epithelial proliferation was frequently accompanied by intraductal papillomatosis, components of fibrocystic disease (apocrine metaplasia and hyperplasia, fibrosis, ductal ectasia), fibroadenoma, adenosis, sclerosing adenosis and chronic mastitis. The median (range) age of the 19 AIDH patients were 46.9 (32-69). In the AIDH group specimens, one was a mastectomy and axillary curettage specimen, one was a partial mastectomy specimen and 17 were excisional biopsies. In five patients, specimens were taken from both breasts and in two of these patients; AIDH was detected in both breasts. One patient underwent mastectomy for invasive ductal carcinoma in the contralateral breast; two patients had a previously diagnosed invasive ductal carcinoma in the contralateral breast. Clinical data could be retrieved in all patients: 12 (65%) had a palpable mass, 4 (20%) had a mass lesion on the mammogram, 2 (10%) had microcalcifications on the mammogram and 1 (5%) had nipple discharge. AIDH was accompanied by severe epithelial proliferation in 18 patients, lobular neoplasia in 6, intraductal papillomatosis in 6, fibroadenoma in 3 and adenosis in 2. Ten of the 19 specimens were obtained between 1995 and 1997: one specimen was sampled totally and one near totally. There was a marked increase in the period 1999–2000. Four specimens smaller than 7 cm were sampled totally and 5 samples larger than 7 cm were sampled subtotally. The sections were examined for cellular and structural features. Two different structural subtypes were identified: classical IDH patterns (secondary lumen formation, bridge formation) and low –grade patterns (micropapillary or cribriform). Of the 19 patients, 11 had IDH, 2 had in situ patterns and 6 had both IDH and in situ patterns. The duct diameters were measured in the 8 specimens that exhibited in situ pattern alone or together with IDH patterns: the results ranged between 0.5 and 1.5 mm. In 6 patients, the cell population was uniform and monotonous; the cell margins were prominent, the cells were round or oval, some had nucleoli; the involved ducts showed both IDH and in situ patterns. In the 6 patients who exhibited the classical IDH pattern, the cell population was uniform; the cells were round and had well-defined margins; the chromatin material was finely organized; some cells were hyperchromatic and some had nucleoli. In 10 other patients with the classical IDH pattern, the cell population was more variable with oval, round or spindle shapes. The cell margins were not well-defined in 5 cases; except for one case, all had prominent nucleoli. It was noted that these patients posed more diagnostic difficulties in comparison with other groups. Two AIDH patients who exhibited DCIS patterns, apocrine features were noted. The sizes of the nucleoli were twice the size of the nucleoli in the normal ductal epithelium. In both patients, pink, eosinophilic secretory material was present in the ductal lumens. In one patient, cells in the papillary projections showed a hobnail appearance. Both specimens were evaluated as atypical apocrine metaplasia. In one patients, the cells lining the lumen in a single layer or multiple layers, had well-defined margins, were hyperchromatic and some cells had multilobulated nuclear structure. A rare variant of AIDHunusual atypical intraductal hyperplasia- was diagnosed. In one patient, the ductal lumens were dilated and filled with secretory material. The stratification resembled the appearance of intraductal papillary carcinoma. None of these patients had the monomorphous clinging DCIS lesion which is included in the DIN Ib group together with AIDH in the DIN classification. Necrosis in the duct lumens were not observed in any patient. Four had calcified material in the lumen. The number of the mitotic figures in the epithelial cells was not taken as a criterion and therefore was not analyzed. There were 30 patients in this group; median (range) age was 50.5 (29-76). The surgical specimen was modified radical mastectomy and axillary curettage in 12 cases, simple mastectomy and curettage in one, quadrantectomy in one, excisional and axillary curettage in 2, excisional biopsy in 14. The surgical margin was positive in 4 patients; these underwent additional procedures. One of the 30 patients had an invasive lobular carcinoma in the contralateral breast. Two patients had a previously diagnosed invasive ductal carcinoma in the contralateral breast. Metastasis was not detected in the 12 axillary curettage material obtained at the time of diagnosis or in a subsequent session. Of the 19 DCIS patients with available clinical data, 8 had microcalcifications on mammography, 4 had palpable masses, 3 had suspicious lesions on mammography, 2 had Paget’s disease of the areola and 2 had nipple discharge. Material for frozen section examination had been sent from 17 patients with a palpable mass or a lesion on mammography. DCIS had been diagnosed in 9 patients (53%). The diagnosis was severe epithelial proliferation in one patient (6%) and benign lesion in 4 (24%). No definite diagnosis could be made in 3 (18%) patients and the decision was deferred to paraffin section examination. Two of the 4 patients with a palpable mass and one of the 8 patients with microcalcifications had a visible mass on macroscopic examination. These three lesions had well-defined margins and on sections ducts containing yellow necrotic material were observed. Microscopic examination showed high-grade comedo carcinoma. Other patients had no visible mass lesion. Of the 30 patients, 2 had multifocal DCIS. The median (range) lesion diameter was 19.2 mm (3-100). Sixteen patients had periductal lymphocyte infiltration, 13 had periductal sclerosis, 7 had lobular cancerization, 4 had mild epithelial proliferation, 6 had severe epithelial proliferation, 2 had AIDH, 1 had fibroadenoma, 1 had Pagetoid extension in the breast skin, two had sclerosing adenosis and 2 had adenosis foci. Thirteen patients had calcifications in the lesion. In the 30 DCIS patients, the pattern was comedo only in 2 patients, comedo and solid in 4, cribriform and micropapillary in 6, heterogeneous consisting of a combination of solid, micropapillary, cribriform and clinging patterns in 18. The 30 DCIS patients were classified into 3 grades according to the DIN system based on the degree of pleomorphic nuclear atypia, presence and as well as extent of necrosis. In this system, grade I corresponds to DIN 1c, grade II to DIN 2 and grade III to DIN 3. Lesions without pleomorphic nuclear atypia and necrosis were grade 1, those with marked pleomorphic nuclear atypia and extensive necrosis were grade III, and those with moderate atypia and moderate necrosis were grade II. Pleomorphic nuclear atypia and necrosis were graded. Of the 30 patients, 3 had grade I, 11 were grade II and 16 were grade III lesions. The 18 lesions with large tumor cells, 10 patients with moderate size tumor cells and 1 patient with small tumor cells were grade II. Two of the grade I had small cells and one had medium size cells. In one of the patients, the in situ carcinoma site in one of the ducts showed solid, papillary and cribriform patterns; the tumor cells had wide cytoplasms, the nucleus was pushed to one side of the cell and the cell gave the impression of signet ring carcinoma. This tumor was classified as DIN 3 (grade III). Of the 94 patients with intraductal proliferative lesions, 45 IDH cases (15 mild, 15 moderate and 15 severe), 19 AIDH cases and 30 DCIS cases were selected for immunohistochemical c-erbB2 staining. In the IDH patients, staining was absent in 37 (82.2%) (Table 1). Two patients with mild epithelial proliferation, four patients with moderate proliferation and one patient with severe proliferation- a total of 7 patients (15.6%) exhibited focal staining. One patient with severe epithelial proliferation (2.2%) exhibited moderate staining (Figure 1). None of the patients in this group showed strong staining. Seventeen (89.5%) of the 19 AIDH lesions showed no staining and 2 (10.5%) showed focal mild staining (Figure 2). None of the patients in this group showed strong staining for c-erbB2. Three (10%) of the 30 DCIS lesions showed no staining, 2 (6.7%) showed focal mild staining, 7 (23.3%) showed moderate staining and 18 (60%)
TABLE 1.
C-erb B2 staining intensities in intraductal proliferative lesions
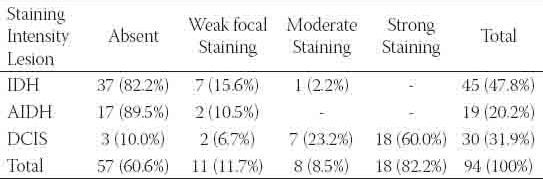
FIGURE 1.
Moderate positive staining for c-erbB2 in a patient with severe epithelial proliferation (×400)
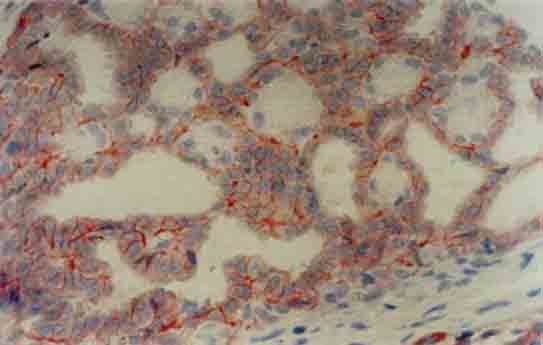
FIGURE 2.
Focal, weak membranous staining for c-erbB2 in atypical ductal hyperplasia (×400)
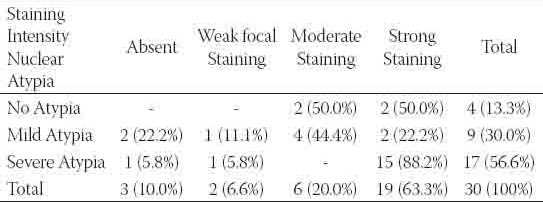
showed strong staining (Figures 3-4). Th e neoplastic intraductal proliferative lesions showed a significantly higher frequency of c-erbB2 staining (p<0.001). The distribution of the staining intensity was summarized in Table 1. There was no statistically significant difference between the c-erbB2 staining intensities of IDH and AIDH patients (p>0.05-Dunn test). However, there was a statistically significant difference between AIDH and DCIS patients (p<0.001-Dunn test). A striking aspect of c-erbB2 staining was that 7 of the 10 IDH lesions containing apocrine metaplasia or hyperplasia showed focal, weak staining. In a patient with DCIS, AIDH in the adjacent areas showed focal weak staining. The relationship between grade and c-erbB2 staining intensity in DCIS patients was described in Table 2. One of the three grade I patients, three of the 10 grade II patients and 13 of the 17 grade II patients showed strong c-erbB2 staining The staining frequency in grade III patients was higher than the frequency in low grade (grades I and II) patients and there was statistically significant difference between high and low grade patients (p=0.016). Cell size was considered as an additional parameter in DCIS patients. Thirteen of the 16 tumors with large cells, two of the 9 tumors with medium size cells and three of the 5 leisons with small cells showed strong staining. The relationship between cell size and c-erbB2 staining intensity in DCIS patients was summarized in Table 3. The frequency of c-erbB2 staining intensity in tumors with large cells was higher than the frequency in tumors with small and medium sized cells and statistically significant results were obtained (p=0.016). Another parameter in DCIS was pleomorphic nuclear atypia. Of the 4 patients without pleomorphic atypia, two had moderate staining and 2 had strong staining. Of the 9 patients with mild atypia, two showed no staining, one showed weak focal staining, 4 showed moderate staining and 2 showed strong staining. Of the 17 patients with severe atypia, one showed no staining; one showed weak, focal staining and 15 (88%) showed strong staining. As shown in Table 4 the frequency of c-erbB2 staining was more frequent in lesions with severe pleomorphic atypia in comparison with those with no or mild atypia and this difference was statistically significant (p=0.030).
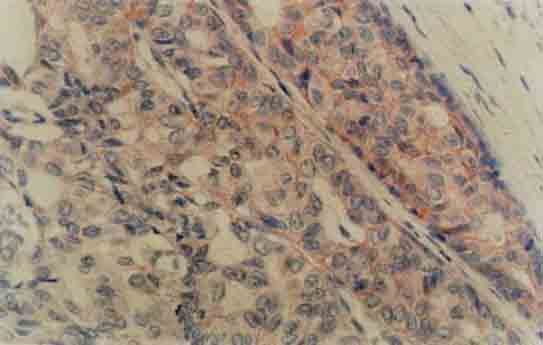
FIGURE 3.
Strong membranous staining for c-erbB2 in a comedo carcinoma and no staining in the adjacent normal ductal epithelium (×100)
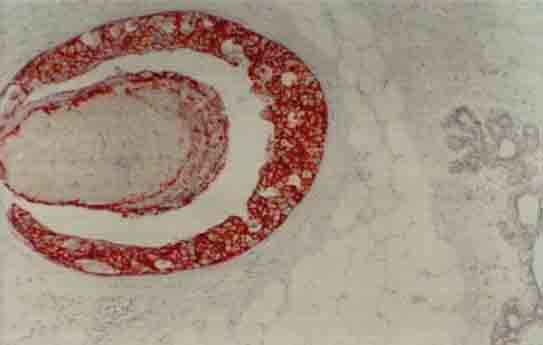
FIGURE 4.
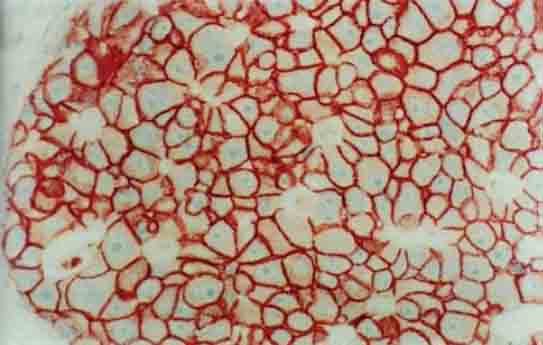
Strong membranous and weak cytoplasmic staining for c-erb-B2 in tumor cells in a grade III ductal carcinoma in situ (×400)
TABLE 2.
The relationship between grade and c-erbB2 staining intensity in DCIS patients
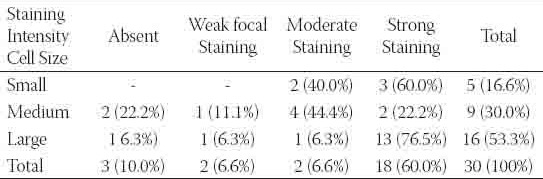
TABLE 3.
The relationship between cell size and c-erbB2 staining intensity in DCIS patients

TABLE 4.
The relationship between pleomorphic nuclear atypia and c-erbB2 staining
DISCUSSION
The risk factors in the etiology of breast carcinoma, the most common malignant tumor in women have been investigated extensively. In the current understanding, intraductal epithelial proliferations are widely accepted as risk factors for breast carcinoma [7]. The widespread availability of mammographic screening techniques in the 1980s dramatically increased the incidence of benign proliferative, i.e. noninvasive breast lesions. In parallel with the rising incidence, the pathologists encounter many “borderline” lesions which cause diagnostic difficulties. Intraductal epithelial proliferations are considered in three categories: IDH, AIDH and DCIS. In the present study, we reexamined our pathology material with these diagnoses according to the DIN system proposed by Tavassoli et al. [8]. The patients were evaluated with respect to age, the method of specimen procurement, clinical presentation and macroscopic features. Whether the expression levels of c-erbB2 oncogene in three main lesion groups differed and whether this marker can serve as adjuncts to morphological evaluation were investigated. The age distribution of the incidence of intraductal hyperplasia varies markedly between races and countries. A study which compared various ethnic and racial groups with respect to the risk of breast cancer revealed that IDH was most frequent in the 35-44 age group in Anglo-Saxon women and in the 45-54 age group in Indians [9]. In another study, the peak incidence in American women was observed in the 40-60 age group [10]. It has been reported that IDHs increase the risk of breast cancer 2-3 times in women older than 45 years old [11]. The median age for AIDHs is approximately 50 which is 5 years older than the median age for IDH and 5 years younger than the median age for DCIS [7]. In the present study, the median age of the IDH patients was 42 and a high percentage belonged to the 41-50 age group. As to the degrees of epithelial proliferation in IDH, the 41-50 age group had the highest percentages- 43%, 47% and 38% in the mild, moderate and severe categories respectively. The calculated median ages of the AIDH and DCIS patients were older- 47 and 51 respectively. The marked increase in the incidence of intraductal proliferative lesions in the 41-50 age group and older, emphasizes the importance of annual mammographic examinations. Excisional biopsy is an adequate treatment for all IDHs and the majority of the AIDHs. If the AIDH is extensive and adjacent to the surgical margin, reexcision is recommended [7]. In the treatment of DCIS, mastectomy was preferred in the USA in the 1980s and early 1990s; there has been a shift towards lumpectomy, lumpectomy and radiation or biopsy only [12]. However, the overall increase in the number of DCIS lesions compensated the decrease in the frequency of radical surgical procedures. Because comedo carcinomas carry an increased risk of recurrence [12, 13], radical surgery is more commonly preferred by the clinicians. The role of axillary curettage in DCIS is controversial. Silverstern et al reported that axillary lymph node metastases may occur in the absence of histologically documented invasion and argued that inadequate sampling may be reason for this [14]. They stated that even though the number of DCIS cases may increase, the frequency of axillary lymph node metastasis will not exceed 1-3% and it is unwise to recommend routine axillary curettage in the face of such a low frequency. The reason may be that standardized patient follow up has not been established in our country. AIDH and IDH are asymptomatic lesions that are detected incidentally on mammography or in the resection specimens of other lesions (fibrocystic change, sclerosing adenosis, fibroadenoma etc.) [7]. Concomitant lesions in IDH patients in the present study were intraductal papillomatosis, components of fibrocystic disease and fibroadenoma. Of the AIDH patients, 13 (54%) presented with a palpable mass and 6 (25%) with mammographic findings. The presence of benign mass lesions such as papillomatosis (6 cases), fibroadenoma (3 cases) and adenosis (2 cases) in the AIDH patients confirms that AIDH is frequently an incidental lesion. DCISs are usually asymptomatic, mostly nonpalpable lesions detected on mammography [14, 15]. Before mammographic screening techniques became widely available, the majority of the DCISs presented with clinical pictures suggesting advanced lesions: palpable mass, Paget disease, nipple discharge. However, with the availability of mammography, the frequency of nonpalpable lesions increased and the patients had the opportunity of early treatment. In the present study, only 4 patients (12.5%) had a palpable mass; the frequency of the patients diagnosed by mammography (37 5%) was higher than the frequency of other presentations such as palpable mass, Paget disease, and nipple discharge. In DCIS patients, ducts exhibiting in situ carcinoma involvement, particularly high grade comedo carcinomas have periductal concentric fibrosis and mild or moderate lymphocyte infiltration [16]. In the present study, the presence of lymphocyte infiltration in 16 patients (50%) and periductal sclerosis in 17 patients (53%) reflect that the majority of the patients (56%) have high grade DCIS. The accuracy of frozen section examination in noninvasive and preinvasive breast lesions is controversial [17]. The majority of the DCISs present with mammographic findings only, i.e. without a palpable mass or a macroscopic lesion. Freezing artifacts complicate the evaluation of nonpalpable or small breast lesions in permanent sections. False negativity rates as high as 76% has been reported for intraoperative frozen section examination [17]; sensitivity may be as low as 46% and this has been ascribed to sampling error. Also, the detection of a palpable lesion or a macroscopic mass, the presence of necrosis, histological grade and the presence of microcalcifications are factors that affect the accuracy of the frozen section examination [17]. Opinions on the size of the lesions appropriate for frozen section examination differ:
Fechner and Millis recommend 0.8 cm, Tinneman et al 0.5 and many groups actually recommend 1 cm as the smallest size [17]. In the present study, 17 of the 30 DCIS lesions had been submitted to frozen section examination. The diagnosis could be made by froze section examination in 9 patients (53%). The false negativity rate was 29% and the number patients in whom the diagnosis could be made on permanent sections was 3 (18%). The sensitivity of the frozen sections was 64%. This may be due to the fact that the majority of the patients did not have a macroscopic mass (only 3 patients). Optimal sampling is of vital importance on the evaluation of AIDH which is a borderline lesion. Bodian et al argued that the incidence of atypical lobular and ductal hyperplasia will increase with examination of increasing number of blocks [18]. Similarly, Schuh et al. [19] and Wang et al. [20] stated that the detection of carcinoma or atypical hyperplasia in benign appearing biopsy materials is related to the number of blocks examined and the frequency of the blocks containing the lesion. In the present study, two cases of AIDH (one in 1996 and one in 1997) were diagnosed as DCIS after examination of serial sections. This suggests that examination of thin serial sections provides a better assessment of atypia. Total or near-total sampling of the material containing AIDH should be performed to exclude in situ carcinoma at another focus. Tavassoli and Norris have established diagnostic criteria for nonapocrine AIDHs [3]. Although these are similar in concept to those established previously by Page et al. [21], they nevertheless include some modifications. These modifications emphasize the cellular structural and quantitative features of AIDH. Tavassoli and Norris define AIDHs as a lesion that exhibits cytologic atypia and is formed by monotonous round cells with slightly increased nucleocytoplasmic ratios and centrally located nuclei that may show hyperchromasia; the cells may show a growth pattern that is distinct form nonnecrotic DCISs [3]. In patients exhibiting the micropapillary and cribriform patterns in AIDHs and non-necrotic DCISs, Tavassoli and Norris proposed the “2-mm rule”. According to this rule, if the diameter of the ducts and ductules exhibiting a DCIS pattern is smaller than 2 mm, the lesion is categorized as an AIDH [3]. Previously, Page et al had stated that atypical proliferation showing in situ carcinoma in ‘a least two areas’ is required for the diagnosis of DCIS [21]. However, the expression ‘two areas’ is not a quantitative evaluation and is open to misinterpretation. This has been a source of discrepancy between pathologists. Recognizing that pathologists are sometimes reluctant in the diagnosis of DCIS in lesions smaller than 2 mm, Tavassoli and Norris proposed the ‘2 mm rule’ for a more standardized approach. They supported this notion by emphasizing that none of their AIDH patients developed invasive carcinoma during follow up [21]. In the present study, there was no difficulty in the diagnosis of IDH lesions whereas the diversity of the patterns and cellular features creates difficulties in the differential diagnosis of AIDHs. In 8 patients, the cribriform pattern- one of the nonnecrotic DCIS patterns- was observed. These patterns were observed in some of the ducts in 6 patients, in all ducts in 2 patients. The diameters of the involved ducts and ductules were measured and results lower than 2 mm were obtained. Therefore, application of the 2-mm-rule circumvented difficulties in diagnosis. The patients who posed the greatest diagnostic problems were cases who did not exhibit DCIS patterns but mimicked intraductal hyperplasia patterns. In 6 patients with classical patterns, the presence of a uniform cell population containing round or oval cells with well-defined margins, in other words, the presence of all cytologic features of AIDH facilitated the diagnosis. However, in 10 patients with the classical IDH patterns, the cell population was more variable and less uniform. Because marked atypia that is never associated with classical IDH was observed on a cytologic basis, inclusion of these cases in the AIDH group was considered more appropriate. In conclusion, the 2-mm-rule facilitated the diagnosis in lesions that exhibited DCIS patterns partially or totally. However, there were difficulties with lesions that did not exhibit these patterns. Since there are major differences in opinion on the borderline lesions, the decisions on individual patients depend on the expertise of the pathologist. Much more effort should be spent to standardize the morphologic evaluation of these lesions. Various classification systems for DCIS have been proposed [22]. The scheme proposed by Bellamy et al. gives priority to patterns but does not emphasize nuclear characteristics [23]. However, the calculation of the percentage is difficult in specimens containing an insufficient number of ducts. Another system, the cytonuclear classification is based solely on cytologic examination and classifies lesions as low, moderate and high grade. For the assessment of the nuclear grade, the cell size is compared with the size of the lymphocyte nucleus; however, the distinctions between low and intermediate and intermediate and high grade lesions are not clear. The van Nuys classification is the one proposed by Silverstein and associates [15]; DCISs are divided as high grade and non-high grade lesions. Irrespective of the comedo necrosis, lesions with high nuclear grade are automatically classified as high grade DCISs. Non-high grade lesions are divided into two categories according to the presence (group 2) and absence (group 1) of comedo necrosis. This system does not include an intermediate grade group and there are no criteria for the extent of comedo necrosis. The Holland system [24] takes into account the cytonuclear and structural differentiation (cell polarization) and defines three grades: poorly differentiated, moderately differentiated and well differentiated. Cell polarization which denotes radial orientation of the cellular apices towards the intercellular lumen is taken as an important criterion in addition to the nuclear grade. Polarization is almost always present in well-differentiated DCISs and almost totally lost in poorly differentiated DCISs. This scheme is unclear on the polarization characteristics of the intermediate grade; in ambiguous cases, the higher grade is designated for the patients. The Nottingham classification is based on pattern and necrosis. DCISs are categorized as those with comedo necrosis, those containing necrosis only and those without necrosis. In patients with mixed patterns, the dominant pattern is designated. This classification does not take into account cytologic characteristics. Jones et al proposed a system based on the extent of necrosis: those with extensive necrosis, those with necrosis only and those without necrosis. Pattern and cytologic features are also taken into consideration. It has been stated that van Nuys classification is the most accurate scheme in reflecting the grade of the invasive component in DCIS cases with concomitant invasive ductal carcinoma [22]. All available classification systems put various degrees of emphasis on patterns, necrosis, nuclear features, cell size, cell structure (polarization). However, DCIS may exhibit more than one of these characteristics. Leninton et al reported on the heterogeneity of the pattern in DCIS [25]. In their series, 46 of the 100 DCIS case showed a mixed type pattern. In the present study, 59% of the patients exhibited more than one patterns. These data suggest that a classification system based solely on pattern characteristics will face difficulties. The weak aspects of various schemes and problems in feasibility were taken into consideration by Tavassoli et al. [3] who were inspired by the breast intraepithelial neoplasia concept described by Rosai and have proposed the DIN classification system. The DIN system envisages a three grade system based on pleomorphic atypia and necrosis. Special patterns and cellular characteristics were included in particular grades. Pleomorphic atypia and necrosis are evaluated separately and the results entered into the scheme. In the present study, 9.37% of the patients were grade I (DIN 1c), 34.7% were grade II (DIN 2) and 56.2% were grade III (DIN 3). In lesions without pleomorphic atypia and necrosis, lesions with cribriform and micropapillary patterns were designated as grade I. Grade III patients had both nuclear pleomorphic atypia and necrosis and did not pose difficulties in grading. One lesion that contained signet cells that stained positively with a mucin stain (more than 75%) was included in the grade III category. The majority of the grade II patients showed micropapillary and cribriform pattern (6 patients). In the DIN classification system, lesions that have the cribriform and micropapillary pattern, exhibit mild pleomorphic atypia (not as marked as that in comedo carcinomas) but do not show necrosis are categorized as intermediate lesions. However, some of our patients with cribriform and micropapillary pattern, showed mild pleomorphic atypia but also extensive necrosis. In such instances, the pleomorphic atypia was given priority and these lesions were included in the grade II category. In the present study, cell size that is not considered in the DIN classification system was evaluated in the grading of the DCIS lesions. We assessed this parameter in the cytonuclear classification system as did another group [26]. Tumor cell size was also taken as a parameter: small (smaller than 3 times the size of a lymphocyte nucleus), medium size (between 3 and 5 times) and large size (larger than 5 times). All 18 lesions with large cells (100%) were grade III, 10 lesions (90.9%) with medium-size lesions were grade II and two lesions (66.6%) with small cells were grade I. In conclusion, there was a close relationship between cell size and grade; therefore, cell size may be used as a criterion in grading. Although we encountered some difficulties in grading, the DIN classification system provides practical, simple, feasible and easily understandable approaches to the grading of DCISs. Amplification of the c-erbB2 oncogene has been demonstrated in kidney, stomach and salivary gland adenocarcinomas. High levels of c-erbB2 protein in breast carcinomas have been reported to be associated with poor prognosis [26]. Intraductal epithelial proliferations are associated with an increased risk of breast carcinoma [1-3]; the potential role of biomarkers such as p53, EGFR, bcl-2, estrogen and progesterone receptor status have been investigated in the differential diagnosis of lesion such as AIDH and DCIS that can not be differentiated easily on morphologic grounds alone. The data on the expression of c-erbB2 in intraductal proliferative lesions are controversial. In the present study, c-erbB2 staining was performed on 45 IDH lesions: 37 showed no staining (82%), 7 showed weak focal staining (15.6%), one showed moderate staining (2.2%). Some authors detected no staining c-erbB2 in any of the IDH cases they investigated [4, 5, 27]. Other studies including ours found a low frequency of staining; this suggests that the negative results may be due to insufficient sampling and consequent missing of the atypical proliferation focus. Among the 19 AIDH patients, 17 patients (89.5%) showed no staining and 2 (10.5%) showed weak focal staining. Some studies found no staining with cerbB2 antibody in atypical epithelial proliferations [4, 5] whereas Tavassoli and Man found a staining frequency of 10% [27]. This approximates to the figure in the present study (10.5%). One important characteristic of the negative studies in the small number of cases investigated: 13 in one study [4] and 15 in another one [5]. The number in the present study was slightly higher -19- and suggests that increasing the number of cases may yield more objective results. Of the 30 DCIS patients, 3 (10%) showed no staining with c-erbB3, 2 showed (6.7%) showed weak focal staining, 7 (23.3%) showed moderate staining, 18 (60%) showed strong staining: in total, 90% of the cases showed some degree of staining with c-erbB2. The reported frequencies in the literature vary between 32 and 56 % [6, 28]. In the present study, there were statistically significant differences in the c-erbB2 staining intensities of IDH, AIDH and DCIS patients lesions (p<0.001). In intergroup comparisons, there was no significant difference between IDH and AIDH lesions (p>0.05); however, there was a significant difference between AIDH and DCIS lesions (p<0.001). These results suggest that c-erbB2 oncogene expression may be useful in the evaluation of intraductal proliferative lesions, particularly in the differentiation of AIDH and DCIS which may be difficult to distinguish on morphologic grounds alone. An interesting finding was that 7 of the 10 IDH patients sections containing foci of apocrine metaplasia or hyperplasia tested weakly positive with c-erbB2. There is a report on a patient with positive staining in apocrine metaplasia [4]. In that particular patient, it has been argued that c-erbB2 antibody realized weak cytoplasmic staining and due to fixation characteristics, staining accumulated near the cytoplasmic membrane. Interestingly, in an immunohistochemical study on bcl-2, normal ducts or lobuli showed positive epithelial staining with bcl-2, the staining was decreased or absent in apocrine metaplasia foci [29]. In a study on estrogen receptors, similarly areas of apocrine metaplasia showed decreased receptor expression [30]. These results suggest that apocrine metaplasia has a biological behavior that is different from those of normal ducts and lobuli. Another striking finding was that in a patient with DCIS, in situ carcinoma area accompanying AIDH showed weak focal staining with the c-erbB2 antibody. The more extensive staining in this particular patient in comparison with other AIDH patients suggests that AIDH may be intermediate lesion in the development of a malignant clone. Strong c-erbB2 staining was detected in 33% of the grade I patients, 30% of the grade II patients and 77% of the grade III patients. The frequency in the grade III patients was significantly higher than the frequency in the two other groups (p=0.016). Many studies investigated the relationship of c-erbB2 staining in DCISs with the extent of DCIS, the degree and extent of necrosis, histologic subtype, nuclear pleomorphism, mitosis and cell size. Other studies examined the relationship between different grading systems with c-erbB2 expression. Zafrani et al used the Holland classification [31] and demonstrated that poorly differentiated (high grade) DCISs had significantly higher levels of expression in comparison with well differentiated lesions (p<0.001). In another study, Leal et al. [32] reported that the frequency of c-erbB2 expression in high grade DCISs may be as high as 45 %. These results suggest that c-erbB2 expression increases with higher grade in DCIS and therefore c-erbB2 may be an adjunct in the prediction of biologic behavior and clinical course of DCISs. In a study that considered cell size, nuclear pleomorphism and mitosis [33], multivariate analysis of the data on 75 patients showed that cell size was the most valuable parameter. In the present study, none of the DCISs with small cells were positive of c-erbB2 whereas 84% of the DCISs with large cells were positive. In two studies on cell size, similar highly significant differences were reported [6, 22]. Although our results are similar to those in the literature, 13 of the 16 specimens (81%) with large cell size showed strong c-erb-B2 staining. There was a statistically significant difference in c-erbB2 staining between DCISs with large cells and those with small or medium size cells (p=0.016). These results show that in DICS, c-erbB2 expression increases with cell size and the clinical course and prognosis of DCISs with large cells may be worse in comparsion with DCISs with small and medium size cells. In immunohistochemistry, 2 of the 4 patients (50%) with no pleomorphic atypia and 15 of the 17 patients with marked atypia (88.2%) showed strong staining with the c-erbB2 antibody (p=0.03). In a study that used cytonuclear differentiation as a parameter, the frequency of c-erb-B2 staining in patients with marked cellular pleomorphism, was precented in 79% of the cases and was statistically significantly higher when compared with cases without marked cellular pleomorphism [33]. In another study, the frequency of c-erbB2 in lesions with poor nuclear differentiation was 73.6% [34]. These results suggest that in DCISs, increased c-erbB2 expression correlates with higher levels of nuclear atypia and thus may serve as an important prognostic factor.
CONCLUSION
IDH, AIDH and DCIS are generally nonpalpable lesions that frequently accompany benign macroscopic breast masses. The 2mm rule proposed in the DIN calcification system facilitates the diagnosis in AIDH patients that exhibit cytologic and structural features of AIDH. However, in lesions that mimick the structural features of intraductal hyperplasia, sometimes cytologic uniformity and monotony are not adequate. The DIN system proposes a practical and simple approach to the grading of DCIS. However, there are still ambiguous aspects in the evaluation of intermediate grades. There were statistically significant differences between the subgroups of intraductal proliferative lesions (IDH, AIDH and DCIS) (p<0.001). Especially the difference between AIDH and DCIS lesions (p<0.001) may be an adjunct to the morphological diagnosis. Although 82.2% of the IDH lesions exhibited no staining, weak focal staining in areas of apocrine metaplasia or hyperplasia (7 of the 10 patients) suggests that this points to a different biological behavior. There was a statistically significant difference in the intensity of the c-erbB2 staining between low grade and high grade DCIS lesions (p=0.016). Staining in DCIS lesions with large cells were found to be significantly more intense than the DCIS lesions with small and medium size cells (p=0.016). Similarly, lesions with marked pleomorphic atypia according to the DIN classification showed more intense staining in comparison to those with mild or no atypia (p=0.03). These results suggest that DCISs with large cells, marked nuclear atypia and high histologic grade may show a worse clinical prognosis.
DECLARATION OF INTEREST
This study was performed with permission of Istanbul University Cerrahpaşa Faculty of Medicine Scientific and Ethics commitee, and supported by Istanbul University Research Fund.
REFERENCES
- [1].Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985;312:146–151. doi: 10.1056/NEJM198501173120303. [DOI] [PubMed] [Google Scholar]
- [2].London SJ, Connolly JL, Schnitt SJ, Colditz GA. A prospective study of benign breast disease and the risk of breast cancer. JAMA. 1992;267:941–944. [PubMed] [Google Scholar]
- [3].Tavassoli FA, Norris HJ. A comparison of the results of long-term follow-up for atypical intraductal hyperplasia and intraductal hyperplasia of the breast. Cancer. 1990;65:518–529. doi: 10.1002/1097-0142(19900201)65:3<518::aid-cncr2820650324>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- [4].Gusterson BA, Machin LG, Gullick WJ, Gibbs NM, Powles TJ, Elliott C, et al. c-erbB-2 expression in benign and malignant breast disease. Br J Cancer. 1988;58(4):453–7. doi: 10.1038/bjc.1988.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Allred DC, Clark GM, Molina R, Tandon AK, Schnitt SJ, Gilchrist KW, et al. Overexpression of HER-2/neu and its relationship with other prognostic factors change during the progression of in situ to invasive breast cancer. Hum Pathol. 1992;23(9):974–9. doi: 10.1016/0046-8177(92)90257-4. [DOI] [PubMed] [Google Scholar]
- [6].De Potter CR, Schelfhout AM, Verbeeck P, Lakhani SR, Brunken R, Schroeter CA, et al. neu overexpression correlates with extent of disease in large cell ductal carcinoma in situ of the breast. Hum Pathol. 1995;26(6):601–6. doi: 10.1016/0046-8177(95)90163-9. [DOI] [PubMed] [Google Scholar]
- [7].Tavassoli FA. Second edition. Appleton & Lange; 1999. Pathology of the breast. [Google Scholar]
- [8].Tavassoli FA. Ductal carcinoma in situ: introduction of the concept of ductal intraepithelial neoplasia. Mod Pathol. 1998;11:140–154. [PubMed] [Google Scholar]
- [9].Bartow SA, Pathak DR, Black WC, Key CR, Teaf SR. Prevalence of benign, atypical, and malignant breast lesions in populations at different risk for breast cancer. A forensic autopsy study. Cancer. 1987;60:2751–2760. doi: 10.1002/1097-0142(19871201)60:11<2751::aid-cncr2820601127>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- [10].Schuerch C, 3rd, Rosen PP, Hirota T, Itabashi M, Yamamoto H, Kinne DW, et al. A pathologic study of benign breast diseases in Tokyo and New York. Cancer. 1982;50(9):1899–903. doi: 10.1002/1097-0142(19821101)50:9<1899::aid-cncr2820500942>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- [11].Page DL, Vander Zwaag R, Rogers LW, Williams LT, Walker WE, Hartmann WH. Relation between component parts of fibrocystic disease complex and breast cancer. J Natl Cancer Inst. 1978;61(4):1055–63. [PubMed] [Google Scholar]
- [12].Ernster VL, Barclay J, Kerlikowske K, Grady D, Henderson C. Incidence of and treatment for ductai carcinoma in situ of the breast. JAMA. 1996;275:913–918. [PubMed] [Google Scholar]
- [13].Morrow M. The natural history of ductal carcinoma in situ. Impiications for clinical decision making. Cancer. 1995;76:1113–1115. doi: 10.1002/1097-0142(19951001)76:7<1113::aid-cncr2820760702>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- [14].Arnesson LG, Smeds S, Fagerberg G, Grontoft O. FolIow-up of two treatment liodalities for ductal cancer in situ of the breast. Br J Surg. 1989;76:672–675. doi: 10.1002/bjs.1800760707. [DOI] [PubMed] [Google Scholar]
- [15].Silverstein MJ, Rosser RJ, Gierson ED, Waisman JR, Gamagami P, Hoffman RS, et al. Axillary lymph node dissection for intraductal breast carcinoma--is it indicated? Cancer. 1987;59(10):1819–24. doi: 10.1002/1097-0142(19870515)59:10<1819::aid-cncr2820591023>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- [16].Rosai J. Breast. In: Rosai J, editor. Ackerman's Surgical Pathology. 8th ed. St. Louis: Mosby; 1996. pp. 1565–1660. [Google Scholar]
- [17].Cheng L, Al-Kaisi NK, Liu AY, Gordon NH. The results of intraoperative consultations in 181 ductal carcinomas in situ of the breast. Cancer. 1997;80:75–79. [PubMed] [Google Scholar]
- [18].Bodian CA, Perzin KH, Lattes R, Hoffmann P. Reproducibility and validity of pathologic classifications of benign breast disease and implications for clinical applications. Cancer. 1993;71(12):3908–13. doi: 10.1002/1097-0142(19930615)71:12<3908::aid-cncr2820711218>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- [19].Schuh ME, Nemoto T, Penetrante RB, Rosner D, Dao TL. Intraductal carcinoma. Analysis of presentation, pathologic findings, and outcome of disease. Arch Surg. 1986;121:1303–1307. doi: 10.1001/archsurg.121.11.1303. [DOI] [PubMed] [Google Scholar]
- [20].Wong JH, Kopaid KH, Morton DL. The impact of microinvasion on axillary node metastases and survival in patients with intraductal breast cancer. Arch Surg. 1990;125:1298–1302. doi: 10.1001/archsurg.1990.01410220082011. [DOI] [PubMed] [Google Scholar]
- [21].Page DL, Dupont WD, Rogers LW, Rados MS. Atypical hyperplastic lesions of the female breast. A long-term follow-up study. Cancer. 1985;55:2698–2708. doi: 10.1002/1097-0142(19850601)55:11<2698::aid-cncr2820551127>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- [22].Douglas-Jones AG, Gupta SK, Attanoos RL, Morgan JM, Mansel RE. A critical appraisal of six modern ciassifications of ductal carcinoma in situ of the breast (DCIS): correlation with grade of associated invasive carcinoma. Histopathology. 1996;29:397–409. doi: 10.1046/j.1365-2559.1996.d01-513.x. [DOI] [PubMed] [Google Scholar]
- [23].Bellamy CO, McDonald C, Salter DM, Chetty U, Anderson TJ. Noninvasive ductal carcinoma of the breast: the relevance of histologic categorization. Hum Pathol. 1993;24:16–23. doi: 10.1016/0046-8177(93)90057-n. [DOI] [PubMed] [Google Scholar]
- [24].Holland R, Peterse JL, Millis RR, Eusebi V, Faverly D, van de Vijver MJ, et al. Ductal carcinoma in situ: a proposal for a new classification. Semin Diagn Pathol. 1994;11(3):167–80. [PubMed] [Google Scholar]
- [25].Lennington WJ, Jensen RA, Dalton LW, Page OL. Ductal carcinoma in situ of the east. Heterogeneity of individual lesions. Cancer. 1994;73:118–124. doi: 10.1002/1097-0142(19940101)73:1<118::aid-cncr2820730121>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- [26].2nd ed. Sheffield: NHS BSP Publications Number 3; 1995. National Coordinating Group for Breast Screening Pathology Pathology reporting in breast cancer screening. [Google Scholar]
- [27].Tavassoli FA, Man YG. Morphofunctional features of intraductal hyperplasia, atypical intraductal hyperplasia and various grades intraductal carcinoma. Breast J. 1995;1:155–162. [Google Scholar]
- [28].Poller DN, Roberts EC, Bell JA, Elston CW, Blamey RW, Ellis IO. p53 protein expression in mammary ductal carcinoma in situ: relationship to immunohistochemical expression of estrogen receptor and c-erbB-2 protein. Hum Pathol. 1993;24(5):463–8. doi: 10.1016/0046-8177(93)90157-c. [DOI] [PubMed] [Google Scholar]
- [29].Siziopikou KP, Prioleau JE, Harris JR, Schnitt SJ. bcl-2 expression in the spectrum of preinvasive breast lesions. Cancer. 1996;77:499–506. doi: 10.1002/(SICI)1097-0142(19960201)77:3<499::AID-CNCR11>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- [30].Barnes R, Masood S. Potential value of hormone receptor assay in carcinoma in situ of breast. Am J Clin Pathol. 1990;94:533–537. doi: 10.1093/ajcp/94.5.533. [DOI] [PubMed] [Google Scholar]
- [31].Zafrani B, Leroyer A, Fourquet A, Laurent M, Trophilme D, Validire P, et al. Mammographically-detected ductal in situ carcinoma of the breast analyzed with a new classification. A study of 127 cases: correlation with estrogen and progesterone receptors, p53 and c-erbB-2 proteins, and proliferative activity. Semin Diagn Pathol. 1994;11(3):208–14. [PubMed] [Google Scholar]
- [32].Leal CB, Schmitt FC, Bento MJ, Maia NC, Lopes CS. Ductal carcinoma in situ of the breast. Histologic categorization and its reiationship to ploidy and immunohistochemical expression of hormone receptors, p53, and c-erbB-2 protein. Cancer. 1995;75:2123–2131. doi: 10.1002/1097-0142(19950415)75:8<2123::aid-cncr2820750815>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- [33].Bobrow LG, Happerfield LC, Gregory WM, Millis RR. Ductal carcinoma in situ: assessment of necrosis and nuclear morphology and their association with biological markers. J Pathol. 1995;176:333–3341. doi: 10.1002/path.1711760404. [DOI] [PubMed] [Google Scholar]
- [34].Bobrow LG, Happerfield LC, Gregory WM, Springall RD, Millis RR. The classification of ductal carcinoma in situ and its association with biological markers. Semin Diagn Pathol. 1994;11:199–207. [PubMed] [Google Scholar]


