Abstract
The aim of this study compares to the increase in tissue temperature and the thermal histological effects of ultrasonic scalpel, bipolar and unipolar electrosurgery incisions in the tongue tissue of rabbits. This study evaluates the histopathological changes related to thermal change and the maximum temperature values in the peripheral tissue brought about by the incisions carried out by the three methods in a comparative way. To assess thermal tissue damage induced by the three instruments, maximum tissue temperatures were measured during the surgical procedure and tongue tissue samples were examined histopathologically following the surgery. The mean maximum temperature values of the groups were 93.93±2.76 C° for the unipolar electrocautery group, whereas 85.07±5.95 C° for the bipolar electrocautery group, and 108.23±7.64 C° for the ultrasonic scalpel group.
There was a statistically significant relationship between the increase in maximum temperature values and the separation among tissue layers, edema, congestion, necrosis, hemorrhage, destruction in blood vessel walls and fibrin accumulation, and between the existence of fibrin thrombus and tissue damage depth (p<0.05).
It was concluded that the bipolar electrocautery use gives way to less temperature increase in the tissues and less thermal tissue damage in comparison to the other methods.
KEY WORDS: Rabbit, tongue, electrocautery, scalpel, temperature, thermal injury
INTRODUCTION
Surgical interventions in tongue tissue frequently include excision of the masses in the tongue or surgical techniques carried out in order to lessen the volume of the tongue in OSAS treatment. Within the framework of these treatments, tools such as radiofrequency, unipolarbipolar electrocautery, laser, and coblator can be used. All these surgical tools cause temperature rise related to the effect of thermal energy, protein denaturation, and tissue destruction. During the use of these tools peripheral area surrounding the incised part is also subjected to temperature rise. This situation may lead to delays in wound healing, decrease in blood circulation, and damage to sensorial nerves and other peripheral vital structures. Thermal tissue damage during the surgery is an important parameter affecting postoperative tissue healing, infection, pain, transition to oral feeding, and hospitalization. Because of the reasons listed above, it is important to pay careful attention to the process of determining the thermal method for surgical dissections that would prevent extreme temperature rise in the tissue and cause minimum spread of temperature to the surrounding tissues [1]. An ample amount of studies carried out on human and animal models have focused on tissue damages related to surgeries using ultrasonic scalpel, bipolar electrocautery, and unipolar electrocautery [2-4]. No study, however, has evaluated the relationship between tissue damage in the tongue tissue and the temperature rise caused by the tools used. The objective of this study is to research the relationship between the maximum temperature rise in the rabbit tongue tissue caused by these three methods and thermal tissue damage, and to determine which method would be more advantageous in reducing thermal tissue damage.
MATERIALS AND METHODS
The study was carried out at Ankara University, Faculty of Veterinary Medicine, Animal Research Lab with the same university's Animal Ethics Board approval dated May 13, 2010 and with the protocol number 539. All the procedures were performed in accordance with the rules set by Ankara University's Animal Ethics Board.
Animals
Sixteen albino rabbits weighing between 2.5 and 3 kg were used in the experiment. The experimental animals were kept alone in separate cages with constant water and food supply at 50% humidity and 23C°degrees.
Procedures
The surgical procedures were done in operating room conditions. The procedures carried out on the experimental animals were done under general anesthesia. Intraperitoneal ketamine (30mg/kg) (KETALAR, Pfizer- Pharmaceutical Company) with Xylazine (5mg/kg) (Rompun, Bayer) were used for general anesthesia. The tongues of all the animals were put out and fixed under general anesthesia. Three full layer incision lines were done along equal length segments from the distal towards the proximal, parallel, perpendicular to the vertical axis of the tongue in the tongue tissue with all three different tools alternately. Special attention was paid to the completion of the incision without applying too much pressure on the tissue while using the tool tips having them on hold until the tongue tissue was cut. Megapower Megadyne Electrosurgical Generator (MEGADYNE MEDICAL PRODUCTS, Inc., Draper, US) cautery was used for electrocauterization procedures. The device was used at ACE (Advance cutting effect) mode for unipolar cauterization. The device has 150 watts of power and is at 400 Hz frequency at this mode. The ACE mode is designed to provide a scalpel-like cutting effect for minimal thermal necrosis and reduced scarring. When the device is used at this mode there is a consistent cutting effect at minimum power settings for improved patient safety. It reaches this effect by adjusting its power to the changing tissue resistance. Rocker Switch Pencil with ACE Blade and Holster were used as cautery pens while 2.5 inches standard blade electrode was used as unipolar cautery tip. The device power was set at 35 watts for bipolar cauterization and Scoville-Greenwood forceps, 1.5 mm tip (17.8 cm) was used as cautery pen. The maximum power of the device was 80 watts and its frequency was 400 Hz for bipolar cautery. Harmonic™ (Ethicon Endosurgery, Inc., Cincinnati, Ohio, USA) was used as the ultrasonic scalpel device. The procedure was performed when the device power was at the third level using ENSEAL® RF60 Generator and with a shaft length of 14 cm and a shaft diameter of 5 mm tips. Deep maximum temperature measurements were done with electrode thermometer device (Fluke 54 II™, Fluke Corporation, Washington, USA) located at a depth of 3-4 mm. The tip of the thermometer's metal probe was fixed in the tongue, 5 mm proximal of the incised line during the incision. The maximum temperature data obtained from the thermometer during the incision were recorded. The same procedure was repeated with all the three devices each time covering enough distance towards the proximal of the tongue. A 10-minute recess was observed among the procedures in order to allow the tongue tissue to return to normal levels. Maximal temperature values were recorded in three different groups. Subsequently, tongue tissue samples were taken and the animals were sacrificed with additional anesthetic doses. Each tissue sample was fixed in 10% buffered formaldehyde taken into separate storage boxes, labeled, and three different groups were formed in a total of 48 bottles. The histopathological analysis of the tissues was done at Ankara Social Security Administration's Teaching Hospital, Pathology Clinic Laboratory. The material was sliced perpendicularly to the incision line in 3-4 mm thickness and all the material was followed. Following the routine tissue follow-ups all the tissue samples were stained with hematoxylin-eosin, having been taken into 5 microns of incisions, and evaluated with the Olympus Bx50 light microscope. Tissue changes that can be seen in thermal tissue damages (thermal damage level) were rated subjectively during the building of pathological rating parameters. The evaluation method was devised according to the parameters set by the pathologist performing the evaluation and was based on the classic histopathological tissue changes known to happen in thermal tissue damage cases [5]. In terms of the followed tissue changes, the histopathological evaluation parameters were set in the Table 1 and Figures 1- to- 13., The results obtained from these tissue parameters were separately scored for each parameter.
TABLE 1.
Histopathological changes after thermal tissue damage.
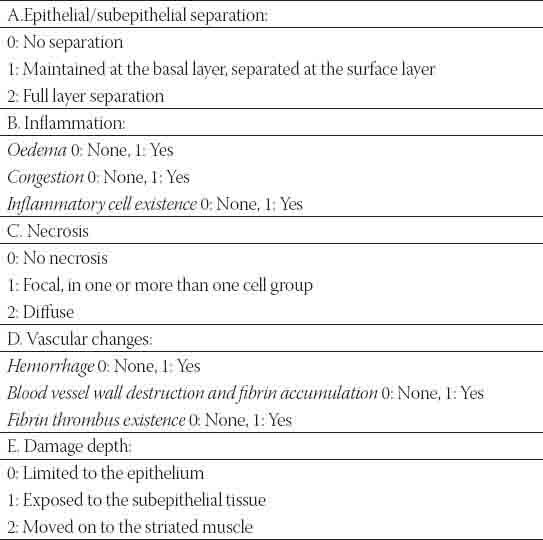
FIGURE 1.

Epithelial/subepithelial separation (H&E×100).
FIGURE 13.

Tissue reaction moving towards total epithelial necrosis and striated muscle tissue in close view (H&E×200).
FIGURE 2.

Full layer epithelial/subepithelial separation (H&E×200).
FIGURE 3.

Separation whereby the basal layer is maintained (H&E×100).
FIGURE 4.
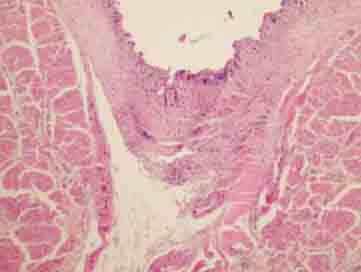
Full layer separation with epidermal necrosis and subepithelial changes (H&E×200).
FIGURE 5.
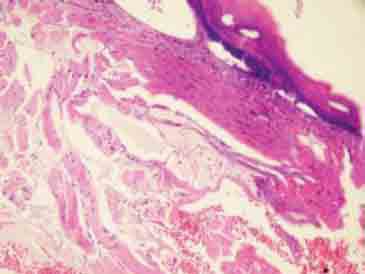
Edema and congestion in the subepithelial tissue (H&E×200).
FIGURE 6.
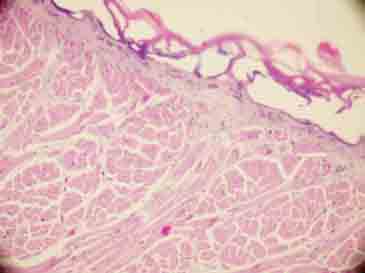
Total epithelial necrosis (H&E×200).
FIGURE 7.
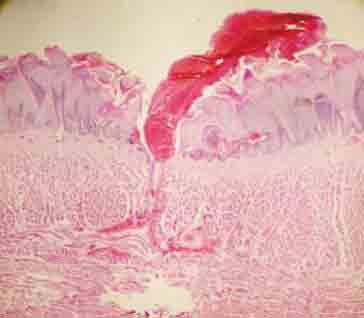
Intraepithelial and subepithelial bleeding in the bipolar group (H&E×100).
FIGURE 8.

Subepithelial bleeding in the coblation group (H&E×100).
FIGURE 9.
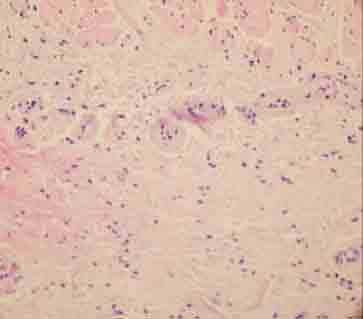
Fibrin accumulation on the blood vessel walls (H&E×400).
FIGURE 10.

Fibrin and necrosis on blood vessel walls forming occlusion in the lumen (H&E×400).
FIGURE 11.
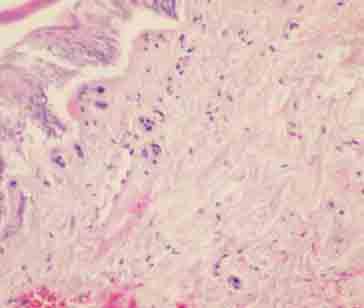
Fibrin and hemorrhage on blood vessel walls (H&E×200).
FIGURE 12.

Tissue reaction moving towards total epithelial necrosis and striated muscle tissue (H&E×100).
RESULTS
Two of the experimental animals died because of infection before the procedure during the feeding phase of the animals. The evaluation of tissue samples of 42 out of a total of 48 samples was completed because of the problems that happened in two animals during the procedures. Temperature measurements and histopathological evaluation of a total of 42 tissue samples were completed and 14 (33.3%) of them were done with unipolar cautery, 15 (35.7%) with bipolar cautery, and 13 (31%) with ultrasonic scalpel. The mean maximum temperature values obtained during the procedures were 93.93±2.76 Co for the unipolar cautery group, 85.07±5.95 Co for the bipolar cautery group, and 108.23±7.64 Co for the ultrasonic scalpel group. The maximum temperature values of each of the three groups were found to be independent of one another according to the ANOVA test. The intergroup differences were evaluated by the Tukey test and a statistical difference was found among the results (p<0.05). The relationship between the surgical methods and the histological parameters was evaluated by the Chi-Square test. The results of histopathological evaluation related to full layer separation between tissue layers, edema, congestion, hemorrhage, blood vessel wall destruction and fibrin accumulation, fibrin thrombus existence, and diffuse necrosis existence changed according to the methods used (p<0.05), (Table 2). The relationship between the obtained maximum temperature values and the histopathological parameters was evaluated by the Independent T-test. There was a statistically significant relationship between the rise in maximum temperature values and the separation among the tissue layers, edema, congestion, necrosis, hemorrhage, blood vessel wall destruction and fibrin accumulation, and fibrin thrombus existence (p<0.05). There was also a statistically significant relationship between the tissue damage depth and the maximum mean tissue temperature rise according to the ANOVAs analysis (p<0.05), (Table 3). The power analysis revealed that the test had a power of 99%.
TABLE 2.
Tissue damage according to the methods used, Chi-Square analysis results, frequency, percentage and p values are displayed.

TABLE 3.
The results of maximal temperature measurement according to the tissue damage parameters, Independent T-test results, frequency, mean ± standard deviation and p values are displayed.

DISCUSSION
Excessive heat is an important cause of tissue injury (burn). Burns used to be classified from the first to the fourth degree, according to the depth of injury (first degree burns being the most superficial). Superficial burns (first degree burns) are confined to the epidermis. Partial thickness burns (second degree burns) involve injury to the dermis. Full thickness burns (third degree burns) extend to the subcutaneous tissue [6]. The surgical tools that work based on the principle that thermal energy is applied to the tissue bring about some changes depending on the level of heat in the tissue. These changes happen in different forms in proportion to the amount and period of the transferred energy to the tissue by the tool used [7, 8]. The destruction in the tissue related to the rise in temperature may cause local and systemic side effects. The burn site is ideal for the growth of microorganisms; the serum and debris provide nutrients, and the burn injury compromises blood flow, blocking effective inflammatory responses. The most common offender is the opportunist, and hospital acquired microorganisms. Cellular and humoral defence against infections are compromised, and both lymphocyte and phagocyte functions are impaired. Direct bacteremic spread and release of toxic substances such as endotoxin from the local site have dire consequences. Pneumonia, septic shock with renal failure and the acute respiratory distress syndrome are the most common serious sequelae [5]. In thermal tissue damage survivors, the development of hypertrophic scars, of both the site of the original burn and at donor graft sites, and itching may become long term, dificult to treat problems. Hypertrophic scars after burn njuries may be a consequence of continuous angiogenesis in the wound caused by excess neuropeptides, such as substance P released from injured nerve endings [5]. High temperature is brought about by this resistance and as a result of the dehydration caused by the generated heat incision is created. The pre-condition for a successful electrocauterization is preventing burns while cutting the tissue. In order to have a burn-free incision the appropriate form of current and dose need to be carefully selected [8]. When the tool is used at the full wave generating mode, the incision made produces an effect similar to the classic scalpel incision in the tissue since there will not be a wide gap between the voltage and power. Besides it reduces bleeding because of the temperature rise in the incised area. At the modulation wave mode the exit voltage of the tool is high but the active power is low. It enables coagulation through generating more heat in the tissue but it also causes burns in the periphery and more tissue damage [9]. Mannes et al. created incisions of 1 mm depth with a speed of 8 mm/sec in rat tongues using the electrocautery method at varying frequencies and wave forms and they carried out a histological evaluation of these incisions. The authors concluded that the modular wave form and low frequency values cause more tissue damage. Therefore, we preferred to use the full wave mode for cautery use in order to have less tissue damage [10]. Electrocautery is divided into two forms; monopolar and bipolar. These systems are different from each other in terms of their working principles and the effects they create. High energy is used in order to obtain the desired results with monopolar units. Bipolar units, on the other hand, need less energy. There are two electrodes, one active one neutral, in monopolar cauterization. The neutral pole is the land line in these types of tools and they are called monopolar since they function passively [11]. Neutral electrodes also have forms like sitting electrodes, hand electrodes, and armband or wristband electrodes [8]. Bipolar cauteries are tools that have low impedance and they exhibit maximum 50 Watts of exit. The reason for this is the fact that the route through which the high-frequency current will complete its circuit is very short; otherwise an arc will be created [12]. The monopolar technique enables the current to be transmitted to the surgical area from the tip of the active electrode. In other words, the monopolar technique uses the patient's body as a circuit complement. With bipolar cautery, on the other hand, the electrical current flows through the distance between the two tips of the cautery. The current spreads into the peripheral tissues on its way to the passive electrode on its way back in the monopolar cautery. Therefore, the monopolar technique is not appropriate to use if the patient has a pacemaker, if he has neural or cardiac problems. Since the bipolar surgical instruments have an electrical resistance within a range of 0-50 Ω, they can be used in the wet areas in intraoral procedures. Moreover, because of the fact that the tissues are cut and coagulated under permanent irrigation also enable the tissues to be kept healthy during the operation they can be specifically preferred. When the intraoral procedures are taken into consideration, the saliva has a poor electrical resistance by nature. Viscous saliva forming a thick layer, however, gives way to the dispersal of a large portion of the current and the desired result might not be obtained. The most appropriate environment is the one that is humidified with normal saline. We performed the procedures in a relatively dry environment having taken the tongue tissues out in our study since our aim was to have the procedures in rabbit tongues under equal conditions with all the three methods. The results of our study also revealed that bipolar cautery causes less thermal tissue damage in the tongue tissue compared to unipolar cautery. The mean values of maximum temperature in the tissue were 85.07±5.95 Co and 93±2.76 Co for bipolar and unipolar cautery respectively. There were statistically meaningful results (p<0.05) in favor of bipolar electrocautery, between separation among tissue layers, edema, congestion, necrosis, hemorrhage, destruction in blood vessel walls and fibrin accumulation, fibrin thrombus existence, and tissue damage depth in proportion to temperature degrees. In the light of the classical information listed above and the results obtained by our study, we can conclude that bipolar cautery is more advantageous regarding the risk of thermal tissue damage in the healthy peripheral tissues when used in the tongue tissue compared to unipolar cautery. On the other hand, the most important advantage of the unipolar method compared to the bipolar one is that unipolar cautery pens’ designs are more ergonomic and therefore it yields more comfort to surgical manipulation during the surgical procedures. Ultrasonic scalpel spreads vibration at so high a frequency that it cannot be perceived by the ear or the eye and this mechanical energy is transmitted to the tissue. It works mechanically with a vibration at 30-60 KHz frequency. The vibrating knife causes protein denaturation by minimal tissue heating through rupturing the tertiary hydrogen ligaments in the protein molecules. The denatured proteins give way to homeostasis by forming adhesive coagulum. In this way, the instrument performs the cutting and coagulation procedures simultaneously [13]. Tissue dissection is created through separation by cutting or forming a cavity. Separation by forming a cavity takes place in collagen-poor areas like the tonsils and the liver, cutting takes place in collagen-rich tissues such as the fascia and tendons [14]. It is stipulated that ultrasonic scalpel forms protein denaturation by mechanically breaking the hydrogen ligaments placed in the protein molecules and therefore enabling less tissue damage by creating less temperature increase around the surgical area as compared to the other methods [15]. The balance between cutting and coagulation is maintained according to tissue tension, power level, and sharpness of the knife. If a sharp knife is used at a high power and is applied to a tense tissue it cuts rapidly with less homeostasis, but if it is applied to a low-tension tissue with an obtuse knife at low power it cuts slower with a strong homeostasis. Since the tongue tissue has a medium tissue tension and because the tongue has well blood supplies, the power level to be used was selected as level three, which is the medium level for the model we used, in order to maintain the balance between cutting and coagulation [3]. In contrast to the information provided above, the results of our study revealed that the mean maximum temperature values of the tongue tissue obtained by the ultrasonic scalpel method were higher than the other electrocautery methods at 108.23±7.64 Co. In line with this result, we found out that the separation among the tissue layers, edema, congestion, necrosis, hemorrhage, destruction in blood vessel walls and fibrin accumulation, fibrin thrombus existence and tissue destruction depth were higher than both electrocautery methods in a statistically meaningful manner (p<0.05). According to these results, we concluded that the ultrasonic scalpel causes more temperature rise and thermal tissue damage in the peripheral tissues than the electrocautery methods. When the difference between the results obtained by each of the three methods is taken into consideration, it is seen that bipolar cautery causes the least temperature rise and thermal tissue damage in the peripheral tissues in rabbit tongue tissue, whereas the ultrasonic scalpel method gives way to the highest temperature rise and the largest tissue damage. This result shows us that the bipolar electrocautery, which is a classical method, is a reliable technique that we can use to prevent thermal damage and complications in procedures performed in the tongue tissue. Since bipolar cautery is a highly successful method in bleeding control there is no need to have an additional method for bleeding control in the tongue tissue, which has very well blood circulation, it is also a time-saving and a convenient method for the surgeon during the procedure. Besides, it is advantageous because it is an inexpensive and widespread method. On the other hand, because the bipolar cautery tips by structure are not designed for surgical dissection they may cause some troubles regarding the surgical technique. We believe that this problem, too, can be overcome through designing new tip for dissection.
CONCLUSION
Bipolar electrocautery is the method which causes the least temperature rise in the surrounding tissues among these 3 methods. In order to minimize complications due to high temperature in the tongue tissue, we believe that bipolar electrocautery should be the method chosen, provided that tip revisions suitable for dissection are made in conventionally used electrocautery tips.
DECLARATION OF INTEREST
There is no financial support received for this present study for all authors, no financial involvement of any kind or affiliation with any organization whose financial interests may be affected by material in the manuscript or which may potentially bias it.
REFERENCES
- [1].Palanker DV, Vankov A, Huie P. Electrosurgery with cellular precision. IEEE Trans Biomed Eng. 2008;55(2 Pt 2):838–841. doi: 10.1109/TBME.2007.914539. [DOI] [PubMed] [Google Scholar]
- [2].Speyer M, Joe J, Davidson JM, Ossoff RH, Reinisch L. Thermal injury patterns and tensile strength of canine oral mucosa after carbon dioxide laser incisions. Laryngoscope. 1996;106(7):845–850. doi: 10.1097/00005537-199607000-00012. [DOI] [PubMed] [Google Scholar]
- [3].Liboon J, Funkhouser W, Terris DJ. A comparison of mucosal incisions made by scalpel, CO2 laser, electrocautery, and constantvoltage electrocautery. Otolaryngol Head Neck Surg. 1997;116(3):379–385. doi: 10.1016/S0194-59989770277-8. [DOI] [PubMed] [Google Scholar]
- [4].Carew JF, Ward RF, LaBruna A, Torzilli PA, Schley WS. Effects of scalpel, electrocautery, and CO2 and KTP lasers on wound healing in rat tongues. Laryngoscope. 1998;108(3):373–380. doi: 10.1097/00005537-199803000-00012. [DOI] [PubMed] [Google Scholar]
- [5].Kane AB, Kumar V. 8th edition. Philadelphia: Saunders Elsevier; 2010. Pathology Basis of Disease, Robbins and Cotran: Common environmental and nutritional occupational exposures, physical environment, thermal injures. [Google Scholar]
- [6].Eisenbeiss W, Marotz J, Schrade JP. Reflection-optical multispectral imaging method for objective determination of burn depth. Burns. 1999;25(8):697–704. doi: 10.1016/s0305-4179(99)00078-9. [DOI] [PubMed] [Google Scholar]
- [7].Livaditis GJ. Comparison of monopolar and bipolar electrosurgical modes for restorative dentistry: a review of the literature. J Prosthet Dent. 2001;86(4):390–399. doi: 10.1067/mpr.2001.118729. [DOI] [PubMed] [Google Scholar]
- [8].Gnanasekhar JD, al-Duwairi YS. Electrosurgery in dentistry. Quintessence Int. 1998;29(10):649–654. [PubMed] [Google Scholar]
- [9].Barba M, Fastenmeier K, Hartung R. Electrocautery: principles and practice. J Endourol. 2003;17(8):541–555. doi: 10.1089/089277903322518545. [DOI] [PubMed] [Google Scholar]
- [10].Maness WL, Roeber FW, Clark RE, Cataldo E, Riis D, Haddad AW. Histologic evaluation of electrosurgery with varying frequency and waveform. J Prosthet Dent. 1978;40(3):304–308. doi: 10.1016/0022-3913(78)90037-9. [DOI] [PubMed] [Google Scholar]
- [11].Smith TL, Smith JM. Electrosurgery in otolaryngology-head and neck surgery: principles, advances, and complications. Laryngoscope. 2001;111(5):769–780. doi: 10.1097/00005537-200105000-00004. [DOI] [PubMed] [Google Scholar]
- [12].Shuman IE. Bipolar versus monopolar electrosurgery: clinical applications. Dent Today. 2001;20(12):74–76. [PubMed] [Google Scholar]
- [13].Glover JL, Bendick PJ, Link WJ, Plunkett RJ. The plasma scalpel: a new thermal knife. Lasers Surg Med. 1982;2(1):101–106. doi: 10.1002/lsm.1900020113. [DOI] [PubMed] [Google Scholar]
- [14].Bokor L, Hajdu Z, Szegedi Z, Kathy S, Bagi R. Laparoscopic hiatal reconstruction and use of harmonic scalpel instrument. Acta Chir Hung. 1997;36(1-4):41–42. [PubMed] [Google Scholar]
- [15].Koch C, Friedrich T, Metternich F, Tannapfel A, Reimann HP, Eichfeld U. Determination of temperature elevation in tissue during the application of the harmonic scalpel. Ultrasound Med Biol. 2003;29(2):301–309. doi: 10.1016/s0301-5629(02)00727-5. [DOI] [PubMed] [Google Scholar]


