Abstract
Hypoxia-inducible factor-1 (HIF-1) regulates the expression of hypoxia-inducible genes by binding erythropoietin (EPO) enhancer fragments. Of these genes, HIF-1 upregulates voltage-gated K+1.2 channels (Kv1.2) in rat PC12 cells. Whether HIF-1 regulates hypoxia-induced Kv channel expression in cultured pulmonary artery smooth muscle cells (PASMCs), however, has not been determined. In this study, we investigated the effects of hypoxia on the expression of Kv1.2 Kv1.5, Kv2.1, and Kv9.3 channels in PASMCs and examined the direct role of HIF-1 by transfecting either wild type or mutant EPO enhancer fragments.
Our results showed that 18 h exposure to hypoxia significantly increased the expression of Kv1.2, Kv1.5, Kv2.1, and Kv9.3; and this hypoxia-induced upregulation was completely inhibited after transfection with the wild type but not mutant EPO enhancer fragment.
These results indicate that HIF-1 regulates hypoxia-stimulated induction of Kv1.2, Kv1.5, Kv2.1, and Kv9.3 channels in cultured PASMCs.
KEY WORDS: hypoxia, pulmonary artery smooth muscle cells (PASMCs), voltage-gated K+ channels, erythropoietin, enhancer hypoxia-inducible factor-1 (HIF-1)
INTRODUCTION
Efficient gas exchange in the lungs is maintained through a mechanism known as hypoxic pulmonary vasoconstriction (HPV), which serves to match local perfusion to ventilation and optimize arterial blood gas exchange. Hypoxia has recently been shown to induce pulmonary artery contraction, even after the endothelium has been denuded [1, 2]. This hypoxia-induced constriction has been demonstrated to occur by inhibition of voltage-gated potassium channels (Kv) at the single pulmonary artery smooth muscle cell level. Voltage-gated potassium channels serve to depolarize the membrane potential, which in turn opens voltage-gated calcium channels. The rise in cytoplasmic calcium concentration within the cell activates the contractile apparatus. Previous studies have shown that Kv channels serve an important role during HPV [1, 3- 5]. At the molecular level, Kv channels are composed of pore-forming α-subunits and the cytoplasmic regulatory (auxiliary) β-subunits [6]. To date, nine families of Kv channels α-subunits have been identified, and each of these subunit groups have multiple subtypes. Based on patch-clamp recording studies using blocking antibodies, four candidate Kv channel α-subunits which could form O2-sensitive channels have been identified: namely, Kv1.2, Kv1.5, Kv2.1, and Kv9.3 [7- 13]. Following in vitro or in vivo exposure to chronic hypoxia of more than 24 h, Kv1.2, Kv1.5, Kv2.1, and Kv9.3 channel gene and protein expression were all reduced. Hong et al recently found that even shorter hypoxia durations of 6 to 24 h in vivo resulted in decreased gene transcription of these four Kv channels [10] However, there have been few studies on how shorter hypoxia exposures alter the mRNA and protein expression of these particular Kv a subunits (Kv1.2, Kv1.5, Kv2.1 and Kv9.3) in cultured rat PASMCs. Furthermore, the molecular mechanisms underlying HPV have not been fully elucidated. Hypoxia-inducible factor-1 (HIF-1) is a key mediator of pulmonary response to hypoxia [14]. HIF-1 is a heterodimeric transcription factor consisting of HIF-1α and HIF-1β subunits. Although HIF-1β is constitutively expressed in the lung, expression of HIF-1α is tightly regulated by O2 tension. Exposure of PASMC to hypoxia significantly increases HIF-1α expression [15]. HIF-1α translocates to the nucleus, dimerizes with HIF-1β, and binds to hypoxia response elements, which contain the erythropoietin (EPO) gene enhancer [16, 17]. HIF-1 recognizes an 8 bp DNA motif 5' TACGTGCT-3' in the EPO enhancer, but not to a mutated sequence containing a 3' nucleotide substitution that eliminates the enhancer function [18, 19]. Hypoxia signal transduction depends to some extent on hypoxia-activated HIF-1 binding to the EPO enhancer or a similar sequence [16, 17]. Thus, we hypothesized that HIF-1 may be involved in the hypoxia-induced oxygen-sensitive Kv channel expression. The aim of the current research was to investigate the effects of hypoxia on the gene and protein expression of Kv1.2, Kv1.5, Kv2.1, Kv9.3 channels in rat PASMCs, using RT-PCR and immunoblotting. Transient transfection with either wild or mutant EPO3'-enhancer fragments was used to determine the role of HIF-1 and reveal HPV-dependent molecular mechanisms.
MATERIALS AND METHODS
Cell culture and hypoxia exposure
Primary cultured PASMCs were prepared from 24 male Sprague-Dawley rats (125-250 g) as described previously [20]. Our animal protocol was approved by the Institutional Animal Care and Use Committee of the Huazhong University of Science and Technology, Tongji Medical School and conforms to current guidelines from the National Institutes of Health and the American Physiological Society for the use and care of laboratory animals. Briefly, the rats were anesthetized with urethane, and the heart and the lungs were removed rapidly and immediately placed in ice-cold D-Hanks solution. The intrapulmonary arteries (4th-6th divisions) were dissected free of adventitia and the endothelium was removed with a cotton swab. The remaining smooth muscle was first digested in 1 mg/ml collagenase (Worthington Biochemical Company) for 1 to 1.5 hours and then in 2.5 mg/ml trypsin (Worthington Biochemical Company) for 8 min at 37°C. DMEM/10% fetal bovine serum (FBS) was added to the solution to stop digestion. Cells were incubated in a humidified atmosphere of 5% CO2 in air at 37°C for 3 to 7 days and fed twice weekly with DMEM/20% FBS supplemented with 100 U/ml penicillin, and 100 μg/ml streptomycin (BioFluids, Camarillo, CA). The primary cultured PASMCs were subcultured at a 1:1 ratio. Passages two to four were used in this study. Twenty four hours before the experiments, the media was replaced with DMEM-0.3% FBS supplemented with 100 U/ml penicillin, and 100 μg/ml streptomycin in order to stop cell proliferation. The purity of the PASMCs in the primary cultures was confirmed by immunocytochemistry using a specific monoclonal antibody raised against smooth muscle α-actin. All the cells reacted with this antibody, indicating that the cultures contained only PASMCs. For hypoxic incubations, plates were transferred to an oxygen-regulated incubator containing a mixture of 92.5% N2, 5% CO2, and 2.5% O2 (partial pressure of oxygen 4.6-6.0 kPa) for 18 h. Control (normoxic) plates were cultured in 74% N2, 5% CO2, and 21% O2. There were no significant changes in pH values of the culture media or in cell viability following 18 h incubation under either normoxic or hypoxic conditions.
Transfection
PASMCs were transiently transfected with Epo enhancer fragments cloned into pRL-SV40 by Lipofectamine method according to manufacturer's protocol(Gibco BRL, Gaithersburg, MD). The EPO enhancer fragments were designed as follows: wild fragments (W18) were: 5'-agcttGCCCTACGTGCTGTCTCAg-3' (forward) and 3'-aCGGGATGCACGACAGAGTCt- taa-5' (reverse), and the mutant fragments (M18) were: 5'-agcttGCCCTAAAAGCTGTCTCAg-3' (forward) and 3'-aCGGGATTTTCGACAGAGTCttaa-5' (reverse).
Preparation of total RNA
Total RNA was obtained from quiescent passage four PASMCs using TRIzol Reagent according to the manufacturer's instructions. Isolated total RNA was dissolved in RNase-free water at 1μg/μl and stored at -70°C.
Semiquantitative reverse transcription polymerase chain reaction
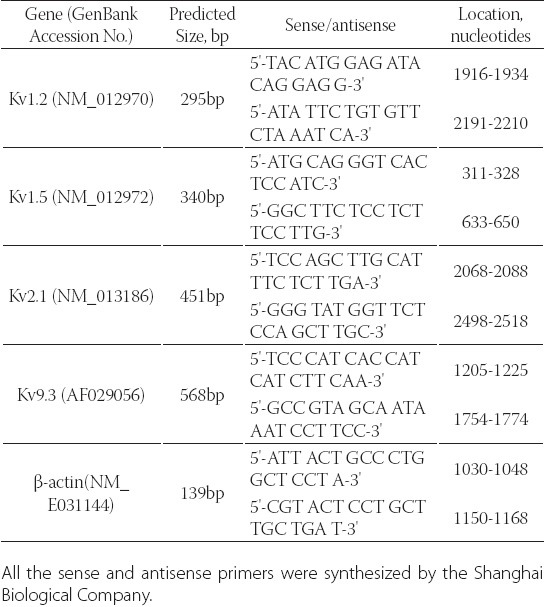
RT-PCR was performed with TaKaRa RNA PCR Kit Ver.2.1 according to manufacturer's instructions (TaKaRa Biotechnology, Co., Ltd. Dalian, China). The sense and antisense PCR oligonuleotide primers (Table 1) for cDNA amplification were specifically designed from coding regions of specific Kv channels as described previously [21].
TABLE 1.
Oligonucleotide sequences of the primers used for RTPCR
The cDNA samples were amplified by denaturing at 94°C for 1 min, annealing at 52- 60°C for 1 min, and extending at 72°C from 20 min. After 26 cycles, the PCR products were electrophoresed through a 1.5% agarose gel, and amplified cDNA bands were visualized by ethidium bromide staining. To quantify the PCR products (the amount of mRNA) of the Kv channels, an invariant mRNA of β-actin was used as an internal control. Immediately after each experiment, the OD values for each band on the gel were measured by a gel documentation system (UVP, Uplant, CA). The OD values of Kv channel signals were normalized to the OD values of the β-actin signals. The normalized values are expressed in arbitrary units for quantitative comparison.
Protein isolation and quantification by immunoblotting
Quiescent passage four PASMCs were washed with PBS, homogenized in M-PER buffer (Pierce company, No.78503), scraped into PBS (2 ml/dish), and centrifuged at 13,000 rpm for 20 min. Protein concentrations were determined by BCA protein assay, using BSA as a standard. For each sample, 40 μg of total protein was separated by SDS-PAGE on 8% gels. The proteins were then transferred onto nitrocellulose membrane, and the efficiency of the transfer was verified by Ponceau-S staining. The membranes were blocked with 5% nonfat dry milk and probed with affinity-purified polyclonal antibodies specific for Kv1.2, Kv1.5, and Kv2.1 (Alomone Laboratories, Jerusalem, Israel and Upstate Biotechnology, Charlottesville, VA). The membranes were washed three times and incubated with anti-rabbit or anti-mouse sheepradish peroxidase-conjugated IgG for 1 h. Enhanced chemiluminescence (ECL; Amersham, Piscataway, NJ) was used for detection of the bound antibody. Fornormalization, all membranes were also probed with α-actin (Sigma, St. Louis, MO).
Statistical analysis
The data were expressed as mean±SEM. Intergroup comparisons are made using a factorial ANOVA with post hoc testing using the Fisher least significant differences test. A p<0.05 was considered statistically significant.
RESULTS
Total RNA was extracted from passage 2-4 rat PASMCs incubated under normoxia or hypoxia for 18 h. Gene transcription (mRNA levels) of Kv channel a sub- units (Kv1.2, Kv1.5, Kv2.1, and Kv9.3) were examined, and the β-actin mRNA level was used as control. The mRNA levels of Kv1.2, Kv1.5, Kv2.1, and Kv9.3 were significantly increased after exposure to hypoxia for 18 h (Figure 1, p<0.05 for all). To understand the role of HIF-1 in hypoxia induced stimulation of K+ channel gene expression, we examined Kv1.2, Kv1.5, Kv2.1, and Kv9.3 mRNA expression in rat PASMCs transfected with either wild type or mutant EPO-enhancer fragments and exposed to hypoxia. Transfection with the wild type EPO-enhancer fragment completely blocked the hypoxia-induced increase in Kv1.2, Kv1.5, Kv2.1, and Kv9.3 mRNA expression. In contrast, transfection with the mutant EPO-enhancer fragment did not affect hypoxia-induced increase in Kv1.2 Kv1.5, Kv2.1, and Kv9.3 mRNA expression (Figure 1). Immunoblotting was used to compare protein levels of the channels in PASMCs incubated under nor- moxia or hypoxia. Since the Kv9.3 antibody was not available, only Kv1.2, Kv1.5, and Kv2.1 protein levels were examined. The α-actin protein level was used as control. Kv1.2, Kv1.5, and Kv2.1 channel protein levels were increased significantly after 18 h of hypoxia, while the protein level of α-actin was unchanged (Figure 2). Transfection with the wild type EPO-enhancer fragment inhibited the hypoxia-induced upregulation of Kv1.2, Kv1.5, and Kv2.1 protein expression. In contrast, transfection with the mutant EPO-enhancer fragment did not affect hypoxia-induced enhancement in Kv1.2, Kv1.5, and Kv2.1 protein expression (Figure 2).
FIGURE 1.
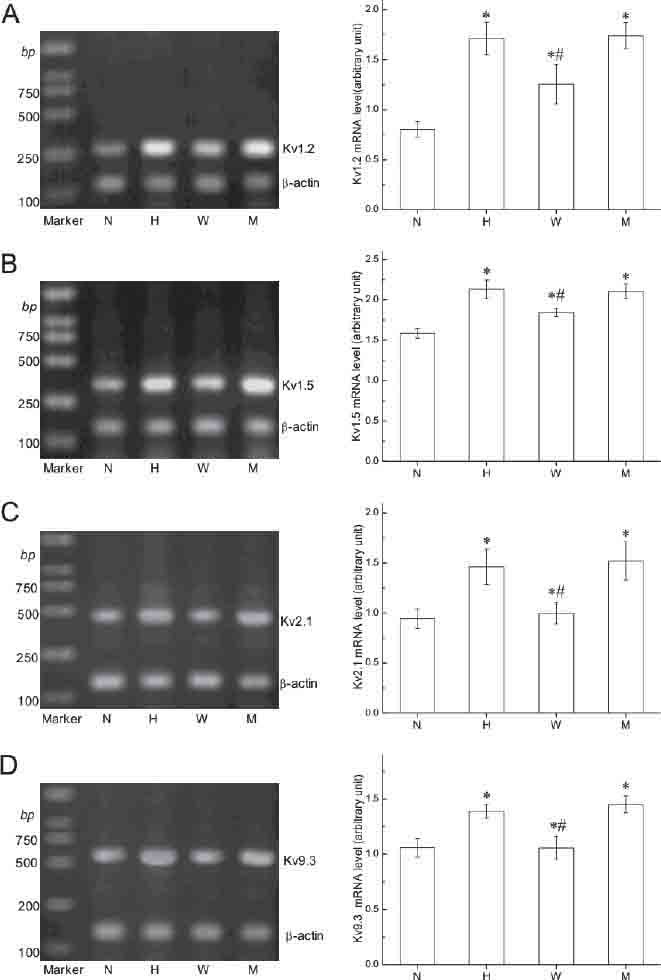
HIF-1 regulates hypoxia induced Kv channels (Kv1.2 Kv1.5, Kv2.1, and Kv9.3) mRNA expression in PASMCs. PCR amplified products are displayed in agarose gel for Kv1.2 (295 bp, A), Kv1.5 (340bp, B), Kv2.1 (451bp, C), Kv9.3 (568 bp, D) and β-actin (139 bp- A, B, C, and D). N, exposure to normoxia for 18 h; H, exposure to hypoxia for 18 h; W, exposed to hypoxia for 18 h after transfection with wildtype EPO-enhancer fragments; M, exposed to hypoxia for 18 h after transfection with mutant-type EPO-enhancer fragments. Right Panels, Data were normalized to the amount of β-actin. Values are presented as mean±SEM (experiments were independently repeated 5-6 times). *p<0.05 compared with N group; #p<0.05 compared to the hypoxia group.
FIGURE 2.
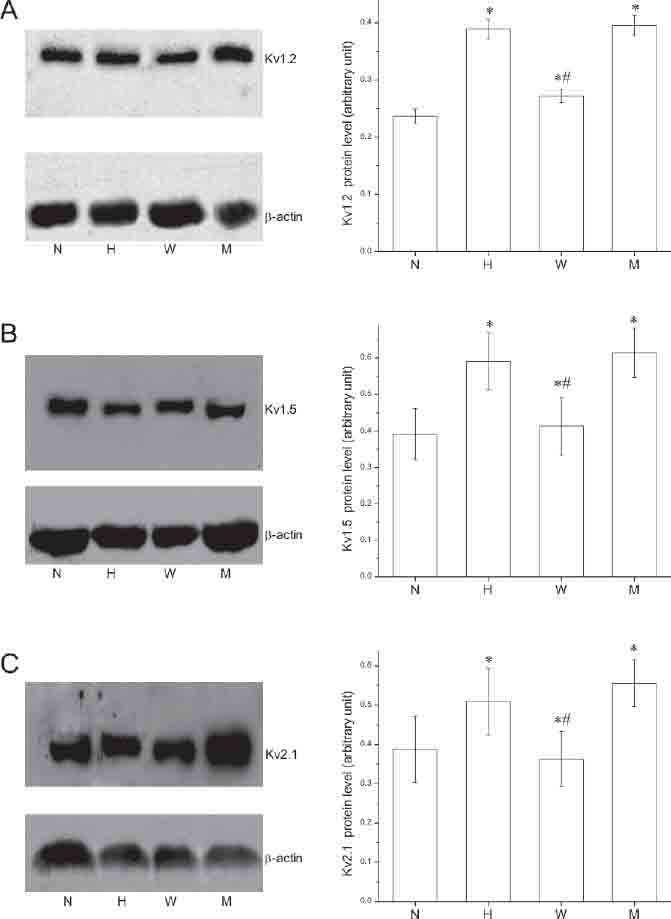
HIF-1 regulates hypoxia induced Kv channel (Kv1.2, Kv1.5, and Kv2.1) protein expression in PASMCs. Western blotting analysis of Kv1.2 (A), Kv1.5 (B) and Kv2.1 (C) channel proteins. Immunoblots of rat PASMCs proteins were incubated with affinity-purified anti-Kv1.2 (A), anti-Kv1.5 (B), antiKv2.1 (C), and anti-α-actin (A, B, and C) N, exposure to normoxia for 18 h; H, exposure to hypoxia for 18 h; W, exposed to hypoxia for 18 h after transfection with wild-type EPO-enhancer fragments; M, exposed to hypoxia for 18 h after transfection with mutant EPO-enhancer fragments. Right Panels, Data were normalized to the amount of α-actin. Values are presented as mean±SEM (experiments were independently repeated 5-6 times). *p<0.05 compared to the normoxia group; and #p<0.05 compared to the hypoxia group.
DISCUSSION
The goals of this study were to evaluate the effects of hypoxia on Kv a subunit expression in PASMCs and to determine the role of the EPO enhancer element on Kv a subunit gene and protein levels. The major findings of this study were that: (a) 18 h hypoxia exposure increased the expression of PASMC Kv a subunits (Kv1.2, Kv1.5, Kv2.1, and Kv9.3) at the transcriptional level; (b) protein expression of Kv1.2, Kv1.5, and Kv2.1 channels were significantly increased after 18 h hypoxia and (c) the effects of 18 h hypoxia were completely blocked following transfection with wild type but not mutant EPO-enhancer fragment. Our results suggest that HIF-1 regulates hypoxia-induced expression of Kv1.2 Kv1.5, Kv2.1, and Kv9.3 channels in cultured PASMCs through EPO enhancer effects. The activity of certain Kv channel subtypes has been shown to be O2 sensitive. Kv channels are important determinants of vascular tone control, and the role of Kv channels have been investigated in several models of chronic hypoxia. Shortly after the first report on the effect of acute hypoxia on K+ channels was published [22], Smirnov et al. [23] demonstrated that PASMCs of adult rats subjected to four weeks in an hypoxic environment have reduced Kv current compared with normoxic rats. It had been proposed that the observed reduction in Kv current amplitude is a result of decreased channel expression. The first evidence for down-regulation of specific Kv channel α-subunits (Kv1.2 and Kv1.5) in cultured rat PASMCs under chronic hypoxia (24-72 h) was provided by Wang et al [24]. More recently, it was reported that chronic hypoxia also decreases the mRNA expression of Kv1.1, Kv1.5, Kv2.1, Kv4.3, and Kv9.3 a subunits in cultured rat PASMCs [20, 25]. Likewise, PASMCs isolated from chronically hypoxic animals also showed reduced levels of these particular channels [26-29]. However, there are no previous reports of the effects of 18 h hypoxia on mRNA and protein expression of Kv a subunits (Kv1.2, Kv1.5, Kv2.1, and Kv9.3) in cultured rat PASMCs. In contrast to the results for the longer exposure times, we found that the expression of Kv1.2, Kv1.5, Kv2.1, and Kv9.3 channels increased at 18 h of hypoxia stimulation. A similar observation has been reported by Conforti and Millhorn [30], who demonstrated that gene expression of Kv1.2 in cultured PC12 cells was significantly upregulated after 18 h exposure to hypoxia. Interestingly, Hong et al. [10] found that mRNA expression of Kv1.2, Kv1.5, and Kv2.1 were all significantly downregulated in freshly isolated rat PASMCs that had previously been exposed to hypobaric hypoxia for 6 h. This difference in expression levels could be attributed to differences in in vivo and in vitro exposure to hypoxia, to differences between cultured and freshly isolated cells, and/or to differences between 6 and 18 h of exposure. Upregulation of Kv1.2, Kv1.5, Kv2.1, and Kv9.3 channel expression by 18 h hypoxia suggests an adaptive response following functional inhibition of Kv in response to acute hypoxia. HIF-1 is a heterodimeric transcription factor responsible for activation of many hypoxia-inducible genes including EPO and endothelin-i [16, 31, 32]. To date, most of the hypoxia-inducible gene expression systems have focused on the hypoxia responsive promoters/enhancer, which harbor HIF-1 binding sites. EPO enhancer fragments are located in the 3'-flanking region, and in combination with HIF-1, promote gene transcription [19, 31]. Since the mechanism underlying hypoxic induction of gene expression via the HIF-1 pathway has been well characterized, we hypothesized that hypoxia induced Kv1.2, Kv1.5, Kv2.1, and Kv9.3 channel gene upregulation could occur through HIF-1. To test our hypothesis, we transfected external EPO-enhancer fragments (wild-type or mutant) to test the effect of the enhancer on Kv channel expression. As reported in a previous study, the external EPO-enhancer fragments can compete with internal EPO-enhancer fragments (or similar sequences) to bind with HIF-1 in the cytoplasm and prevent HIF-1 entry into the nucleus, thereby inhibiting gene transcription [33]. The mutant EPO-enhancer fragments, with a “CGT” to “AAA” substitution in its sequence, eliminates HIF-1 binding as demonstrated by Semenza and colleagues [18, 19, 31]. The current study found that the upregulation of Kv1.2, Kv1.5, Kv2.1, and Kv9.3 channel expression after exposure to 18 h hypoxia was completely blocked by transfection with the wild type of EPO-enhancer fragment, but their expression was unaffected by transfection with the mutant EPO- enhancer fragment. These results are in agreement with a previous study, which showed that expression of HIF-1 is required for hypoxia-induced alterations in the Kv current [34]. In a conclusion, our results demonstrate that exposure to 18 h of hypoxia upregulated the expression of Kv1.2, Kv1.5, Kv2.1, and Kv9.3 channels in PASMCs, and this increase in gene expression is regulated through a HIF-1-dependent mechanism.
DECLARATION OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (No 30971243 and No 81170164) to Dr. Du. We thank Medjaden Bioscience Limited for editorial assistance in the preparation of this manuscript.
REFERENCES
- [1].Archer S, Michelakis E. The mechanism(s) of hypoxic pulmonary vasoconstriction: potassium channels, redox O2 sensors, and controversies. News Physiol Sci. 2002;17:131–137. doi: 10.1152/nips.01388.2002. [DOI] [PubMed] [Google Scholar]
- [2].Murray TR, Chen L, Marshall BE, Macarak EJ. Hypoxic contraction of cultured pulmonary vascular smooth muscle cells. Am J Respir Cell Mol Biol. 1990;3(5):457–465. doi: 10.1165/ajrcmb/3.5.457. [DOI] [PubMed] [Google Scholar]
- [3].Olschewski A, Hong Z, Nelson DP, Weir EK. Graded response of K+current, membrane potential, and [Ca2+]i to hypoxia in pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2002;283(5):1143–1150. doi: 10.1152/ajplung.00104.2002. [DOI] [PubMed] [Google Scholar]
- [4].Bonnet S, Archer SL. Potassium channel diversity in the pulmonary arteries and pulmonary veins: implications for regulation of the pulmonary vasculature in health and during pulmonary hypertension. Pharmacol Ther. 2007;115(1):56–69. doi: 10.1016/j.pharmthera.2007.03.014. [DOI] [PubMed] [Google Scholar]
- [5].Platoshyn O, Yu Y, Ko EA, Remillard CV, Yuan JX. Heterogeneity of hypoxia-mediated decrease in I(K(V)) and increase in [Ca2+](cyt) in pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;293(2):L402–416. doi: 10.1152/ajplung.00391.2006. [DOI] [PubMed] [Google Scholar]
- [6].Hille B. Ion Channels of Excitable Membranes. 3rd ed. Sunderland: Sinauer Associates Inc; 2001. Potassium channels and chloride channels; pp. 131–167. [Google Scholar]
- [7].Archer SL, Michelakis ED, Thébaud B, Bonnet S, Moudgil R, Wu XC, et al. A central role for oxygen-sensitive K+channels and mitochondria in the specialized oxygen-sensing system. Novartis Found Symp. 2006;272:157–171. discussion 171-175, 214-217. [PubMed] [Google Scholar]
- [8].Conforti L, Bodi I, Nisbet JW, Millhorn DE. O2-sensitive K+channels: role of the Kv1.2-subunit in mediating the hypoxic response. J Physiol. 2000;524(Pt 3):783–793. doi: 10.1111/j.1469-7793.2000.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hogg DS, Davies AR, McMurray G, Kozlowski RZ. Kv2.1 channels mediate hypoxic inhibition of I(Kv) in native pulmonary arterial smooth muscle cells of the rat. Cardiovasc Res. 2002;55(2):349–360. doi: 10.1016/s0008-6363(02)00411-x. [DOI] [PubMed] [Google Scholar]
- [10].Hong Z, Weir EK, Nelson DP, Olschewski A. Subacute hypoxia decreases voltage-activated potassium channel expression and function in pulmonary artery myocytes. Am J Respir Cell Mol Biol. 2004;31(3):337–343. doi: 10.1165/rcmb.2003-0386OC. [DOI] [PubMed] [Google Scholar]
- [11].Hulme JT, Coppock EA, Felipe A, Martens JR, Tamkun MM. Oxygen sensitivity of cloned voltage-gated K+channels expressed in the pulmonary vasculature. Circ Res. 1999;85(6):489–497. doi: 10.1161/01.res.85.6.489. [DOI] [PubMed] [Google Scholar]
- [12].Patel AJ, Lazdunski M, Honoré E. Kv2.1/Kv9.3, a novel ATP-dependent delayed-rectifier K+channel in oxygen-sensitive pulmonary artery myocytes. Embo J. 1997;16(22):6615–6625. doi: 10.1093/emboj/16.22.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pozeg ZI, Michelakis ED, McMurtry MS, Thébaud B, Wu XC, Dyck JR, et al. In vivo gene transfer of the O2-sensitive potassium channel Kv1.5 reduces pulmonary hypertension and restores hypoxic pulmonary vasoconstriction in chronically hypoxic rats. Circulation. 2003;107(15):12037–2044. doi: 10.1161/01.CIR.0000062688.76508.B3. [DOI] [PubMed] [Google Scholar]
- [14].Semenza GL, Agani F, Iyer N, Jiang BH, Leung S, Wiener C, et al. Hypoxia-inducible factor 1: from molecular biology to cardiopulmonary physiology. Chest. 1998;114(1 Suppl):40S–45S. doi: 10.1378/chest.114.1_supplement.40s. [DOI] [PubMed] [Google Scholar]
- [15].Yu AY, Frid MG, Shimoda LA, Wiener CM, Stenmark K, Semenza GL. Temporal, spatial, and oxygen-regulated expression of hypoxia-inducible factor-1 in the lung. Am J Physiol. 1998;275(4 Pt 1):L818–826. doi: 10.1152/ajplung.1998.275.4.L818. [DOI] [PubMed] [Google Scholar]
- [16].Weidemann A, Johnson RS. Biology of HIF-1alpha. Cell death and differentiation. 2008;15:4, 621–627. doi: 10.1038/cdd.2008.12. [DOI] [PubMed] [Google Scholar]
- [17].Benizri E, Ginouvès A, Berra E. The magic of the hypoxia-signaling cascade. Cell Mol Life Sci. 2008;65(7-8):1133–1149. doi: 10.1007/s00018-008-7472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Semenza GL. Regulation of erythropoietin production. New insights into molecular mechanisms of oxygen homeostasis. Hematol Oncol Clin North Am. 1994;8(5):863–884. [PubMed] [Google Scholar]
- [19].Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci U S A. 1993;90(9):4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Platoshyn O, Yu Y, Golovina VA, McDaniel SS, Krick S, Li L, et al. Chronic hypoxia decreases Kv channel expression and function in pulmonary artery myocytes. Am J Physiol Lung Cell Mol Physiol. 2001;280(4):L801–812. doi: 10.1152/ajplung.2001.280.4.L801. [DOI] [PubMed] [Google Scholar]
- [21].Yuan XJ, Wang J, Juhaszova M, Golovina VA, Rubin LJ. Molecular basis and function of voltage-gated K+channels in pulmonary arterial smooth muscle cells. Am J Physiol. 1998;274(4 Pt 1):L621–635. doi: 10.1152/ajplung.1998.274.4.L621. [DOI] [PubMed] [Google Scholar]
- [22].Yuan XJ, Goldman WF, Tod ML, Rubin LJ, Blaustein MP. Hypoxia reduces potassium currents in cultured rat pulmonary but not mesenteric arterial myocytes. Am J Physiol. 1993;264(2 Pt 1):L116–123. doi: 10.1152/ajplung.1993.264.2.L116. [DOI] [PubMed] [Google Scholar]
- [23].Smirnov SV, Robertson TP, Ward JP, Aaronson PI. Chronic hypoxia is associated with reduced delayed rectifier K+ current in rat pulmonary artery muscle cells. Am J Physiol. 1994;266(1 Pt 2):H365–370. doi: 10.1152/ajpheart.1994.266.1.H365. [DOI] [PubMed] [Google Scholar]
- [24].Wang J, Juhaszova M, Rubin LJ, Yuan XJ. Hypoxia inhibits gene expression of voltage-gated K+channel alpha subunits in pulmonary artery smooth muscle cells. J Clin Invest. 1997;100(9):2347–2353. doi: 10.1172/JCI119774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sweeney M, Yuan JX. Hypoxic pulmonary vasoconstriction: role of voltage-gated potassium channels. Respir Res. 2000;1(1):40–48. doi: 10.1186/rr11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Osipenko ON, Alexander D, MacLean MR, Gurney AM. Influence of chronic hypoxia on the contributions of non-inactivating and delayed rectifier K currents to the resting potential and tone of rat pulmonary artery smooth muscle. Br J Pharmacol. 1998;124(7):1335–1337. doi: 10.1038/sj.bjp.0702006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shimoda LA, Sylvester JT, Sham JS. Chronic hypoxia alters effects of endothelin and angiotensin on K+currents in pulmonary arterial myocytes. Am J Physiol. 1999;277(3 Pt 1):L431–439. doi: 10.1152/ajplung.1999.277.3.L431. [DOI] [PubMed] [Google Scholar]
- [28].Reeve HL, Sylvester JT, Sham JS. Alterations in a redox oxygen sensing mechanism in chronic hypoxia. J Appl Physiol. 2001;90(6):2249–2256. doi: 10.1152/jappl.2001.90.6.2249. [DOI] [PubMed] [Google Scholar]
- [29].Li KX, Fouty B, McMurtry IF, Rodman DM. Enhanced ETA-receptor-mediated inhibition of Kv channels in hypoxic hypertensive rat pulmonary artery myocytes. Am J Physiol. 1999;277(1 Pt 2):H363–370. doi: 10.1152/ajpheart.1999.277.1.H363. [DOI] [PubMed] [Google Scholar]
- [30].Conforti L, Millhorn DE. Selective inhibition of a slow-inactivating voltage-dependent K+channel in rat PC12 cells by hypoxia. J Physiol. 1997;502(Pt 2):293–305. doi: 10.1111/j.1469-7793.1997.293bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Molecular and cellular biology. 1992;12(12):5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hu J, Discher DJ, Bishopric NH, Webster KA. Hypoxia regulates expression of the endothelin-1 gene through a proximal hypoxiainducible factor-1 binding site on the antisense strand. Biochem Biophys Res Commun. 1998;245(3):894–899. doi: 10.1006/bbrc.1998.8543. [DOI] [PubMed] [Google Scholar]
- [33].Ye H, Hao TL, Jin XR. Effect of erythropoietin 3-enhancer on proliferation of pulmonary artery smooth muscle cells induced by hypoxia. Sheng Li Xue Bao. 2000;52(5):355–359. [PubMed] [Google Scholar]
- [34].Shimoda LA, Manalo DJ, Sham JS, Semenza GL, Sylvester JT. Partial HIF-1alpha deficiency impairs pulmonary arterial myocyte electrophysiological responses to hypoxia. Am J Physiol Lung Cell Mol Physiol. 2001;281(1):202–208. doi: 10.1152/ajplung.2001.281.1.L202. [DOI] [PubMed] [Google Scholar]


