Abstract
Brain natriuretic peptide (BNP) is released from ventricular myocites due to their stretching and volume overload. In heart failure there is BNP release. Aim of this study was to observe BNP release in acute myocardial infarction (AMI).
We measured BNP in 75 patients with AMI. Control group (n=61) was similar by age and gender to AMI group.
We found statistically significant elevation of BNP compared to controls (462.875 pg/ml vs 35.356 pg/ml,p< 0.001). Patients with severe systolic dysfunction had the highest BNP levels, while patients with the preserved systolic function had the lowest BNP levels (Group with EF<30% BNP= 1129.036 pg/ml vs Group with EF31-40 % BNP= 690.177 pg/ml vs Group with EF 41-50% BNP= 274.396 pg/ml vs Group with EF>51% BNP= 189.566 pg/ml, p< 0.001). We found statistically significant light positive correlation between BNP and left ventricle end-diastolic diameter (LVDd) (r= 0.246,p<0.05). and real positive correlation between BNP and peak troponin levels (r= 0.441,p < 0.05). BNP levels were higher in anteroseptal allocation of AMI compared to inferior allocation (835.80 pg/ml vs 243.03 pg/ml, p< 0.001) and in patients who were treated with heparin compared to fibrinolitic therapy (507.885 pg/ml vs 354.73 pg/ml, p< 0.05).
BNP is elevated in AMI and is a quantitative biochemical marker related to the extent of infarction and the left ventricle systolic dysfunction. Besides echocardiographic calculation, elevation of BNP could be used for quick and easy determination of the left ventricle systolic dysfunction.
KEY WORDS: brain natriuretic peptide, acute myocardial infarction, left ventricle systolic dysfunction
INTRODUCTION
In 1988 de Bold discovered BNP in blood of patients with congestive heart failure [1]. This peptide was named after porcine brain where is first isolated, but after it was realised that heart was its main source [2]. BNP is released from cardiac myocites due to their stretching, volume overload and high filling pressure. All of this actions result in high wall stress who is initiating release of BNP precursor Pre–Pro-BNP- it cleaves first to pro-BNP, then to the biologically active BNP and the inactive aminoterminal fragment, N-terminal prohormone of BNP- NT-proBNP [3, 4]. In the failling heart BNP release is a part of the compensatory action such as activation of renin-angiotensin-aldosteron system (RAAS) and symphathetic nervous system. Besides its role of extreme mechanic pump, the heart has now become new endocrine organ- by releasing BNP the heart expresses it's suffering. In numerous clinical and epidemiological studies [5, 6, 7] it was proved direct corellation between reduction of systolic function of the left ventricule and elevation of natriuretic peptides – this enables posibble biochemical diagnosis of the heart failure. In the heart with a good systolic function level of BNP is up to 100 pg/ml. If BNP level is is 100-500 pg/ml that requires further diagnostic evaluation («grey zone»). If BNP is higher than 500 pg/ml there is high probability of the heart failure [8]. Today the most frequent cause of the failing heart is coronary artery disease- acute myocardial infarction. In this work we wanted to observe BNP release in acute myocardial infarction and investigate its correlation with the left ventricle ejection fraction, left ventricle dimension and peak value of troponin, localization of infarction, modus of therapy and survival. There are only few studies about BNP release in AMI, still precise mechanism for it secretion has not been clear. In today reports have been many controversies and conflicts. It seems that besides chronical hemodinamic cause- high wall stress, ischaemia “per se” is also important contributor of its release [9, 10].
MATERIALS AND METHODS
Patients
In AMI group were involved 75 patients hospitalised in the acute phase of ST-segment elevation myocardial infarction in Intensive Coronary Care Unit, Clinic for heart disease and rheumatism, Sarajevo. Structure of control group (n=61) was similar by age and gender to AMI group – participants didn’t suffer from arterial hypertension nor stable angina pectoris and their echocardiographic exam was normal (without signs of the left ventricle wall hypertrophy or systolic or diastolic dysfunction). Prior the recruitment of participants, this study was approved by the Ethics Committee of Clinical Center University of Sarajevo.
Methods
The blood samples for BNP analysis in AMI group were taken within first 48 hours from the beginning of the symptoms. BNP was determinated with AxSYM assay (Abbott Laboratory) from venous blood collected in EDTA plastic tubes (Ethylenediaminetetraacetic acid). Left ventricle ejection fraction (LVEF) was determined by transthoracic echocardiographic examination by calculation method of Teicholtz and Simpson. The examinations were performed on TOSHIBA POWER VISION 7000 ultrasound device.
Statistical analysis
Data are expressed as a X±SEM or median and interval. For parametric variables not belonging to the same population Student t-test was used while for nonparametric variables we used X2-test. Mann-Whitney U-test is a test for assessing whether two of observations come from the same distribution. Pearson’s correlation test was used to assess association between measured parameters. p-values less than <0.05 was considered as statistically significant. For the statistical evaluation of data was used computer program Sigma Stat® 2.11 and Mathematica® 5.0.
RESULTS
We calculated body mass index (BMI) for AMI and control group and didn’t found out significant difference in the number of obese individuals (BMI > 30kg/m2) (p NS) (Table 1). Patients with AMI had significantly higher serum creatinine levels compared to controls (76.96 μmol/l vs 106.48μmol/l, p < 0.001) (Table 2).
TABLE 1.
Adiposity in AMI and control group

TABLE 2.
Serum creatinine in control and AMI group

In AMI group we found BNP levels significantly higher compared to control group (X=462.875 pg/ml vs controls 35.356 pg/ml, p < 0.001) (Table 3, Figure 1).
TABLE 3.
Plasma BNP level in control and AMI group

FIGURE 1.

Range of BNP levels in AMI and control group (minmax: minimal and maximal value, 25 %- 75%: 25th to 75th percentile, median value)
All patients were divided according to their LVEF in 4 groups: Group I-preserved LV function (EF >51%), Group II-mild LV dysfunction (EF 41-50%), Group III-moderate LV dysfunction (EF 31-40%) and Group IV-severe LV dysfunction (EF <30%). Patients in the group with severe left ventricle dysfunction had the highest level of BNP and vice versa- the patients with the preserved systolic function had the lowest levels of BNP (Table 4, Figure 2). We investigated possible correlation of BNP level and the left ventricle end-diastolic diameter (LVDd) in AMI by correlation test and found statistically significant light positive correlation (p<0.05) with weak correlation coefficient (r=0.246) (Figure 3). We also tested possible correlation of BNP and creatine kinase (CK) and peak troponin value- test of correlation showed statistically significant positive correlation (p<0.05) with medium strength of association -correlation coefficient r= 0.332 for CK and r= 0.441 for troponin (Figure 4 and 5). We investigated BNP levels in groups formed by different allocation of the left ventricle wall infarction and found statistically significant higher levels in anteroseptal allocation compared to inferior allocation (Table 5). We measured BNP in patients who were treated with fibrinolitic therapy and found statistically significant lower levels in comparation to the patients who were treated with low-weight molecular heparin (Table 6).
TABLE 4.
BNP value in the patients group with a different LVEF

FIGURE 2.

Range of BNP values in the different class of LVEF (minmax: minimal and maximal value, 25 %- 75%: 25th to 75th percentile, median value)
FIGURE 3.

Correlation of BNP and LVDd in AMI
FIGURE 4.

Correlation of BNP and Creatine kinase value in AMI
FIGURE 5.
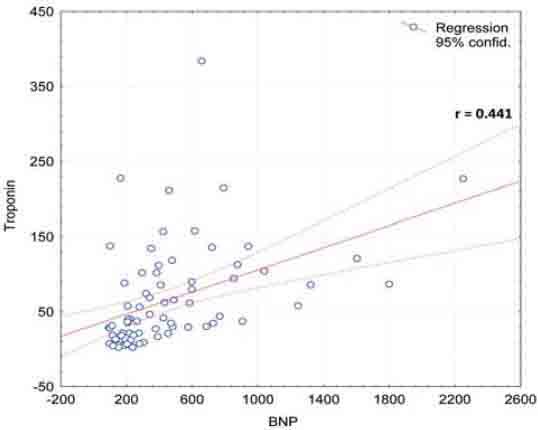
Correlation of BNP and peak troponin value in AMI
TABLE 5.
BNP levels according to allocation of infarct

TABLE 6.
BNP levels in patients treated with fibrinolitic and heparin therapy

DISCUSSION
We found statisticaly significant elevated levels of plasma BNP in acute myocardial infarction. Indeed, Morita and al. [11] and Richards and al. [12] by studying patients in AMI found out also significantly higher BNP plasma levels in comparation with healthy controls. Talwar et al. [13] also found elevated N-terminal pro BNP in acute myocardial infarction. In our study we didn’t found out significant difference in the number of obese individuals in AMI and control group. Recent studies have shown that obese and overweight individuals have considerably lower circulating natriuretic peptide levels compared with individuals with a normal body mass index (BMI). The lower levels of BNP relative to lean patients seem to persist even when obese patients are in HF, despite a similar severity of HF [14, 15, 16]. We excluded obesity as a potential reason for lower BNP levels in controls. Patients with AMI had significantly higher serum creatinine levels compared to controls (76.96 μmol/l vs 106.48μmol/l, p<0.001). There is an important interrelationship between cardiac and renal dysfunction. About one third of patients with chronic heart failure have renal insufficiency, defined by an eGFR (estimated glomerular filtration rate) less than 60 ml/min. Current data suggest that the cause of elevated natriuretic peptide levels in renal failure is multifactorial, representing in part a true counter-regulatory response from the heart to the kidney, and not simply diminished passive renal clearance. In order to maintain optimal diagnostic performance, the cut point for detecting HF may need to be raised when eGFR is less than 60 ml/min, and suggested cut –point is 200 pg/ml [8]. Potential reasons for higher creatinine levels in AMI groups are co-morbidities (arterial hypertension, diabetes mellitus) who affect renal function but elevation is possibly caused by cardio-renal syndrome type 1 (acute worsening of heart function -acute heart failure or acute coronary syndrome leading to kidney injury and/or dysfunction)[17]. Therefore higher BNP levels in AMI could be partly due to higher creatinine levels that are not only simple consequence of renal dysfunction “per se” but also consequence of heart dysfunction and pre-renal azotemia (cardio-renal syndrome type 1). We found that patients with severe systolic dysfunction (LVEF < 30%) had the highest levels of BNP, while group with the normal systolic function (LVEF > 51%) has the lowest level. Our data are consistent with those of Groenning et al. [6] which showed direct correlation between increase of BNP and decrease of LVEF. Dilic et al. [18] tested correlation of BNP and LVEF and found statistically real negative correlation, large strength of association between BNP levels and LVEF (r= –0.684 (p<0.05) -this finding has implications for the potential use of BNP as a marker for LV dysfunction following AMI. Easy and quick determination of BNP in AMI gives us important information of LVEF. While the current ’gold standard’ for the presence of LV dysfunction is the echocardiogram, provision of this investigation is by no means routine after AMI and adequate images may not be obtained in a proportion of patients (adipose patients, thoracic deformities, chronic opstructive pulmonary disease). We found out statistically significant negative correlation between BNP levels and left ventricule end-diastolic diameter (LVDd), small strength of association (r= 0.246, p< 0.05). We speculated that this correlation was weak because we measured LVDd in acute phase of AMI (it was too short period of the observation and LV had not enough time to became dilatated) and dilatation will start in months will come. Nilsson and al. [19] by studying patients with signs of LV dilatation one year after AMI confirmed that they had elevated BNP in acute phase. These patients could be identified earlier if their BNP was measured in acute phase of infarction. Therefore BNP could be use as an early predictor of LV dilation in future. Necrosis and apoptosis of myocites in AMI are contributors of progressive left ventricule dysfunction- we hypothesised that levels of BNP could be correlated with peak value of marker of myocites necrocis troponin. In our study we confirmed positive correlation of BNP and peak troponin levels, with medium strength of association (r= 0.441, p< 0.05). In literature Karciauskaite et al. [20] reported strong correlation between BNP and peak troponin, strenght of association was large (r = 0.72). The possible reason for lower correlation coefficient values obtained in our study may be due to fact that BNP gene transcription is increased both in infracted tissue and it surrounding ischemic but viable myocardium whose extent differs [21]. Therefore BNP could be used as a marker of extensibility of necrotic myocardium but also ishemic viable myocardium at risk. Our results are consistent with those of Talwar et al. [22] who found BNP elevated to a greater extent in anterior compared to inferior infarction suggesting that a larger area of myocardium was at risk in anterior allocation. We found lower BNP levels in patient treated with fibrinolitic therapy compared to heparin probably due to greater reperfusion and salvation of myocardium obtained by streptokinase. Jeong at al. [23] reported that angiographic ‘no-reflow’ phenomenon in ST segment elevation AMI can be predicted by BNP levels on admission.
CONCLUSION
BNP levels are significantly higher in AMI group compared to controls and its rise is proportional to decrease of LVEF. BNP could be used as a biochemical marker of LV systolic function in AMI. BNP was correlated with CK and peak troponin levels and were higher in anteroseptal allocation of infarct. Degree of BNP rise could be use for quick and easy estimation of infarction size. BNP levels were higher in patients who were treated with heparin compared to fibrinolitic-besides greater reduction of LVEF the reason for it could be more extensive coronary artery disease. We speculated that BNP could be used for the estimation of severity of coronary artery disease – this could be an interesting topic for the future studies.
DECLARATION OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- [1].de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extracts in rats. Life Sci. 1981;28:89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- [2].Sudoh T, Kangawa K, Minamino N, Matsuo H. A new natriuretic peptide in porcine brain. Nature. 1988;332:78–81. doi: 10.1038/332078a0. [DOI] [PubMed] [Google Scholar]
- [3].Thygesen K, Mair J, Mueller C, Huber K, Weber M, Plebani M, et al. Recommendations for the use of natriuretic peptides in acute cardiac care, A position statement from the Study Group on Biomarkers in Cardiology of the ESC Working Group on Acute Cardiac Care. Eur Heart J. 2011 doi: 10.1093/eurheartj/ehq509. doi: 10.1093/eurheartj/ehq509. [DOI] [PubMed] [Google Scholar]
- [4].Sudoh T, Maekawa K, Kojima M, Minamino M, Kangawa N, Matsuo H. Cloning and sequence analysis of cDJNA encoding a precursor for human brain natriuretic peptide in plasma. Clin Chim Acta. 2002;316:129–35. doi: 10.1016/0006-291x(89)92269-9. [DOI] [PubMed] [Google Scholar]
- [5].Sagnella GA. Measurement and importance of plasma brain natriuretic peptide and related peptides. Ann Clin Biochem. 2001;38:83–93. doi: 10.1258/0004563011900317. [DOI] [PubMed] [Google Scholar]
- [6].Groenning BA, Nilsson JC, Sondergaard L, Kjaer A, Larsson HB, Hildebrant PR. Evaluation of impaired left ventricular ejection fraction and increased dimension by multiple neurohumoral plasma concentrations. Eur J Heart Fail. 2001;3:699–708. doi: 10.1016/s1388-9842(01)00181-7. [DOI] [PubMed] [Google Scholar]
- [7].Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–167. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- [8].Maisel A, Mueller C, Adams K, Jr, Anker DS, Aspromonte N, Cleland JG, et al. Review: State of the art: Using natriuretic peptide levels in clinical practice. Eur J Heart Fail. 2008;10(9):824–839. doi: 10.1016/j.ejheart.2008.07.014. [DOI] [PubMed] [Google Scholar]
- [9].Goetz JP. Pro-BNP-derived peptides in cardiac disease. Scand J Clin Lab Invest. 2004;64:497–510. doi: 10.1080/00365510410002913. [DOI] [PubMed] [Google Scholar]
- [10].Nogales JM, Fuentes ME, Morales A, Martinez L, Marzal D, Alonso R. Neurohormonal prediction of post-infarction ventricular dysfunction and coronary disease. Rev Esp Cardiol. 2004;57:472–475. [PubMed] [Google Scholar]
- [11].Morita E, Yashue H, Yoshimura M, Ogawa H, Jougasaki M, Matsura T, et al. Increased plasma levels of BNP in patients with acute myocardial infarction. Circulation. 1993;88:82–91. doi: 10.1161/01.cir.88.1.82. [DOI] [PubMed] [Google Scholar]
- [12].Richards MA, Nicholls MG, Yandle TG, Ikram H, Espiner EA, Turner JG, et al. Neuroendocrine prediction of left ventricular function and heart failure after acute myocardial infarction. Heart. 1999;81:114–120. doi: 10.1136/hrt.81.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Talwar S, Squire BI, Downie PF, McCullough AM, Campton MC, Davies JE, et al. Profile of plasma N-terminal pro-BNP following acute myocardial infarction. Eur Heart J. 2000;21:1514–1521. doi: 10.1053/euhj.1999.2045. [DOI] [PubMed] [Google Scholar]
- [14].Daniels LB, Clopton P, Bhalla V, Krishnaswamy P, Nowak RM, James McCord. How obesity affects the Cut-Points for B-Type Natriuretic Peptide in the diagnosis of acute heart failure. Am Heart J. 2006;151(5):999–1005. doi: 10.1016/j.ahj.2005.10.011. [DOI] [PubMed] [Google Scholar]
- [15].Mehra MR, Uber PA, Park MH, Scott RL, Ventura HO, Harris BC, et al. Obesity and suppressed B-type natriuretic peptide levels in heart failure. J Am Coll Cardiol. 2004;43:1590–1595. doi: 10.1016/j.jacc.2003.10.066. [DOI] [PubMed] [Google Scholar]
- [16].McCord J, Mundy BJ, Hudson MP, Maisel AS, Hollander JE, Abraham WT, et al. Relationship between obesity and B-type natriuretic peptide levels. Arch Intern Med. 2004;164:2247–2252. doi: 10.1001/archinte.164.20.2247. [DOI] [PubMed] [Google Scholar]
- [17].Ronco C, McCullough P, Anker SD, Anand I, Aspromonte N, Bagshaw SM, et al. Cardiorenal syndromes: report from the consensus conference of the Acute Dialysis Quality Initiative. Eur Heart J. 2010;31:703–711. doi: 10.1093/eurheartj/ehp507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dilic M, Nalbantic DA, Arslanagic A, Huskic J, Brdjanovic S, Kulic M, et al. Biphasic and monophasic pattern of BNP release in acute myocardial infarction. Coll Antropol. 2011;35(1):155–159. [PubMed] [Google Scholar]
- [19].Nilsson J, Groenning B, Nielsen G, Fritz-Hansen T, Trawinski J, Hildebrandt P, et al. Left ventricular remodeling in the first year after acute myocardial infarction and the predictive value of N-terminal pro brain natriuretic peptide. Am Heart J. 2002;143:696–702. doi: 10.1067/mhj.2002.120293. [DOI] [PubMed] [Google Scholar]
- [20].Karciauskaite D, Grybauskiene R, Grybauskas P, Janenaite J. Brain natriuretic peptide and other cardiac markers in predicting left ventricular remodeling in patients with the first myocardial infarction. Medicina (Kaunas) 2004;(40):949–956. [PubMed] [Google Scholar]
- [21].Morrow A.D, Cannon PC, Jesse LR, Newby LK, Ravkilde J, Storrow B.A, et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: Clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation. 2007;115:356–375. doi: 10.1161/CIRCULATIONAHA.107.182882. [DOI] [PubMed] [Google Scholar]
- [22].Talwar S, Squire BI, Downie PF, McCullough AM, Campton MC, Davies JE, et al. Profile of plasma N-terminal pro BNP following acute myocardial infarction. Eur Heart J. 2000;21:1514–1521. doi: 10.1053/euhj.1999.2045. [DOI] [PubMed] [Google Scholar]
- [23].Jeong YH, Kim WJ, Park DW, Choi BR, Lee SW, Kim YH, et al. Serum B-type natriuretic peptide on admission can predict the ‘no-reflow’ phenomenon after primary drug-eluting stent implantation for ST-segment elevation myocardial infarction. Int J Cardiol. 2010;141(2):175–181. doi: 10.1016/j.ijcard.2008.11.189. [DOI] [PubMed] [Google Scholar]


